

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (2): 246-255.DOI: 10.11983/CBB24143 cstr: 32102.14.CBB24143
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Zheng Guo1, Xiangjun Shao2, Haiwen Lu1, Dan Hou1, Simeng Kong1, Xiangyu Li1, Huaqian Liu1, Xinchun Lin1,*( )
)
Received:2024-09-18
Accepted:2024-11-15
Online:2025-03-10
Published:2024-11-26
Contact:
Xinchun Lin
Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper[J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255.
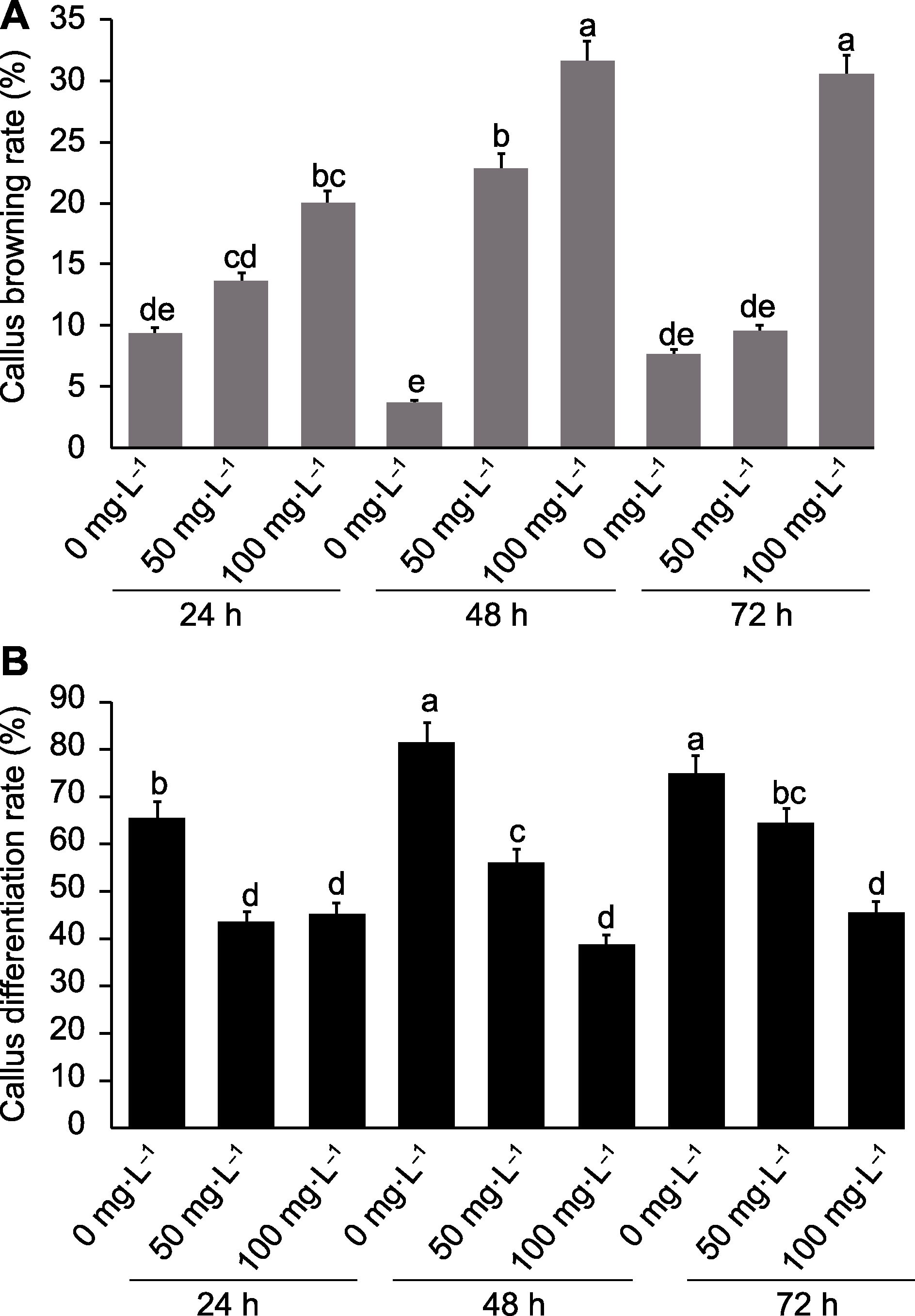
Figure 1 Results of colchicine treatment of Dendrocalamus asper calluses based on liquid suspension method (A) Callus browning rate at different colchicine concentrations and treatment times; (B) Callus differentiation rate at different colchicine concentrations and treatment times. Data are means ± SD (n=10), and different lowercase letters indicate significant differences among different treatments.
