

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (5): 605-613.DOI: 10.11983/CBB21020 cstr: 32102.14.CBB21020
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Yaqian Xiong1,2, Xianbao Deng1,3, Huihui Zhang1, Dong Yang1,3, Heng Sun1, Juan Liu1, Mei Yang1,3,*( )
)
Received:2021-01-26
Accepted:2021-05-27
Online:2021-09-01
Published:2021-08-31
Contact:
Mei Yang
Yaqian Xiong, Xianbao Deng, Huihui Zhang, Dong Yang, Heng Sun, Juan Liu, Mei Yang. In Vitro Rapid Propagation of Nelumbo nucifera[J]. Chinese Bulletin of Botany, 2021, 56(5): 605-613.
| Types | NAA (mg∙L-1) | 6-BA (mg∙L-1) | Sucrose (g∙L-1) | Activated charcoal (g∙L-1) |
|---|---|---|---|---|
| I | 0.5 | 0.5 | 30 | 0.5 |
| II | 0.5 | 1 | 30 | 0.5 |
| III | 0.5 | 0.5 | 80 | 0.5 |
| IV | 0.5 | 0.5 | 30 | 1 |
Table 1 The component of the primary culture medium
| Types | NAA (mg∙L-1) | 6-BA (mg∙L-1) | Sucrose (g∙L-1) | Activated charcoal (g∙L-1) |
|---|---|---|---|---|
| I | 0.5 | 0.5 | 30 | 0.5 |
| II | 0.5 | 1 | 30 | 0.5 |
| III | 0.5 | 0.5 | 80 | 0.5 |
| IV | 0.5 | 0.5 | 30 | 1 |
| Types | NAA (mg∙L-1) | 6-BA (mg∙L-1) |
|---|---|---|
| 1 | 0.5 | 0.5 |
| 2 | 0.3 | 0.5 |
| 3 | 0.1 | 0.5 |
| 4 | 0.5 | 1.5 |
| 5 | 1 | 0.5 |
| 6 | 0.3 | 1 |
Table 2 The combinations of plant hormones in subculture medium
| Types | NAA (mg∙L-1) | 6-BA (mg∙L-1) |
|---|---|---|
| 1 | 0.5 | 0.5 |
| 2 | 0.3 | 0.5 |
| 3 | 0.1 | 0.5 |
| 4 | 0.5 | 1.5 |
| 5 | 1 | 0.5 |
| 6 | 0.3 | 1 |

