

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (4): 451-461.DOI: 10.11983/CBB20205 cstr: 32102.14.CBB20205
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Qian Luo, Yansha Zhang, Jing Ou*( )
)
Received:2020-12-19
Accepted:2021-05-07
Online:2021-07-01
Published:2021-06-30
Contact:
Jing Ou
Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’[J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461.
| Concentrations of plant hormones (mg·L-1) | Treatment code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 6-BA | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2,4-D | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 |
| TDZ | 0.01 | 0.1 | 0.5 | 0.1 | 0.5 | 0.01 | 0.5 | 0.01 | 0.1 |
Table 1 Orthogonal design of different plant hormones in callus differentiation of Cerasus serrulata
| Concentrations of plant hormones (mg·L-1) | Treatment code | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 6-BA | 0.5 | 0.5 | 0.5 | 1 | 1 | 1 | 2 | 2 | 2 |
| 2,4-D | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 | 0.1 | 0.5 | 1 |
| TDZ | 0.01 | 0.1 | 0.5 | 0.1 | 0.5 | 0.01 | 0.5 | 0.01 | 0.1 |
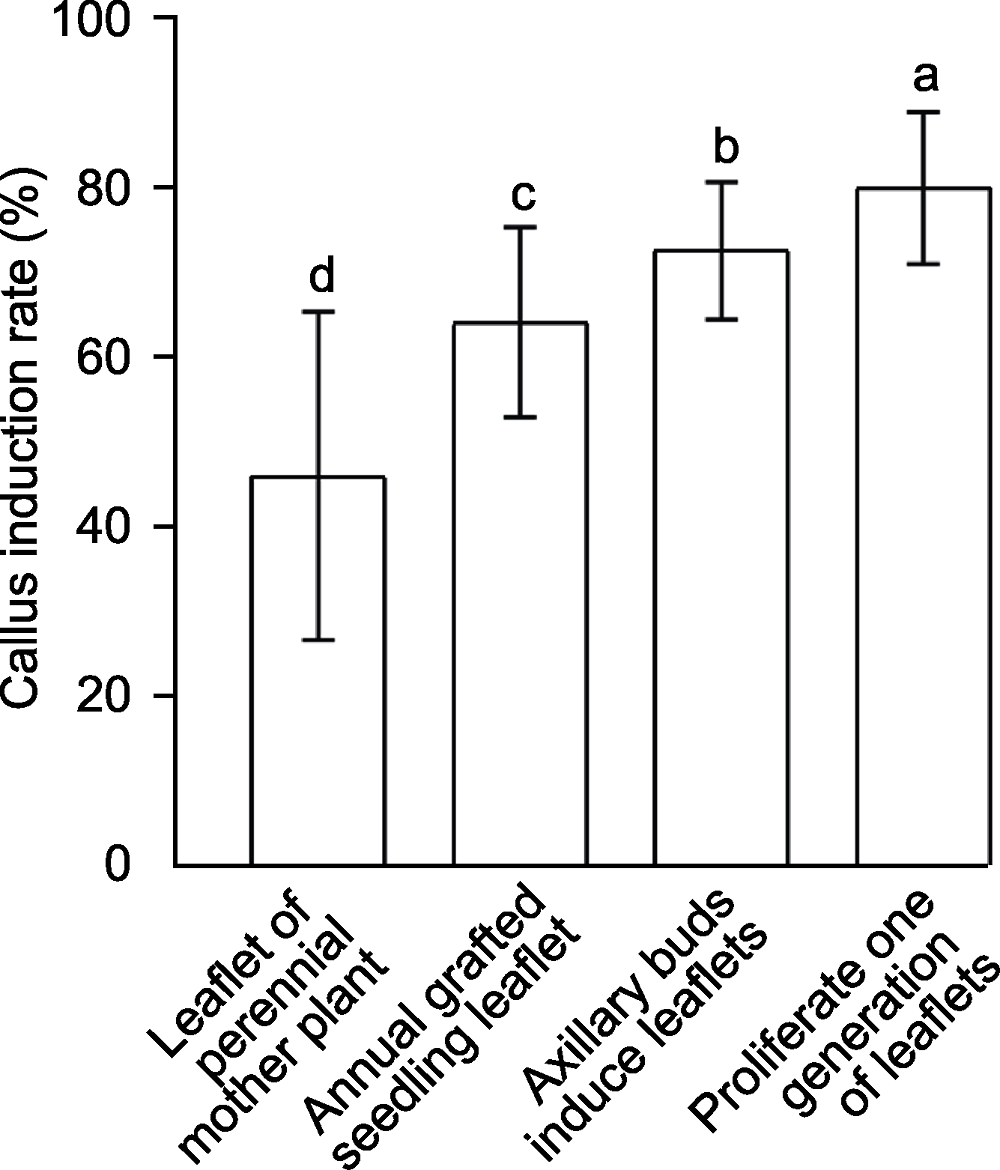
Figure 1 Average callus induction rates in different explants of Cerasus serrulata Different lowercase letters indicate significant differences among different treatments (P<0.05).