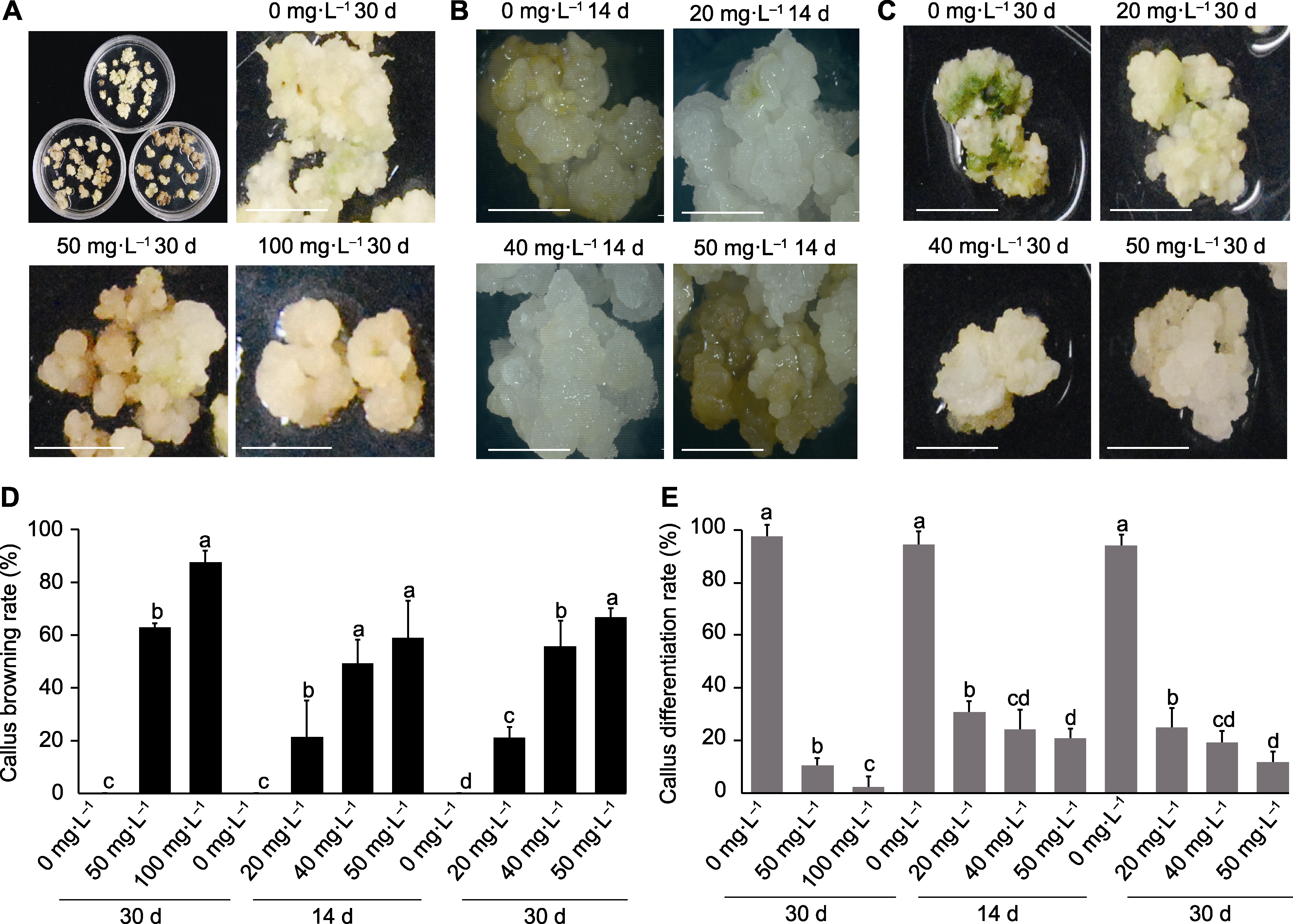
Figure 2 Results of colchicine treatment of Dendrocalamus asper calluses based on solid medium mixed culture method (A) Treat with 0, 50, and 100 mg∙L-1 colchicine for 30 days; (B) Treat with 0, 20, 40, and 50 mg∙L-1 colchicine for 14 days; (C) Treat with 0, 20, 40, and 50 mg∙L-1 colchicine for 30 days; (D) Callus browning rate; (E) Callus differentiation rate. Data are means ± SD (n=10), and different lowercase letters indicate significant differences among different treatments. Bars=5 mm
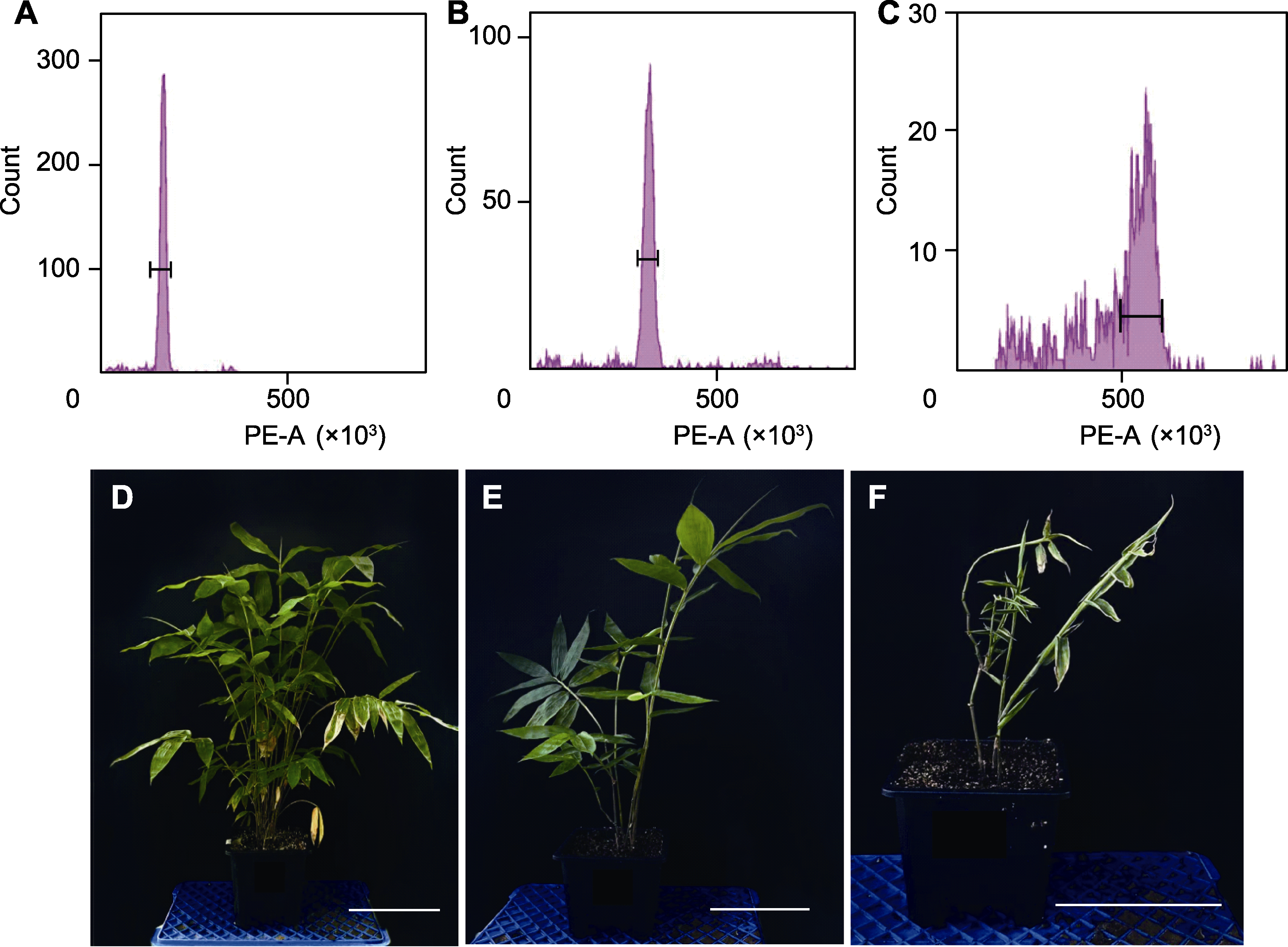
Figure 3 Results of flow cytometry analysis and morphological observation of different polyploids in Dendrocalamus asper (A), (D) Control group of hexaploid (6×); (B), (E) Experimental group of dodecaploid (12×); (C), (F) Experimental group of octaploid (18×). Bars=20 cm
