

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (2): 235-245.DOI: 10.11983/CBB24061 cstr: 32102.14.CBB24061
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Xuemin Cao1,3,†, Ying Bao2,†, Yuexin Zhang1,3, Ruijie Li1,3, Jianxin Su1,3, Wei Zhang1,3,*( )
)
Received:2024-04-24
Accepted:2024-08-20
Online:2025-03-10
Published:2024-08-22
Contact:
Wei Zhang
About author:First author contact:†These authors contributed equally to this paper
Xuemin Cao, Ying Bao, Yuexin Zhang, Ruijie Li, Jianxin Su, Wei Zhang. Tissue Culture, Rapid Propagation and Efficient Transient Expression Systems of Rosa multiflora[J]. Chinese Bulletin of Botany, 2025, 60(2): 235-245.
| Reagents | Required solvent dosage |
|---|---|
| 0.2 mol∙L-1 NaH2PO4 | 38 mL |
| 0.2 mol∙L-1 Na2HPO4 | 62 mL |
| 100 mmol∙L-1 K3Fe(CN)6 | 1 mL |
| 100 mmol∙L-1 K4Fe(CN)6 | 1 mL |
| 0.5 mol∙L-1 NaEDTA | 4 mL |
| 0.1% Triton X-100 | 200 μL |
| 20% methanol | 20 mL |
| X-Gluc | 200 mg |
Table 1 GUS staining solution formulation
| Reagents | Required solvent dosage |
|---|---|
| 0.2 mol∙L-1 NaH2PO4 | 38 mL |
| 0.2 mol∙L-1 Na2HPO4 | 62 mL |
| 100 mmol∙L-1 K3Fe(CN)6 | 1 mL |
| 100 mmol∙L-1 K4Fe(CN)6 | 1 mL |
| 0.5 mol∙L-1 NaEDTA | 4 mL |
| 0.1% Triton X-100 | 200 μL |
| 20% methanol | 20 mL |
| X-Gluc | 200 mg |
| Treatments | 75% ethanol immersion time (s) | NaClO (%) | NaClO immersion time (min) |
|---|---|---|---|
| A | 30 | 5 | 10 |
| B | 30 | 5 | 15 |
| C | 30 | 5 | 20 |
| D | 30 | 10 | 10 |
| E | 30 | 10 | 15 |
| F | 30 | 10 | 20 |
| G | 30 | 15 | 10 |
| H | 30 | 15 | 15 |
| I | 30 | 15 | 20 |
Table 2 Explant disinfection scheme
| Treatments | 75% ethanol immersion time (s) | NaClO (%) | NaClO immersion time (min) |
|---|---|---|---|
| A | 30 | 5 | 10 |
| B | 30 | 5 | 15 |
| C | 30 | 5 | 20 |
| D | 30 | 10 | 10 |
| E | 30 | 10 | 15 |
| F | 30 | 10 | 20 |
| G | 30 | 15 | 10 |
| H | 30 | 15 | 15 |
| I | 30 | 15 | 20 |
| Treatments | Basic medium | 6-BA (mg∙L-1) | NAA (mg∙L-1) | GA3 (mg∙L-1) |
|---|---|---|---|---|
| A1 | MS | 0.5 | 0.01 | 0 |
| A2 | MS | 1.0 | 0.01 | 0 |
| A3 | MS | 0.5 | 0.1 | 0 |
| A4 | MS | 1.0 | 0.1 | 0 |
| A5 | MS | 0.5 | 0.01 | 0.1 |
| A6 | MS | 1.0 | 0.01 | 0.1 |