Figure 1 Primary culture of Qiuhongyang immature embryo explants from developing seeds of 18 days after pollination (A) Immature embryo explants from developing seeds of 18 days after pollination; (B) Immature embryo cultured one day after inoculation; (C) The rooted plantlets at 10 days after primary culture; (D) The aseptic seedling at 60 days after primary culture. Bars=1 cm
| Types | The amount of inoculation | Rooting rate (%) | The amount of nodes | The amount of apical bud | The amount of leaves |
|---|---|---|---|---|---|
| I | 331 | 100 | 3.9±0.36 a | 0.9±0.03 a | 4.3±0.48 a |
| II | 134 | 98 | 4.0±0.05 a | 0.7±0.04 b | 3.8±0.27 a |
| III | 122 | 95 | 3.2±0.19 b | 0.7±0.01 b | 4.3±0.13 a |
| IV | 300 | 95 | 3.6±0.33 ab | 0.9±0.13 a | 3.9±0.22 a |
Table 3 Effects of medium components on the growth of Qiuhongyang aseptic seedlings in the primary culture (60 days after inoculation)
| Types | The amount of inoculation | Rooting rate (%) | The amount of nodes | The amount of apical bud | The amount of leaves |
|---|---|---|---|---|---|
| I | 331 | 100 | 3.9±0.36 a | 0.9±0.03 a | 4.3±0.48 a |
| II | 134 | 98 | 4.0±0.05 a | 0.7±0.04 b | 3.8±0.27 a |
| III | 122 | 95 | 3.2±0.19 b | 0.7±0.01 b | 4.3±0.13 a |
| IV | 300 | 95 | 3.6±0.33 ab | 0.9±0.13 a | 3.9±0.22 a |
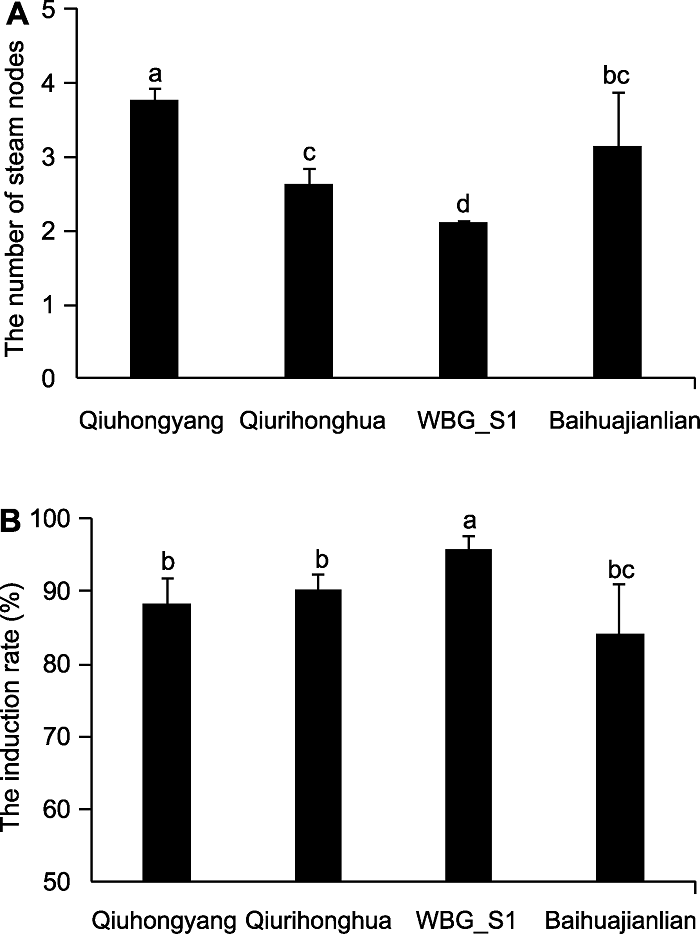
Figure 2 Effects of lotus varieties on the number of stem nodes (A) and the induction rate (B) of lotus aseptic seedlings in the primary culture Different lowercase letters indicate significant differences (P<0.05).
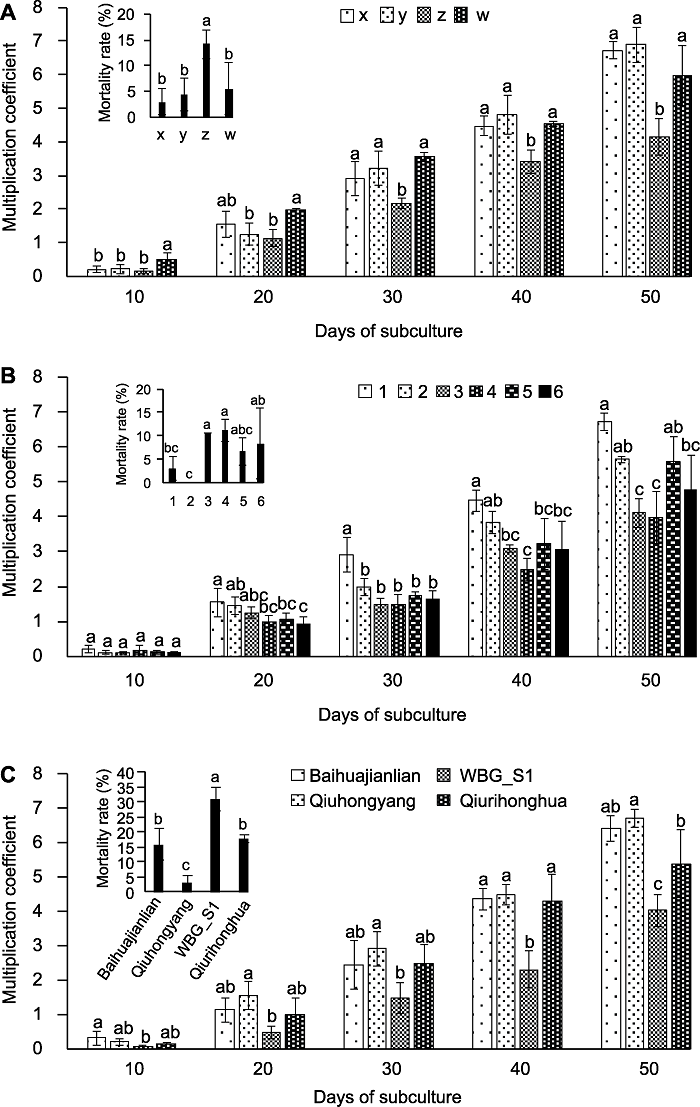