| Colchicine concentration and treatment time | Number of chromosome doubling | Number of regenerated plantlets obtained | Doubling rate (%) | |
|---|---|---|---|---|
| Liquid suspension method | 50 mg∙L-1, 24 h | 0 | 7 | 0 |
| 50 mg∙L-1, 48 h | 2 | 7 | 28.57 | |
| 50 mg∙L-1, 72 h | 3 | 8 | 37.50 | |
| 100 mg∙L-1, 24 h | 0 | 5 | 0 | |
| 100 mg∙L-1, 48 h | 6 | 11 | 54.54 | |
| 100 mg∙L-1, 72 h | 2 | 6 | 33.33 | |
| Solid medium mixed culture method | 40 mg∙L-1, 14 d | 1 | 1 | 100 |
| 40 mg∙L-1, 30 d | 1 | 1 | 100 | |
| 50 mg∙L-1, 14 d | 1 | 1 | 100 |
Table 1 Chromosome doubling rate of regenerated plantlets of Dendrocalamus asper
| Colchicine concentration and treatment time | Number of chromosome doubling | Number of regenerated plantlets obtained | Doubling rate (%) | |
|---|---|---|---|---|
| Liquid suspension method | 50 mg∙L-1, 24 h | 0 | 7 | 0 |
| 50 mg∙L-1, 48 h | 2 | 7 | 28.57 | |
| 50 mg∙L-1, 72 h | 3 | 8 | 37.50 | |
| 100 mg∙L-1, 24 h | 0 | 5 | 0 | |
| 100 mg∙L-1, 48 h | 6 | 11 | 54.54 | |
| 100 mg∙L-1, 72 h | 2 | 6 | 33.33 | |
| Solid medium mixed culture method | 40 mg∙L-1, 14 d | 1 | 1 | 100 |
| 40 mg∙L-1, 30 d | 1 | 1 | 100 | |
| 50 mg∙L-1, 14 d | 1 | 1 | 100 |
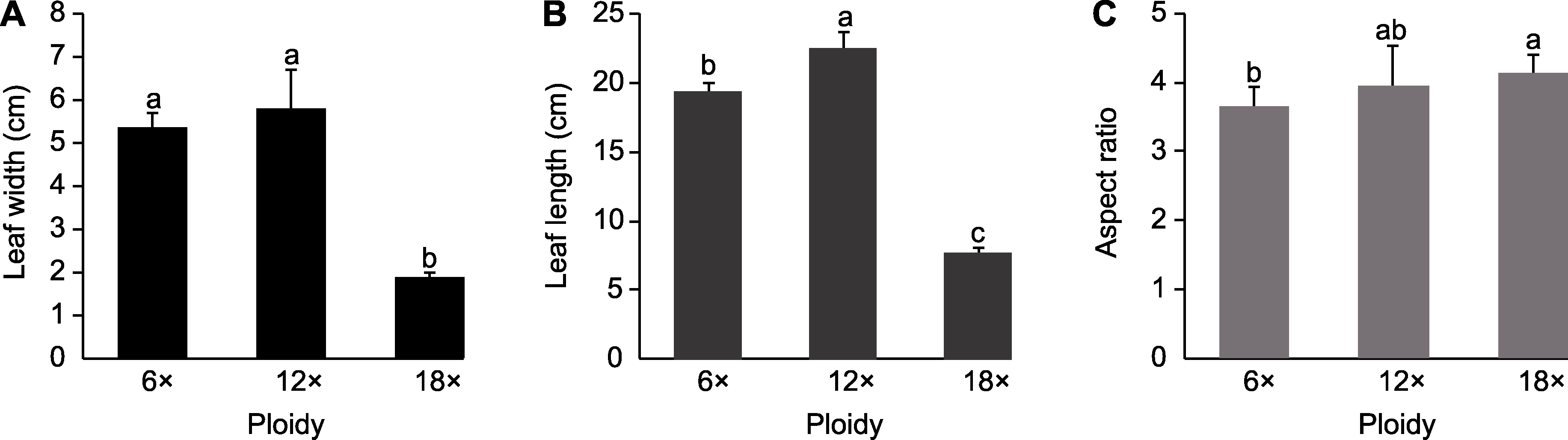
Figure 4 Morphological characteristics of Dendrocalamus asper leaves with different ploidy (A) Comparison of leaf width; (B) Comparison of leaf length; (C) Comparison of aspect ratio. Data are means ± SD (n=10), and different lowercase letters indicate significant differences among the three ploidies.
| Ploidy | Blade thickness (μm) | Upper skin thickness (μm) | Lower skin thickness (μm) |