| A7 | MS | 0.5 | 0.1 | 0.1 |
| A8 | MS | 1.0 | 0.1 | 0.1 |
Table 3 Treatment schemes for the initiation culture of Rosa multiflora
| Treatments | Basic medium | 6-BA (mg∙L-1) | NAA (mg∙L-1) | GA3 (mg∙L-1) |
|---|---|---|---|---|
| A1 | MS | 0.5 | 0.01 | 0 |
| A2 | MS | 1.0 | 0.01 | 0 |
| A3 | MS | 0.5 | 0.1 | 0 |
| A4 | MS | 1.0 | 0.1 | 0 |
| A5 | MS | 0.5 | 0.01 | 0.1 |
| A6 | MS | 1.0 | 0.01 | 0.1 |
| A7 | MS | 0.5 | 0.1 | 0.1 |
| A8 | MS | 1.0 | 0.1 | 0.1 |
| Treatments | Basic medium | Sucrose (%) | Agar (%) |
|---|---|---|---|
| B1 | MS | 3 | 0.75 |
| B2 | 1/2MS | 3 | 0.75 |
| B3 | QL | 3 | 0.75 |
| B4 | 1/2QL | 3 | 0.75 |
| B5 | WPM | 3 | 0.75 |
| B6 | B5 | 3 | 0.75 |
Table 4 Treatment schemes for the propagation culture of Rosa multiflora
| Treatments | Basic medium | Sucrose (%) | Agar (%) |
|---|---|---|---|
| B1 | MS | 3 | 0.75 |
| B2 | 1/2MS | 3 | 0.75 |
| B3 | QL | 3 | 0.75 |
| B4 | 1/2QL | 3 | 0.75 |
| B5 | WPM | 3 | 0.75 |
| B6 | B5 | 3 | 0.75 |
| Treatments | Basic medium | NAA (mg∙L-1) | IBA (mg∙L-1) |
|---|---|---|---|
| C1 | MS | 0.05 | 0 |
| C2 | MS | 0.1 | 0 |
| C3 | 1/2MS | 0.05 | 0 |
| C4 | 1/2MS | 0.1 | 0 |
| C5 | MS | 0 | 0.5 |
| C6 | MS | 0 | 1.0 |
| C7 | 1/2MS | 0 | 0.5 |
| C8 | 1/2MS | 0 | 1.0 |
Table 5 Treatment schemes for the rooting culture of Rosa multiflora
| Treatments | Basic medium | NAA (mg∙L-1) | IBA (mg∙L-1) |
|---|---|---|---|
| C1 | MS | 0.05 | 0 |
| C2 | MS | 0.1 | 0 |
| C3 | 1/2MS | 0.05 | 0 |
| C4 | 1/2MS | 0.1 | 0 |
| C5 | MS | 0 | 0.5 |
| C6 | MS | 0 | 1.0 |
| C7 | 1/2MS | 0 | 0.5 |
| C8 | 1/2MS | 0 | 1.0 |
| Treatments | Bacterium OD600 | Vacuum pressure (MPa) | Vacuum processing time (min) |
|---|---|---|---|
| D1 | 0.6 | -0.06 | 5 |
| D2 | 0.8 | -0.08 | 5 |
| D3 | 1.0 | -0.10 | 5 |
| D4 | 1.0 | -0.08 | 10 |
| D5 | 0.8 | -0.06 | 10 |
| D6 | 0.6 | -0.10 | 10 |
| D7 | 0.6 | -0.08 | 15 |
| D8 | 0.8 | -0.10 | 15 |
| D9 | 1.0 | -0.06 | 15 |
Table 6 Experimental design for transient expression of sterile re-generated plantlets of Rosa multiflora
| Treatments | Bacterium OD600 | Vacuum pressure (MPa) | Vacuum processing time (min) |
|---|---|---|---|
| D1 | 0.6 | -0.06 | 5 |
| D2 | 0.8 | -0.08 | 5 |