Figure 3 The multiplication coefficient and mortality rate of subculture of aseptic seedlings (A) Effect of dividing methods in Qiuhongyang aseptic seedlings on the multiplication coefficient and mortality rate of subculture (x: Cuttings with two nodes, y: Cuttings with one node and one growing tip, z: Cuttings with one nodes, w: Cuttings with one growing tip); (B) Effects of plant hormone combinations in Qiuhongyang on the multiplication coefficient and mortality rate of subculture, combinations of plant hormones (No.1-6) are in Table 2; (C) Effect of lotus varieties on the multiplication coefficient and mortality rate of subculture. Different lowercase letters indicate significant differences at P<0.05.
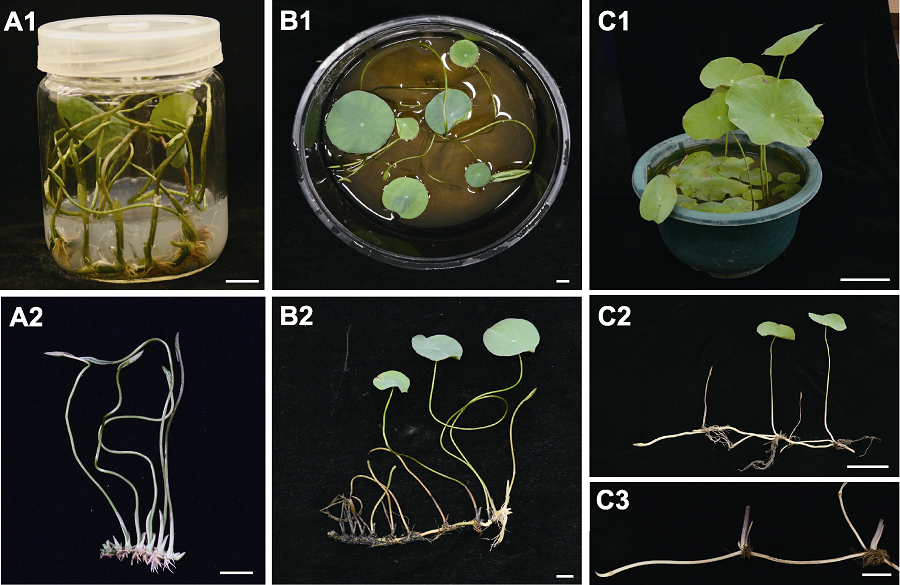
Figure 4 Hardening and transplanting of Qiuhongyang aseptic seedlings (A1), (A2) Aseptic seedlings at 50 days of subculture; (B1), (B2) Plantlets at 10 days after transplanting; (C1) Standing leaves started to emerge at 40 days after transplanting; (C2) Rhizomes at 40 days after transplanting; (C3) Rhizome taken from plants grew in ponds. (A1), (A2), (B1), (B2) Bars=1 cm, (C1)-(C3) Bars=10 cm
| Lotus varieties | Survival rate (%) | The amount of expanded leaves | The amount of standing leaves | ||
|---|---|---|---|---|---|
| 10 d | 20 d | 10 d | 20 d | 50 d | |
| Qiuhongyang | 93.7±3.0 a | 83.9±1.6 a | 0.4±0.10 b | 1.8±0.07 b | 0.7±0.04 a |
| Qiurihonghua | 93.3±6.7 a | 93.3±6.7 a | 0.7±0.07 a | 2.7±0.15 a | 0.5±0.04 ab |
| WBG_S1 | 95.8±7.2 a | 93.3±6.3 a | 0.5±0.00 b | 1.7±0.05 b | 0.3±0.06 b |