|---|---|---|---|
| 6× | 82.83±7.19 c | 9.47±0.56 c | 7.19±0.30 b |
| 12× | 121.86±5.30 b | 15.19±0.86 b | 8.02±1.58 b |
| 18× | 168.74±11.47 a | 23.12±0.53 a | 15.06±0.50 a |
Table 2 Analysis of leaf tissue parameters of Dendrocalamus asper with different ploidy
| Ploidy | Blade thickness (μm) | Upper skin thickness (μm) | Lower skin thickness (μm) |
|---|---|---|---|
| 6× | 82.83±7.19 c | 9.47±0.56 c | 7.19±0.30 b |
| 12× | 121.86±5.30 b | 15.19±0.86 b | 8.02±1.58 b |
| 18× | 168.74±11.47 a | 23.12±0.53 a | 15.06±0.50 a |
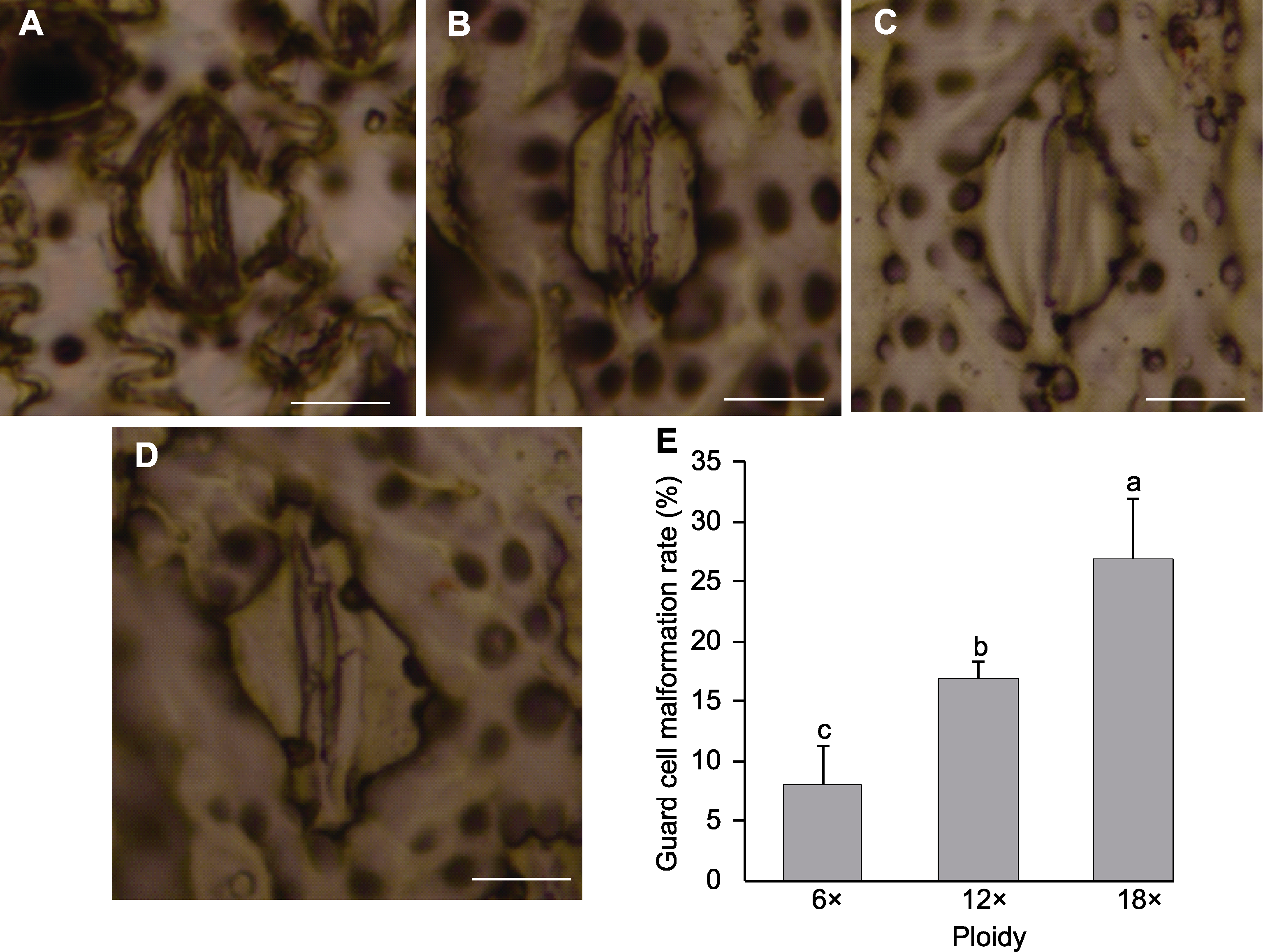
Figure 5 Observation of guard cells of different ploidy of Dendrocalamus asper (A) Control group of hexaploid (6×); (B) Experimental group of dodecaploid (12×); (C) Experimental group of octaploid (18×); (D) Malformed guard