| D3 | 1.0 | -0.10 | 5 |
| D4 | 1.0 | -0.08 | 10 |
| D5 | 0.8 | -0.06 | 10 |
| D6 | 0.6 | -0.10 | 10 |
| D7 | 0.6 | -0.08 | 15 |
| D8 | 0.8 | -0.10 | 15 |
| D9 | 1.0 | -0.06 | 15 |
| Treatments | Contamination rate (%) | Browning rate (%) | Survival rate (%) |
|---|---|---|---|
| A | 11.11±0.00 a | 59.26±6.42 b | 29.60±6.42 a |
| B | 0.00±0.00 b | 92.59±6.42 a | 7.41±6.42 b |
| C | 7.41±6.42 ab | 100.00±0.00 a | 0.00±0.00 b |
| D | 0.00±0.00 b | 92.59±6.42 a | 7.41±6.42 b |
| E | 3.70±6.42 b | 100.00±0.00 a | 0.00±0.00 b |
| F | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| G | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| H | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| I | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
Table 7 Effects of different disinfection methods on the stem segments with apical buds of Rosa multiflora
| Treatments | Contamination rate (%) | Browning rate (%) | Survival rate (%) |
|---|---|---|---|
| A | 11.11±0.00 a | 59.26±6.42 b | 29.60±6.42 a |
| B | 0.00±0.00 b | 92.59±6.42 a | 7.41±6.42 b |
| C | 7.41±6.42 ab | 100.00±0.00 a | 0.00±0.00 b |
| D | 0.00±0.00 b | 92.59±6.42 a | 7.41±6.42 b |
| E | 3.70±6.42 b | 100.00±0.00 a | 0.00±0.00 b |
| F | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| G | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| H | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| I | 0.00±0.00 b | 100.00±0.00 a | 0.00±0.00 b |
| Treatments | Contamination rate (%) | Browning rate (%) | Survival rate (%) |
|---|---|---|---|
| A | 3.70±6.42 a | 18.52±6.42 ab | 77.78±11.11 a |
| B | 7.41±6.42 a | 25.93±6.42 a | 66.67±0.00 a |
| C | 3.70±6.42 a | 14.81±6.42 ab | 81.48±6.42 a |
| D | 7.41±6.42 a | 11.11±0.00 b | 81.48±6.42 a |
| E | 14.81±16.97 a | 14.81±6.42 ab | 70.37±16.97 a |
| F | 3.70±6.42 a | 0.00±0.00 c | 96.30±6.42 a |
| G | 11.11±11.11 a | 14.81±6.42 ab | 74.07±16.97 a |
| H | 0.00±0.00 a | 25.93±6.42 a | 74.07±6.42 a |
| I | 18.52±12.83 a | 11.11±0.00 b | 70.37±12.83 a |
Table 8 Effects of different disinfection methods on the stem segments with axillary buds of Rosa multiflora
| Treatments | Contamination rate (%) | Browning rate (%) | Survival rate (%) |