Figure 2 Callus induction, differentiation and proliferation of Cerasus serrulata (A) Axillary buds induce leaflets; (B1)-(B3) Leaflet callus induction and differentiation of perennial mother plant; (C1)-(C7) Leaflet callus induction, adventitious bud differentiation and proliferation of one-year grafted seedlings; (D1)-(D7) The process of callus induction, adventitious bud differentiation and proliferation induced from axillary buds; (E1)-(E7) The process of callus induction, adventitious bud differentiation and proliferation of the proliferative generation of leaflets. Bars=1 cm
| No. | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Callus induction rate (%) | |||
|---|---|---|---|---|---|---|
| Leaflet of perennial mother plant | Annual grafted seedling leaflet | Axillary buds induce leaflets | Proliferate one generation of leaflets | |||
| 1 | 0 | 0.5 | 0 l | 42.87±1.51 i | 66.09±0.72 g | 66.51±0.33 i |
| 2 | 0 | 1.0 | 23.91±0.51 j | 49.32±1.01 h | 68.46±1.00 ef | 72.86±0.59 g |
| 3 | 0 | 1.5 | 46.28±0.58 h | 58.92±0.72 f | 67.64±0.80 f | 76.23±0.96 ef |
| 4 | 0 | 2.0 | 57.35±0.68 e | 68.63±0.60 d | 72.52±1.16 d | 80.77±0.59 d |
| 5 | 0 | 3.0 | 57.15±0.32 e | 61.31±1.34 e | 69.61±0.67 e | 77.63±0.71 e |
| 6 | 0.5 | 0.5 | 16.68±1.45 k | 55.08±0.82 g | 63.67±0.54 h | 71.12±0.20 h |
| 7 | 0.5 | 1.0 | 60.09±0.98 d | 61.43±1.40 e | 69.04±0.70 ef | 96.22±0.94 a |
| 8 | 0.5 | 1.5 | 49.31±0.70 g | 74.11±0.78 c | 83.07±0.73 c | 94.63±0.42 b |
| 9 | 0.5 | 2.0 | 68.55±1.04 a | 80.71±0.49 a | 88.74±0.45 a | 91.40±0.80 c |
| 10 | 0.5 | 3.0 | 62.52±1.09 c | 77.62±1.11 b | 86.17±0.72 b | 77.05±0.77 ef |
| 11 | 1 | 0.5 | 27.85±0.69 i | 62.06±0.73 e | 68.09±0.23 f | 76.63±0.82 ef |
| 12 | 1 | 1.0 | 45.64±0.99 h | 69.25±0.42 d | 73.28±0.64 d | 81.36±1.09 d |
| 13 | 1 | 1.5 | 51.03±1.17 f | 73.81±0.92 c | 83.64±1.06 c | 92.18±2.00 c |
| 14 | 1 | 2.0 | 65.46±0.61 b | 60.95±0.30 e | 67.87±0.98 f | 75.57±0.90 f |