cells; (E) Guard cell malformation rate (different lowercase letters indicate significant differences among the three ploidies.). Bars=10 μm
| Ploidy | Guard cells | |
|---|---|---|
| Length (μm) | Width (μm) | |
| 6× | 20.25±1.30 c | 7.04±1.02 b |
| 12× | 28.37±2.20 b | 8.40±0.81 a |
| 18× | 36.54±5.58 a | 8.96±1.32 a |
Table 3 Stomatal parameters of Dendrocalamus asper with different ploidy
| Ploidy | Guard cells | |
|---|---|---|
| Length (μm) | Width (μm) | |
| 6× | 20.25±1.30 c | 7.04±1.02 b |
| 12× | 28.37±2.20 b | 8.40±0.81 a |
| 18× | 36.54±5.58 a | 8.96±1.32 a |
| [1] | 曹嘉雯 (2022). 软枣猕猴桃多倍体诱导及倍性鉴定. 硕士论文. 长春: 吉林农业大学. pp. 42-44. |
| [2] | 陈松河, 马丽娟, 丁振华, 罗祺, 刘婧 (2018). 5种牡竹属笋用竹竹笋营养成分之比较. 竹子学报 37(4), 4-8, 19. |
| [3] |
符勇耀, 蔡莉, 李丰耀, 杨文俊, 徐文姬, 姜思佳, 杨利平 (2024). 兰州百合多倍体离体诱导及其分子细胞鉴定. 草业学报 33(7), 172-181.