|---|---|---|---|
| A | 3.70±6.42 a | 18.52±6.42 ab | 77.78±11.11 a |
| B | 7.41±6.42 a | 25.93±6.42 a | 66.67±0.00 a |
| C | 3.70±6.42 a | 14.81±6.42 ab | 81.48±6.42 a |
| D | 7.41±6.42 a | 11.11±0.00 b | 81.48±6.42 a |
| E | 14.81±16.97 a | 14.81±6.42 ab | 70.37±16.97 a |
| F | 3.70±6.42 a | 0.00±0.00 c | 96.30±6.42 a |
| G | 11.11±11.11 a | 14.81±6.42 ab | 74.07±16.97 a |
| H | 0.00±0.00 a | 25.93±6.42 a | 74.07±6.42 a |
| I | 18.52±12.83 a | 11.11±0.00 b | 70.37±12.83 a |
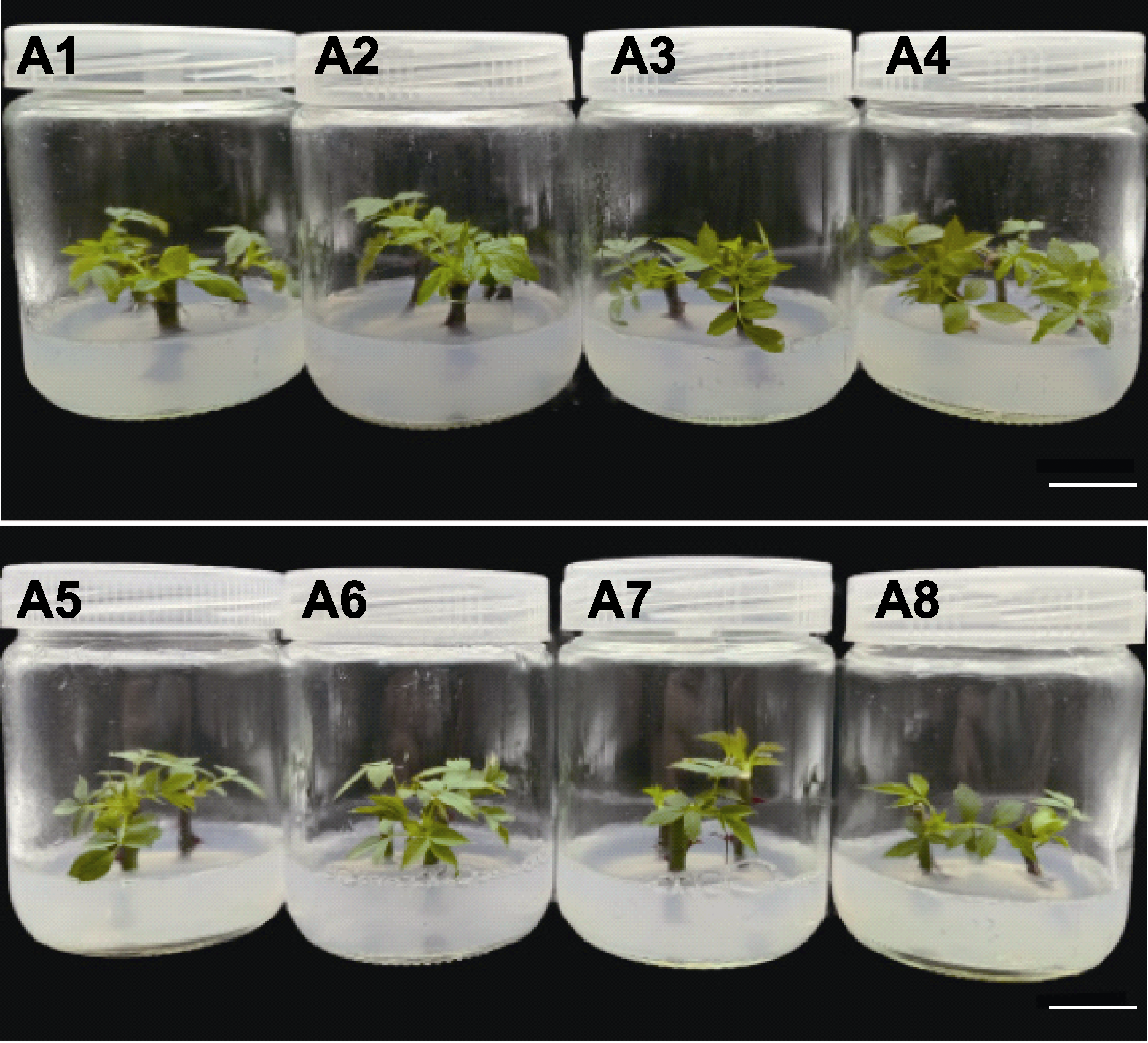
Figure 1 Growth condition of Rosa multiflora on different initiation media A1-A8 are the same as shown in Table 3. Each treatment is inoculated with 10 vials, each vial is inoculated with 3 explants, and each treatment is repeated 3 times. Bars=1.5 cm
| Treatments | Germination rate (%) | Plant height (cm) | Number of compound leaves (slice) |
|---|---|---|---|
| A1 | 92.22±7.70 a | 1.30±0.50 bc | 4.37±1.39 cd |
| A2 | 97.78±1.92 a | 1.52±0.59 a | 4.94±1.72 abc |
| A3 | 96.67±3.34 a | 1.20±0.59 bc | 4.45±1.46 cd |
| A4 | 98.89±1.92 a | 1.25±0.50 bc | 5.17±1.41 a |
| A5 | 98.89±1.92 a | 1.11±0.39 c | 4.74±1.64 abcd |
| A6 | 98.89±1.92 a | 1.35±0.45 b | 5.07±1.61 ab |
| A7 | 97.78±1.92 a | 1.15±0.48 bc | 4.17±1.71 d |
| A8 | 96.67±5.77 a | 1.25±0.41 bc | 4.69±1.77 abcd |
Table 9 Effects of different plant growth regulator types and concentrations on the germination of Rosa multiflora
| Treatments | Germination rate (%) | Plant height (cm) | Number of compound leaves (slice) |
|---|---|---|---|
| A1 | 92.22±7.70 a | 1.30±0.50 bc | 4.37±1.39 cd |
| A2 | 97.78±1.92 a | 1.52±0.59 a | 4.94±1.72 abc |
| A3 | 96.67±3.34 a | 1.20±0.59 bc | 4.45±1.46 cd |
| A4 | 98.89±1.92 a | 1.25±0.50 bc | 5.17±1.41 a |
| A5 | 98.89±1.92 a | 1.11±0.39 c | 4.74±1.64 abcd |
| A6 | 98.89±1.92 a | 1.35±0.45 b | 5.07±1.61 ab |
| A7 | 97.78±1.92 a | 1.15±0.48 bc | 4.17±1.71 d |
| A8 | 96.67±5.77 a | 1.25±0.41 bc | 4.69±1.77 abcd |
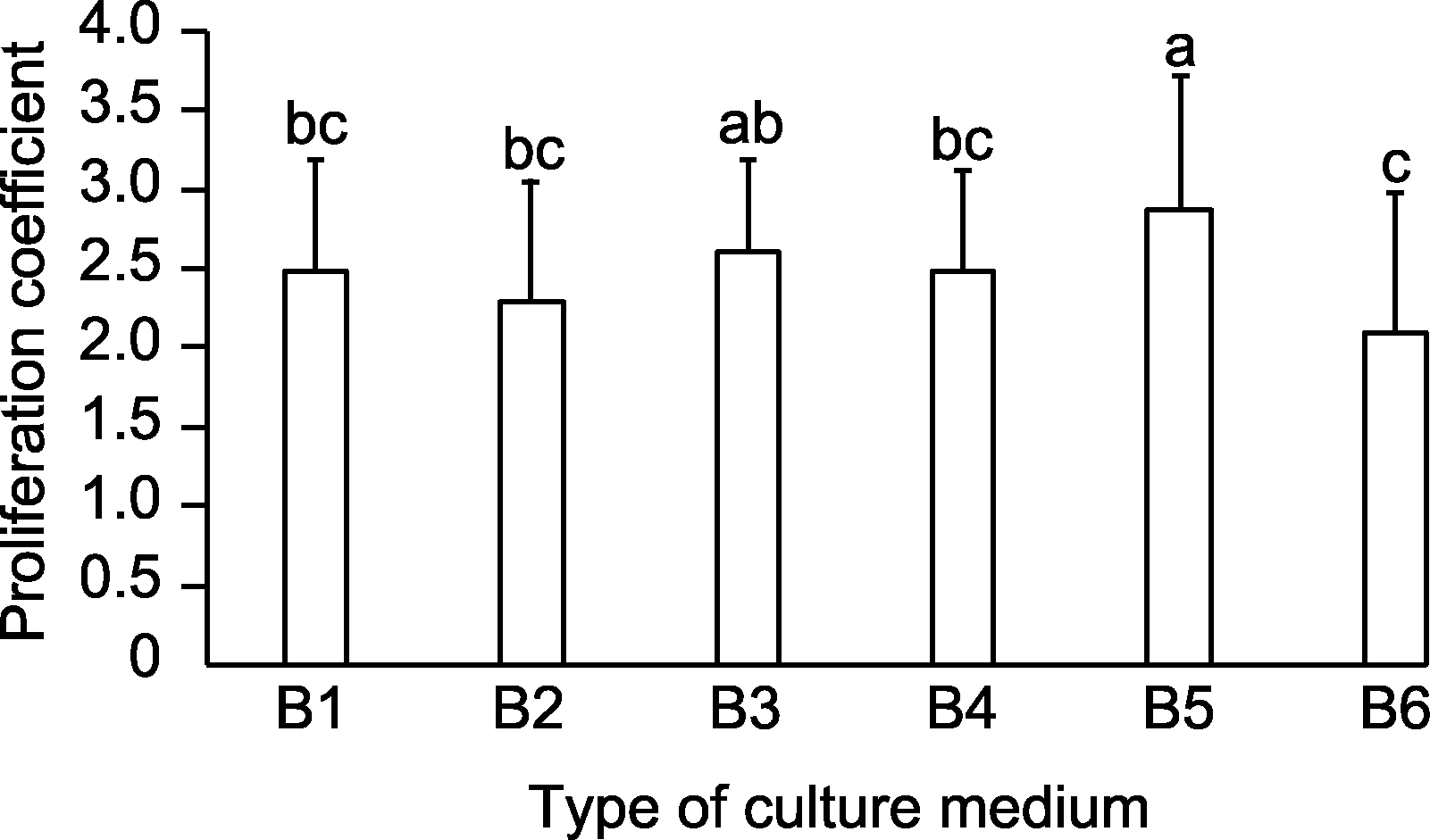
Figure 2 Effects of basal medium types on the proliferation coefficient of Rosa multiflora B1-B6 are the same as shown in Table 4. Different lowercase letters indicate significant differences (P<0.05).