| Baihuajianlian | 87.8±6.3 a | 87.2±18.0 a | 0.5±0.11 b | 2.5±0.40 a | 0.5±0.13 ab |
Table 4 Effects of lotus varieties on the survival rate and the number of leaves developed after transplanting
| Lotus varieties | Survival rate (%) | The amount of expanded leaves | The amount of standing leaves | ||
|---|---|---|---|---|---|
| 10 d | 20 d | 10 d | 20 d | 50 d | |
| Qiuhongyang | 93.7±3.0 a | 83.9±1.6 a | 0.4±0.10 b | 1.8±0.07 b | 0.7±0.04 a |
| Qiurihonghua | 93.3±6.7 a | 93.3±6.7 a | 0.7±0.07 a | 2.7±0.15 a | 0.5±0.04 ab |
| WBG_S1 | 95.8±7.2 a | 93.3±6.3 a | 0.5±0.00 b | 1.7±0.05 b | 0.3±0.06 b |
| Baihuajianlian | 87.8±6.3 a | 87.2±18.0 a | 0.5±0.11 b | 2.5±0.40 a | 0.5±0.13 ab |
| [1] | 蔡颖欣, 汤宇环, 邵晓宇, 黄霞 (2017). 莲子胚培养再生植株及原生质体分离的研究. 种子 36(3), 125-127, 134. |
| [2] | 何碧珠, 曾明星, 赵时端, 王家福, 赖钟雄 (2002). 建莲茎尖离体培养研究初报. 福建农林大学学报(自然科学版) 31, 59-61. |
| [3] |
黄宁珍, 付传明, 赵志国, 唐凤鸾, 石云平 (2010). 桂林小花苣苔离体快速繁殖技术. 植物学报 45, 744-750.
DOI |
| [4] | 柯卫东, 彭静, 刘玉平, 黄新芳 (2001). 试管藕诱导技术研究. 武汉植物学研究 19, 173-175. |
| [5] | 孔德政, 李艳妮, 杨秋生, 刘广甫 (2007). 荷花胚组织培养的初步研究. 河南科学 25, 593-595. |
| [6] | 李峰, 周雄祥, 柯卫东, 黄新芳, 朱红莲, 钟兰, 宗义湘, 吴曼, 彭静, 李双梅, 袁田垚 (2020). 湖北省莲产业发展调研报告. 湖北农业科学 59(23), 101-106, 109. |
| [7] | 刘建平, 王芳, 杜彩娴, 梁少丽, 曾莉莎, 郑芝波 (2016). 荷花幼胚组织培养技术研究. 现代农业科技 (15), 140, 142. |
| [8] | 刘义满, 柯卫东 (2012). 关于提高莲产业效益的建议. 长江蔬菜 (16), 134-137. |
| [9] | 彭燕, 张玲莉, 杨小青, 阳静, 李娜, 宋金春 (2017). 莲子心总生物碱对人肝癌细胞的抑制作用. 中国药师 20, 1009-1012. |
| [10] |
唐凤鸾, 赵健, 赵志国, 夏科, 仇硕 (2019). 走马胎的组织培养与快速繁殖. 植物学报 54, 378-384.