DOI |
| [4] | 高志民 (2023). 竹类植物遗传育种研究进展. 世界竹藤通讯 21, 1-9. |
| [5] | 郭恒琳, 勾晓婉 (2024). 作物倍性育种研究进展. 现代农业科技 (2), 20-26. |
| [6] | 国家林业和草原局 (2021). 林业和草原统计年鉴2021. 北京: 中国林业出版社. pp. 10-11. |
| [7] |
李高科, 陈琦, 孟鑫, 林海建 (2021). 秋水仙素加倍玉米单倍体胚性愈伤组织的初步研究. 植物遗传资源学报 22, 1606-1614.
DOI |
| [8] | 李霖锋, 刘宝 (2019). 植物多倍化与多倍体基因组进化研究进展. 中国科学: 生命科学 49, 327-337. |
| [9] | 李淑洁, 裴怀弟, 刘新星, 陈军, 江晶, 张朝巍 (2023). 植物染色体加倍的表型和遗传学效应研究进展. 寒旱农业科学 2, 1079-1084. |
| [10] | 罗静, 周厚成, 王永清 (2005). 园艺植物化学诱变与抗性突变体筛选研究进展. 中国农学通报 21(8), 302-305. |
| [11] | 石庆华, 刘平, 刘孟军 (2012). 果树倍性育种研究进展. 园艺学报 39, 1639-1654. |
| [12] | 唐军荣, 李斌, 朱丽娜, 张俊, 何承忠, 李贤忠, 辛培尧 (2016). 滇杨多倍体苗期叶片形态及光合生理比较分析. 林业科学研究 29, 103-109. |
| [13] | 陶抵辉, 刘明月, 肖君泽, 邓建平 (2007). 生物多倍体诱导方法研究进展. 生命科学研究 (S1), 6-13. |
| [14] |
王江银, 徐婉宁, 苏洋, 张博 (2023). 干旱胁迫下紫花苜蓿和黄花苜蓿实生苗叶片形态及解剖结构变化. 华北农学报 38(S1), 228-236.
DOI |
| [15] | 徐克凡, 彭忠明, 皮培尧, 周明兵, 汤定钦 (2024). 开花雷竹埋鞭繁育竹种间杂交育种的开花小苗. 分子植物育种 1-11. http://kns.cnki.net/kcms/detail/46.1068.s.20240131.1413.004.html. |
| [16] | 徐鹏飞, 杨艳红, 张毓婷, 陈云, 汤定钦 (2020). 毛竹四倍体诱导及初步鉴定. 林业科学 56(8), 55-62. |
| [17] |
张俊芳, 刘庆华, 王奎玲, 刘庆超, 孙阳 (2009). 秋水仙素诱导青岛百合四倍体研究. 核农学报 23, 454-457.
DOI |
| [18] | 张立荣, 陈南 (2009). 分离植物叶片上、下表皮新方法. 生物学通报 44(12), 49-50, 63. |
| [19] | 张云洁, 蔡昌杨, 冉取丙, 高鸿烨, 朱强 (2021). 竹子遗传改良技术研究进展. 世界林业研究 34(5), 26-31. |
| [20] |
周慧文, 冯斗, 严华兵 (2015). 秋水仙素离体诱导多倍体研究进展. 核农学报 29, 1307-1315.