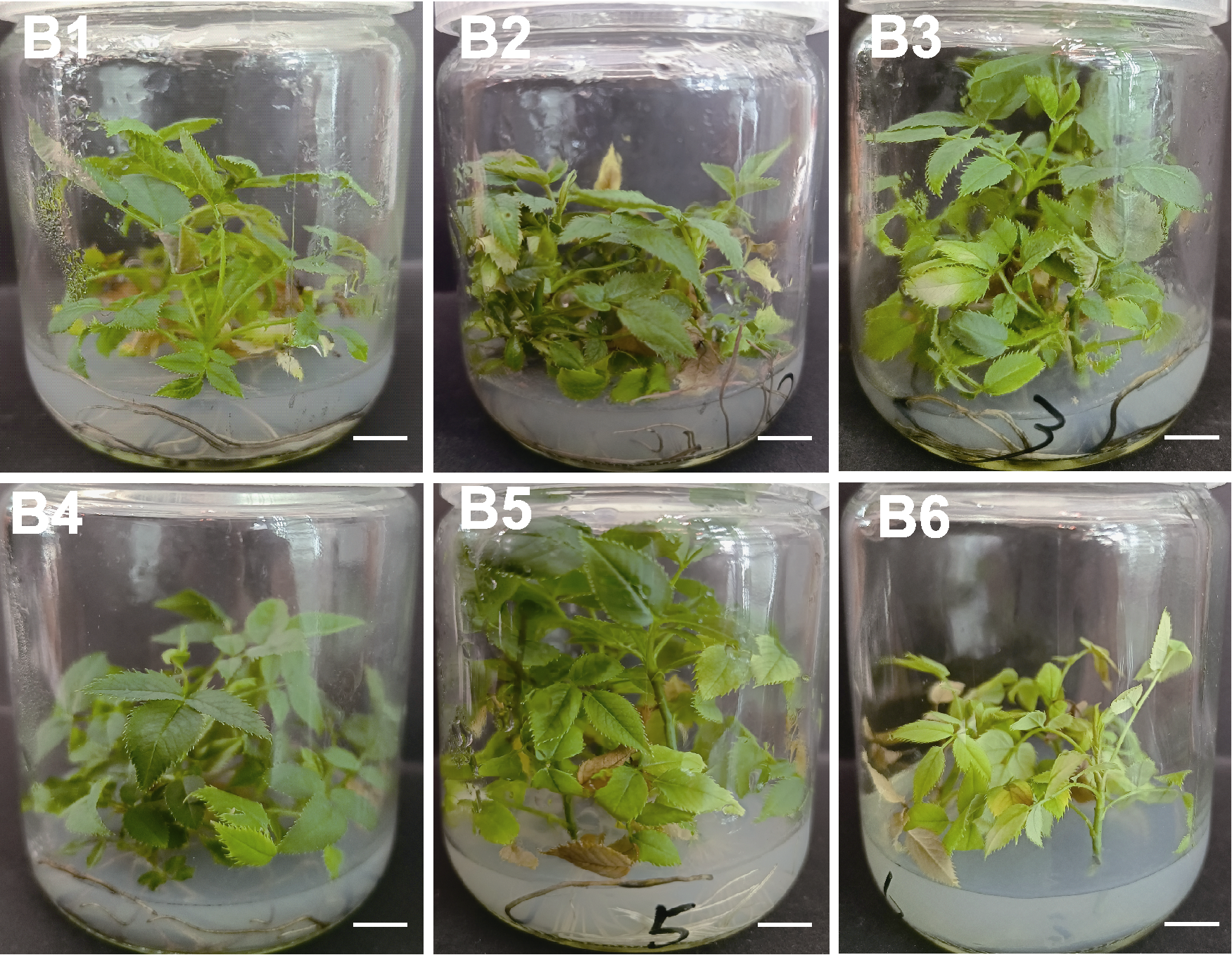
Figure 3 Growth condition of Rosa multiflora on different proliferation media B1-B6 are the same as shown in Table 4. Each treatment is inoculated with 5 vials, each vial is inoculated with 3 explants, and each treatment is repeated 3 times. Bars=1 cm
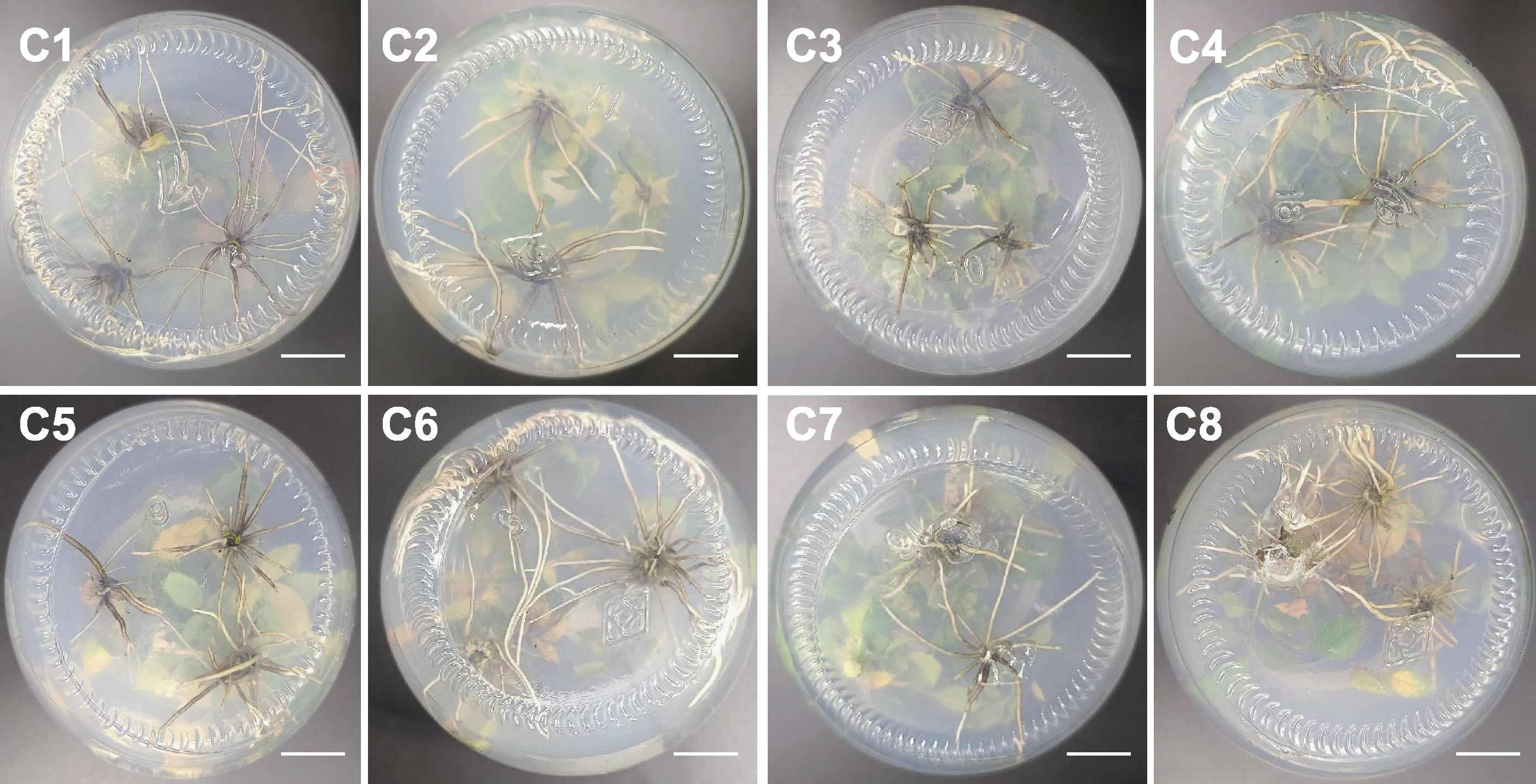
Figure 4 Root condition of Rosa multiflora on different rooting media C1-C8 are the same as shown in Table 5. Each treatment is inoculated with 5 vials, each vial is inoculated with 3 explants, and each treatment is repeated 3 times. Bars=1 cm
| Treatments | Rooting rate (%) | Average number of roots (root) | Root status |
|---|---|---|---|
| C1 | 88.33±2.89 ab | 8.22 | Short roots, weak growth |
| C2 | 88.33±2.89 ab | 9.30 | Strong roots, grow well |
| C3 | 93.33±7.64 ab | 9.78 | Short roots, weak growth |
| C4 | 93.33±2.89 a | 8.85 | Strong roots, grow well |
| C5 | 81.67±5.77 ab | 8.25 | Short roots, weak growth |
| C6 | 81.67±2.89 ab | 8.47 | Strong roots, grow well |
| C7 | 78.33±5.77 b | 6.68 | Short roots, weak growth |
| C8 | 86.67±5.77 ab | 7.18 | Short roots, weak growth |
Table 10 Effects of different media formulations on the rooting of Rosa multiflora
| Treatments | Rooting rate (%) | Average number of roots (root) | Root status |
|---|---|---|---|
| C1 | 88.33±2.89 ab | 8.22 | Short roots, weak growth |
| C2 | 88.33±2.89 ab | 9.30 | Strong roots, grow well |
| C3 | 93.33±7.64 ab | 9.78 | Short roots, weak growth |
| C4 | 93.33±2.89 a | 8.85 | Strong roots, grow well |
| C5 | 81.67±5.77 ab | 8.25 | Short roots, weak growth |
| C6 | 81.67±2.89 ab | 8.47 | Strong roots, grow well |
| C7 | 78.33±5.77 b | 6.68 | Short roots, weak growth |
| C8 | 86.67±5.77 ab | 7.18 | Short roots, weak growth |