DOI |
| [11] |
唐嘉瓅, 邱杰, 黄学辉 (2020). 基因组学技术大发展助力园艺植物研究取得新进展. 植物学报 55, 1-4.
DOI |
| [12] | 王其超, 张行言 (1998). 二元分类法在荷花品种分类中的应用. 北京林业大学学报 20(2), 33-37. |
| [13] | 王其超, 张行言 (2005). 中国荷花品种图志. 北京: 中国林业出版社. pp. 34-43. |
| [14] | 徐君, 李静会, 李欣, 周玉珍, 韦庆华, 姜红卫 (2013). 荷花顶芽初代组织培养. 江苏农业科学 41(3), 38-39. |
| [15] | 岳建华, 董艳, 王小画, 孙佩霞, 王思颖, 张新年, 张琰 (2020). 早花百子莲叶片器官发生和胚胎发生再生体系的建立. 植物学报 55, 588-595. |
| [16] | 曾明星, 何碧珠, 罗银华 (2005). 建莲藕茎尖离体培养快繁技术及应用. 福建农业科技 (3), 13-14. |
| [17] | 张建福, 王锋 (2002). 莲藕组织培养与微繁殖技术初探. 上海农业科技 (6), 17-18. |
| [18] |
张文婷, 何燕红, 舒宁, 邢景景, 刘宝骏, 包满珠, 刘国锋 (2019). 金黄花滇百合植株再生与离体快繁技术体系的建立. 植物学报 54, 773-778.
DOI |
| [19] | 赵芹, 李效尊, 徐国鑫, 阴筱, 尹静静, 吴修 (2016). 莲组织培养与分子生物学研究进展. 分子植物育种 14, 1587-1594. |
| [20] |
Abe T, Futsuhara Y (1986). Genotypic variability for callus formation and plant regeneration in rice ( Oryza sativa L.). Theor Appl Genet 72, 3-10.
DOI PMID |
| [21] |
Arunyanart S, Chaitrayagun M (2005). Induction of somatic embryogenesis in lotus ( Nelumbo nucifera Geartn.). Sci Horticult 105, 411-420.
DOI URL |
| [22] |
Buathong R, Saetiew K, Phansiri S, Parinthawong N, Arunyanart S (2013). Tissue culture and transformation of the antisense DFR gene into lotus (Nelumbo nucifera Gaertn.) through particle bombardment. Sci Horticult 161, 216-222.
DOI URL |
| [23] |
Deng XB, Xiong YQ, Li J, Yang D, Liu J, Sun H, Song HY, Wang YM, Ma JY, Liu YL, Yang M (2020). The establishment of an efficient callus induction system for lotus ( Nelumbo nucifera). Plants 9, 1436.
DOI URL |
| [24] |
Guo HB (2009). Cultivation of lotus ( Nelumbo nucifera Gaertn. ssp. nucifera) and its utilization in China. Genet Resour Crop Evol 56, 323-330.
DOI URL |
| [25] |
Jun MY, Karki R, Paudel KR, Sharma BR, Adhikari D, Kim DW (2016). Alkaloid rich fraction from Nelumbo nucifera targets VSMC proliferation and migration to suppress restenosis in balloon-injured rat carotid artery. Atherosclerosis 248, 179-189.
DOI URL |
| [26] |
La-ongsri W, Trisonthi C, Balslev H (2009). Management and use of Nelumbo nucifera Gaertn. in Thai wetlands. Wetlands Ecol Manage 17, 279-289.
DOI URL |
| [27] |
Liu QQ, Zhang DS, Liu FL, Qin M, Tian DK (2019). Micropropagation of Nelumbo nucifera ‘Weishan Hong’ through germfree mature embryos. In Vitro Cell Dev Biol Plant 55, 305-312.