DOI |
| [21] | Charoenphun N, Pakeechai K (2023). Effect of thickness on qualities of dried sweet bamboo shoots (Dendrocalamus asper Backer) products. J Food Health Bioenviron Sci 14, 1-8. |
| [22] | Correia S, Braga A, Martins J, Correia B, Pinto G, Canhoto J (2023). Effects of polyploidy on physiological performance of acclimatized Solanum betaceum Cav. plants under water deficit. Forests 14, 208. |
| [23] | Jin Y, Zhao Y, Ai S, Chen X, Liu X, Wang H, Han Y, Ma F, Li C (2022). Induction of polyploid Malus prunifolia and analysis of its salt tolerance. Tree Physiol 42, 2100-2115. |
| [24] | Kong CK, Tan YN, Chye FY, Sit NW (2020). Nutritional compositions, biological activities, and phytochemical contents of the edible bamboo shoot, Dendrocalamus asper, from Malaysia. Int Food Res J 27, 546-556. |
| [25] | Li XY, Zhang LY, Wei XC, Datta T, Wei F, Xie ZQ (2024). Polyploidization: a biological force that enhances stress resistance. Int J Mol Sci 25, 1957. |
| [26] | Mangena P (2023). Impact of polyploidy induction for salinity stress mitigation in soybean (Glycine max L. Merrill). Plants 12, 1356. |
| [27] | Mustafa AA, Derise MR, Yong WTL, Rodrigues KF (2021). A concise review of Dendrocalamus asper and related bamboos: germplasm conservation, propagation and molecular biology. Plants 10, 1897. |
| [28] | Qiao GR, Liu MY, Song KL, Li HY, Yang HQ, Yin YF, Zhuo RY (2017). Phenotypic and comparative transcriptome analysis of different ploidy plants in Dendrocalamus latiflorus Munro. Front Plant Sci 8, 1371. |
| [29] | Sun HY, Wang JF, Li H, Li TK, Gao ZM (2023). Advancements and challenges in bamboo breeding for sustainable development. Tree Physiol 43, 1705-1717. |
| [30] | Wang P, Mu X, Gao YG, Zhang J, Du J (2020). Successful induction and the systematic characterization of tetraploids in Cerasus humilis for subsequent breeding. Sci Hortic 265, 109-216. |
| [31] | Zang QL, Liu QQ, Zhuge F, Wang XQ, Lin XC (2019). In vitro regeneration via callus induction in Dendrocalamus asper (Schult.) Backer. Propag Ornamental Plants 19, 66-71. |
| [32] |
Zhao HS, Sun S, Ding YL, Wang Y, Yue XH, Du X, Wei Q, Fan GY, Sun HY, Lou YF, Yang HM, Wang J, Xu X, Li LC, Yang KB, Xu H, Wang JL, Zhu CL, Wang SN, Shan XM, Hou YG, Wang Y, Fei BH, Liu X, Jiang ZH, Gao ZM (2021). Analysis of 427 genomes reveals moso bamboo population structure and genetic basis of property traits. Nat Commun 12, 5466.
DOI PMID |
| [33] | Zhou J, Guo F, Fu J, Xiao Y, Wu J (2020). In vitro polyploid induction using colchicine for Zingiber officinale Roscoe cv. ‘Fengtou’ ginger. Plant Cell Tissue Organ Cult 142, 87-94. |
| [1] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [2] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [3] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [4] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [5] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [6] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [7] | Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum [J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235. |
| [8] | Yanmin Li, Hui Jiang, Zhenzhu Fu, Jing Zhang, Xin Yuan, Huijuan Wang, Jie Gao, Xiaoyu Dong, Limin Wang, Hechen Zhang. Callus Induction and Somatic Embryogenesis in Anther Culture of Paeonia lactiflora [J]. Chinese Bulletin of Botany, 2021, 56(4): 443-450. |
| [9] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [10] | Pengfei Du, Yu Wang, Yingping Cao, Song Yang, Zhichao Sun, Decai Mao, Jiajun Yan, Daxu Li, Meizhen Sun, Chunxiang Fu, Shiqie Bai. Establishment of Biolistic Mediated Transformation System for Elymus sibiricus [J]. Chinese Bulletin of Botany, 2021, 56(1): 62-70. |
| [11] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [12] | Jianfei Liu, Yan Liu, Kejian Liu, Yang Chi, Zhifa Huo, Yonghong Huo, Xiangling You. Optimization of the Regeneration System from Somatic Embryogenesis in Larix olgensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 605-612. |
| [13] | Yan Xiao,Zhenxing Wang,Dongming Li,Yanhua Qi, Enhebayaer. Optimization of Tissue Culture and Plant Regeneration System of Mature Embryo of Leymus chinensis [J]. Chinese Bulletin of Botany, 2020, 55(2): 192-198. |
| [14] | Ying Feng,Lianwen Qian,Qingliang Lin. The Effect of Different Hormones on Explant Browning and Callus Browning in Cyclocarya paliurus [J]. Chinese Bulletin of Botany, 2019, 54(5): 634-641. |
| [15] | Xiaomei Liu,Lili Sun,Xiangdong Fu,Hong Liao. An Effective Method for the Rooting of Tea Cuttings [J]. Chinese Bulletin of Botany, 2019, 54(4): 531-538. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||