Figure 5 The transplanting process of rooted plantlets of Rosa multiflora (A) Rooted sterile regenerated plantlets; (B) Plantlets on the first day of transplanting; (C) Plantlets transplanted 30 days later. Transplant 40 rooted plantlets per replicate and repeat 3 times. Bars=1 cm
| Treatments | Transient expression efficiency (%) | Staining effect |
|---|---|---|
| D1 | 14.07±12.24 d | Pale blue, flake area blue |
| D2 | 30.63±2.44 cd | Pale blue, flake area blue |
| D3 | 65.27±17.71 b | Blue, flake area blue |
| D4 | 52.38±10.82 bc | Pale blue, leaf blue |
| D5 | 15.87±5.72 d | Blue, flake area blue |
| D6 | 52.89±17.16 bc | Blue, leaf blue |
| D7 | 4.76±8.25 d | Blue, leaf blue |
| D8 | 96.30±6.42 a | Blue, leaf blue |
| D9 | 31.19±7.84 cd | Blue, flake area blue |
Table 11 Effects of different treatments on the transient expression efficiency of Rosa multiflora
| Treatments | Transient expression efficiency (%) | Staining effect |
|---|---|---|
| D1 | 14.07±12.24 d | Pale blue, flake area blue |
| D2 | 30.63±2.44 cd | Pale blue, flake area blue |
| D3 | 65.27±17.71 b | Blue, flake area blue |
| D4 | 52.38±10.82 bc | Pale blue, leaf blue |
| D5 | 15.87±5.72 d | Blue, flake area blue |
| D6 | 52.89±17.16 bc | Blue, leaf blue |
| D7 | 4.76±8.25 d | Blue, leaf blue |
| D8 | 96.30±6.42 a | Blue, leaf blue |
| D9 | 31.19±7.84 cd | Blue, flake area blue |

Figure 6 Effects of different treatments on GUS staining of sterile regenerated plantlets of Rosa multiflora D1-D9 are the same as shown in Table 6. Each treatment is inoculated with 3 vials, each vial is inoculated with 3 explants, and each treatment is repeated 3 times. Bars=1 cm
| [1] | 丁萌 (2012). 野蔷薇再生体系的建立及其遗传转化的研究. 硕士论文. 武汉: 华中农业大学. pp. 14-38. |
| [2] | 龚维红 (2022). 大马士革玫瑰组培与快繁技术研究. 安徽农学通报 28, 19-21. |