| 15 | 1 | 3.0 | 59.89±1.22 d | 54.64±1.00 g | 65.07±0.82 f | 73.85±0.63 g |
Table 2 Effects of different hormone combinations and explants on Cerasus serrulata callus induction (means±SE)
| No. | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Callus induction rate (%) | |||
|---|---|---|---|---|---|---|
| Leaflet of perennial mother plant | Annual grafted seedling leaflet | Axillary buds induce leaflets | Proliferate one generation of leaflets | |||
| 1 | 0 | 0.5 | 0 l | 42.87±1.51 i | 66.09±0.72 g | 66.51±0.33 i |
| 2 | 0 | 1.0 | 23.91±0.51 j | 49.32±1.01 h | 68.46±1.00 ef | 72.86±0.59 g |
| 3 | 0 | 1.5 | 46.28±0.58 h | 58.92±0.72 f | 67.64±0.80 f | 76.23±0.96 ef |
| 4 | 0 | 2.0 | 57.35±0.68 e | 68.63±0.60 d | 72.52±1.16 d | 80.77±0.59 d |
| 5 | 0 | 3.0 | 57.15±0.32 e | 61.31±1.34 e | 69.61±0.67 e | 77.63±0.71 e |
| 6 | 0.5 | 0.5 | 16.68±1.45 k | 55.08±0.82 g | 63.67±0.54 h | 71.12±0.20 h |
| 7 | 0.5 | 1.0 | 60.09±0.98 d | 61.43±1.40 e | 69.04±0.70 ef | 96.22±0.94 a |
| 8 | 0.5 | 1.5 | 49.31±0.70 g | 74.11±0.78 c | 83.07±0.73 c | 94.63±0.42 b |
| 9 | 0.5 | 2.0 | 68.55±1.04 a | 80.71±0.49 a | 88.74±0.45 a | 91.40±0.80 c |
| 10 | 0.5 | 3.0 | 62.52±1.09 c | 77.62±1.11 b | 86.17±0.72 b | 77.05±0.77 ef |
| 11 | 1 | 0.5 | 27.85±0.69 i | 62.06±0.73 e | 68.09±0.23 f | 76.63±0.82 ef |
| 12 | 1 | 1.0 | 45.64±0.99 h | 69.25±0.42 d | 73.28±0.64 d | 81.36±1.09 d |
| 13 | 1 | 1.5 | 51.03±1.17 f | 73.81±0.92 c | 83.64±1.06 c | 92.18±2.00 c |
| 14 | 1 | 2.0 | 65.46±0.61 b | 60.95±0.30 e | 67.87±0.98 f | 75.57±0.90 f |
| 15 | 1 | 3.0 | 59.89±1.22 d | 54.64±1.00 g | 65.07±0.82 f | 73.85±0.63 g |
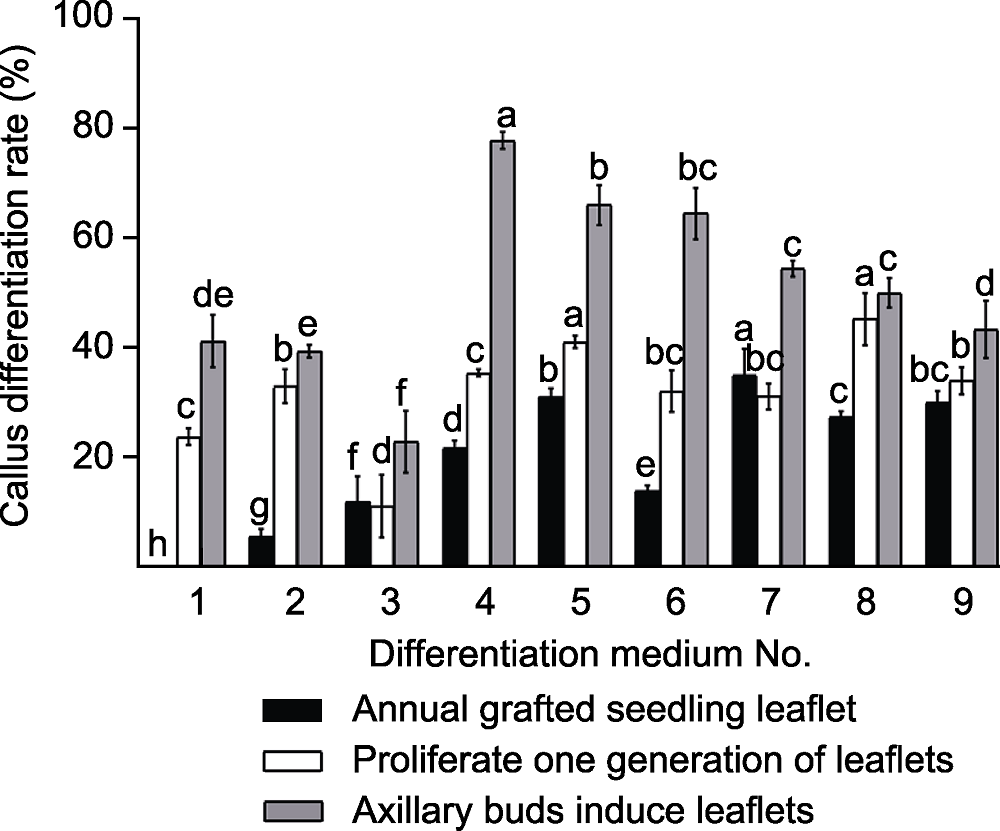
Figure 3 Effects of different plant hormones on Cerasus serrulata callus differentiation in different explants Differentiation medium No. 1-9 are the same as Table 1. Different lowercase letters indicate significant differences at P<0.05 among different treatments.