DOI URL |
| [28] | Mahmad N, Taha RM, Othman R, Saleh A, Hasbullah NA, Elias H (2014). Effects of NAA and BAP, double-layered media, and light distance on in vitro regeneration of Nelumbo nucifera Gaertn. (lotus), an aquatic edible plant. The Scientific World J 2014, 745148. |
| [29] |
Shou SY, Miao LX, Zai WS, Huang XZ, Guo DP (2008). Factors influencing shoot multiplication of lotus ( Nelumbo nucifera). Biol Plantarum 52, 529-532.
DOI URL |
| [30] | Sridhar KR, Bhat R (2007). Lotus-A potential nutraceutical source. J Agric Technol 3, 143-155. |
| [31] |
Yang M, Zhu LP, Pan C, Xu LM, Liu YL, Ke WD, Yang PF (2015). Transcriptomic analysis of the regulation of rhizome formation in temperate and tropical lotus ( Nelumbo nucifera). Sci Rep 5, 13059.
DOI PMID |
| [32] |
Zhang XY, Wang XY, Wu TT, Li BX, Liu TQ, Wang R, Liu Q, Liu ZJ, Gong YQ, Shao CS (2015). Isoliensinine induces apoptosis in triple-negative human breast cancer cells through ROS generation and p38 MAPK/JNK activation. Sci Rep 5, 12579.
DOI PMID |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Xuemin Cao, Ying Bao, Yuexin Zhang, Ruijie Li, Jianxin Su, Wei Zhang. Tissue Culture, Rapid Propagation and Efficient Transient Expression Systems of Rosa multiflora [J]. Chinese Bulletin of Botany, 2025, 60(2): 235-245. |
| [3] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [4] | Liu Xiaofei, Sun Yingbo, Huang Lili, Yang Yuchai, Zhu Genfa, Yu Bo. Efficient Plant Regeneration via Somatic Embryogenesis in Alocasia reginula cv. ‘Black Velvet’ [J]. Chinese Bulletin of Botany, 2023, 58(5): 750-759. |
| [5] | Jiming Cheng, Huimin He, Hongyu Niu, Hongmao Zhang. Research progress on the effect of intraspecific personality differences on seed dispersal in rodents [J]. Biodiv Sci, 2023, 31(4): 22446-. |
| [6] | Lü Xiuli, Yu Zequn, Chen Xiangbo, Fu Renjie, Miao Shanshan, Du An. Rapid Propagation Technology and Field Production of Hemerocallis fulva cv. ‘Fenmeiren’ [J]. Chinese Bulletin of Botany, 2022, 57(3): 350-357. |
| [7] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [8] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [9] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [10] | Xifu Yang, Hongmao Zhang, Zhibin Zhang. Mast seeding and its relationship to animal hoarding behaviour [J]. Biodiv Sci, 2020, 28(7): 821-832. |
| [11] | Yan Xiao,Zhenxing Wang,Dongming Li,Yanhua Qi, Enhebayaer. Optimization of Tissue Culture and Plant Regeneration System of Mature Embryo of Leymus chinensis [J]. Chinese Bulletin of Botany, 2020, 55(2): 192-198. |
| [12] | Xianjun Lai,Yizheng Zhang,Yinghong Gu,Lang Yan. Transformation of Insect Derived Antifreeze Gene into Sweet Potato (Ipomoea batatas) and Enhanced Its Freeze-tolerance [J]. Chinese Bulletin of Botany, 2020, 55(1): 9-20. |
| [13] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| [14] | Yue Xu,Yingping Cao,Yu Wang,Chunxiang Fu,Shaojun Dai. Agrobacterium rhizogenes-mediated Transformation System of Spinacia oleracea [J]. Chinese Bulletin of Botany, 2019, 54(4): 515-521. |
| [15] | Jia Guo,Yansu Li,Chaoxing He,Yan Yan,Xianchang Yu. Establishing a High-efficiency Regeneration System in Pumpkin (Cucurbita moschata) [J]. Chinese Bulletin of Botany, 2019, 54(4): 539-546. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||