| [3] | 李刚, 宋平丽, 王翔, 马青翠, 张海霞, 张玉星, 许建锋, 亓宝秀 (2021). 农杆菌介导的杜梨叶片瞬时转化方法的建立. 果树学报 38, 2006-2013. |
| [4] |
李惠玲, 罗玉兰, 章漳, 尹丽娟, 李圃锦, 张冬梅 (2020). 细梗蔷薇种子发芽及组织培养技术研究. 中国农学通报 36 (13), 89-93.
DOI |
| [5] |
李佳慧, 叶维雁, 朱鹏锦, 庞新华, 张继, 唐毓玮, 韦俏宇 (2022). 猫须草无菌短枝组织培养与快速繁殖体系的建立. 热带作物学报 43, 2063-2070.
DOI |
| [6] | 李静, 陈敏, 刘现伟, 沈法富, 王鹏 (2006). 莴苣高效瞬时表达体系的建立. 园艺学报 33, 405-407. |
| [7] | 李晓亮, 张军云, 张钟, 董春富, 杨世先, 王文智, 张建康, 张翠萍 (2017). 滇红食用玫瑰生根培养基的试验筛选研究. 西南农业学报 30, 656-663. |
| [8] | 李心悦, 张克闯, 张道远, 李进, 王玉成 (2018). 新疆野苹果瞬时遗传转化方法建立及初步验证. 分子植物育种 16, 7315-7321. |
| [9] | 李增武, 钟玲, 赵梁军 (2011). 野蔷薇种子休眠和萌发整齐度研究. 种子 30(10), 42-44. |
| [10] |
刘雪梅, 王蕾, 文添龙, 何鹏, 俞嘉宁 (2014). 农杆菌介导的棉花子叶瞬时表达系统的建立. 植物学报 49, 587-594.
DOI |
| [11] | 刘亚娟, 杨小艳, 谢树章, 吴红, 高立均 (2018). 芳纯月季组培快繁技术研究. 西南师范大学学报(自然科学版) 43(8),52-56. |
| [12] | 鲁艺, 史文君, 黄淑丹, 肖明 (2022). 苦水玫瑰组培快繁技术研究. 青海大学学报 40(3), 41-46. |
| [13] | 穆建鑫 (2021). ‘中梨一号’梨再生体系建立及秋子梨遗传转化体系探索. 硕士论文. 南京: 南京农业大学. pp. 13-24. |
| [14] | 彭奎莉, 孟繁博, 周志达, 郑关任, 张金柱, 杨涛, 车代弟 (2018). 不同基因型丰花月季快繁体系差异的研究. 见:张启翔. 中国观赏园艺研究进展2018. 北京: 中国林业出版社. pp. 480-486. |
| [15] | 强泽宇 (2015). 中华金叶榆繁殖技术研究. 硕士论文. 保定: 河北农业大学. pp. 6-12. |
| [16] | 任菲宏, 王姣, 李晓松, 石丽姝, 任红艳, 吴茂宏 (2023). 外植体取样时间和消毒方式对沙子空心李诱导培养的影响. 现代农业科技 (12), 77-79. |
| [17] | 宋常美, 文晓鹏 (2015). 4种贵州樱桃的高效离体再生. 西南大学学报(自然科学版) 37(9),19-24. |
| [18] | 王悦, 吴艳菊, 孟大伟, 郎晨婧, 赖薪宇, 刘佳, 葛禹凡, 杨丽萍 (2020). 植物瞬时表达体系真空侵染法的优化. 分子植物育种 18, 6743-6748. |
| [19] | 徐立军, 李志斌, 蒋淑磊, 李振勤 (2015). 大马士革玫瑰组织培养技术研究. 河北林业科技 (2), 19-21. |
| [20] | 闫海霞, 蒋月喜, 黄昌艳, 邓杰玲, 何荆洲, 王晓国, 卜朝阳 (2016). 月季‘卡罗拉’的组培快繁技术. 热带作物学报 37, 1741-1746. |
| [21] | 闫允青, 姜桦韬, 谷超, 吴俊 (2017). ‘雪花梨’扩繁和叶片再生体系的建立. 南京农业大学学报 40, 68-75. |
| [22] | 杨丽萍, 许亚男, 刘雨晴, 孟大伟, 金太成 (2016). 利用根部真空侵染法在烟草中瞬时表达外源蛋白. 分子植物育种 14, 3385-3389. |
| [23] |
张燕红, 黄乐平, 周小云, 王冬梅 (2008). 农杆菌真空渗透法转化棉花花粉的初步研究. 棉花学报 20, 354-358.
DOI |
| [24] | 赵月玲, 王汉海, 程贯召, 杜延飞 (2001). 枣组织培养中外植体取材部位的比较研究. 潍坊学院学报 1(2), 53-54, 43. |
| [25] | 周玉洁, 韦雪芬, 申长青, 李焜钊, 孙朝辉, 黄久香 (2019). 濒危植物四药门花的组培快繁. 植物生理学报 55, 635-641. |
| [26] |
Lu J, Bai MJ, Ren HR, Liu JY, Wang CQ (2017). An efficient transient expression system for gene function analysis in rose. Plant Methods 13, 116.
DOI PMID |
| [27] |
Nakamura N, Hirakawa H, Sato S, Otagaki S, Matsumoto S, Tabata S, Tanaka Y (2018). Genome structure of Rosa multiflora, a wild ancestor of cultivated roses. DNA Res 25, 113-121.
DOI PMID |
| [28] |
Zhang Y, Zhao MJ, Zhu W, Shi CM, Bao MZ, Zhang W (2021). Nonglandular prickle formation is associated with development and secondary metabolism-related genes in Rosa multiflora. Physiol Plant 173, 1147-1162.
DOI PMID |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [3] | Lü Xiuli, Yu Zequn, Chen Xiangbo, Fu Renjie, Miao Shanshan, Du An. Rapid Propagation Technology and Field Production of Hemerocallis fulva cv. ‘Fenmeiren’ [J]. Chinese Bulletin of Botany, 2022, 57(3): 350-357. |
| [4] | Yaqian Xiong, Xianbao Deng, Huihui Zhang, Dong Yang, Heng Sun, Juan Liu, Mei Yang. In Vitro Rapid Propagation of Nelumbo nucifera [J]. Chinese Bulletin of Botany, 2021, 56(5): 605-613. |
| [5] | Hua Zhao,Guangda Shao,Wenxin Gao,Biao Gu. The Application of Double-barreled Particle Bombardment for Transient Gene Expression in Arabidopsis [J]. Chinese Bulletin of Botany, 2020, 55(2): 182-191. |
| [6] | Yan Zhao,Jianmin Zhou. Luciferase Complementation Assay for Detecting Protein Interactions [J]. Chinese Bulletin of Botany, 2020, 55(1): 69-75. |
| [7] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| [8] | Lijing Liu,Qingzhen Zhao,Qi Xie,Feifei Yu. An Quick and Efficient Assay for In Vivo Protein Ubiquitination [J]. Chinese Bulletin of Botany, 2019, 54(6): 753-763. |
| [9] | Aihua Song, Wenbin Zhang, Shulan Sun, Lingfei Li, Xiaojing Wang. Preparation of Protoplast and Establishment of Transient Expression System in Gerbera hybrida [J]. Chinese Bulletin of Botany, 2017, 52(4): 511-519. |
| [10] | Xiao Li, Haiyan Sun, Mengbin Ruan, Peihong Wang, Ming Peng. Studies of the Transient Expression and Transformation of Cloned Thermostable α-Amylase Genes from Bacillus licheniformis in Tobacco and Arabidopsis [J]. Chinese Bulletin of Botany, 2015, 50(3): 354-362. |
| [11] | Xuemei Liu, Lei Wang, Tianlong Wen, Peng He, Jianing Yu. The Establishment of Agrobacterium-mediated Transient Expression System in Cotton Cotyledons [J]. Chinese Bulletin of Botany, 2014, 49(5): 587-594. |
| [12] | Ningzhen Huang, Chuanming Fu, Zhiguo Zhao, Fengluan Tang, Yunping Shi. Rapid Propagation in Vitro of Chiritopsis repanda var. guilinensis [J]. Chinese Bulletin of Botany, 2010, 45(06): 744-750. |
| [13] | Xiaofeng Fan;Xiaoqiang Guo;Zhaoxia Xiao;Lingxia Liu. Callus Induction and Rapid Propagation of Chaenomeles speciosa [J]. Chinese Bulletin of Botany, 2009, 44(06): 725-727. |
| [14] | Daojie Wang, Cuiling Yang, Ming Lu. Transformation of Brassica napus by Vacuum Infiltration [J]. Chinese Bulletin of Botany, 2009, 44(02): 216-222. |
| [15] | Wei Xie Chaoyin Yue Zhenghong Guo Zhipeng Dai Min Liu Wei Yao. Transient Expression of GUS Gene Controlled by Different Regulator Sequences of Tobacco [J]. Chinese Bulletin of Botany, 2007, 24(04): 452-458. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||