| Different explants | Source | df | Sum of squares | Mean square | F | P |
|---|---|---|---|---|---|---|
| Annual grafted seedling leaflet | 6-BA | 2 | 2622.375 | 1311.187 | 77.591 | 0.000** |
| 2,4-D | 2 | 57.819 | 28.910 | 1.711 | 0.206 | |
| TDZ | 2 | 128.789 | 64.394 | 3.811 | 0.040* | |
| Error | 20 | 337.975 | 16.899 | - | - | |
| Sun | 27 | 13723.723 | - | - | - | |
| Axillary buds induce leaflets | 6-BA | 2 | 1476.646 | 738.323 | 42.619 | 0.000** |
| 2,4-D | 2 | 144.656 | 72.328 | 4.175 | 0.031* | |
| TDZ | 2 | 19.924 | 9.962 | 0.575 | 0.572 | |
| Error | 20 | 346.474 | 17.324 | - | - | |
| Sun | 27 | 29649.142 | - | - | - | |
| Proliferate one generation of leaflets | 6-BA | 2 | 3360.867 | 1680.433 | 29.026 | 0.000** |
| 2,4-D | 2 | 600.965 | 300.483 | 5.19 | 0.015* | |
| TDZ | 2 | 182.009 | 91.005 | 1.572 | 0.232 | |
| Error | 20 | 1157.866 | 57.893 | - | - | |
| Sun | 27 | 79113.749 | - | - | - |
Table 3 Variance analysis of Cerasus serrulata callus differentiation of different explants with different plant hormones
| Different explants | Source | df | Sum of squares | Mean square | F | P |
|---|---|---|---|---|---|---|
| Annual grafted seedling leaflet | 6-BA | 2 | 2622.375 | 1311.187 | 77.591 | 0.000** |
| 2,4-D | 2 | 57.819 | 28.910 | 1.711 | 0.206 | |
| TDZ | 2 | 128.789 | 64.394 | 3.811 | 0.040* | |
| Error | 20 | 337.975 | 16.899 | - | - | |
| Sun | 27 | 13723.723 | - | - | - | |
| Axillary buds induce leaflets | 6-BA | 2 | 1476.646 | 738.323 | 42.619 | 0.000** |
| 2,4-D | 2 | 144.656 | 72.328 | 4.175 | 0.031* | |
| TDZ | 2 | 19.924 | 9.962 | 0.575 | 0.572 | |
| Error | 20 | 346.474 | 17.324 | - | - | |
| Sun | 27 | 29649.142 | - | - | - | |
| Proliferate one generation of leaflets | 6-BA | 2 | 3360.867 | 1680.433 | 29.026 | 0.000** |
| 2,4-D | 2 | 600.965 | 300.483 | 5.19 | 0.015* | |
| TDZ | 2 | 182.009 | 91.005 | 1.572 | 0.232 | |
| Error | 20 | 1157.866 | 57.893 | - | - | |
| Sun | 27 | 79113.749 | - | - | - |
| No. | 6-BA (mg·L-1) | NAA (mg·L-1) | Proliferation coefficient | ||
|---|---|---|---|---|---|
| Annual grafted seedling leaflets | Axillary buds induce leaflets | Proliferate one generation of leaflets | |||
| 1 | 0.5 | 0 | 0 e | 2.42±0.04 g | 3.32±0.46 e |
| 2 | 0.5 | 0.1 | 0.88±0.77 d | 3.52±0.07 f | 2.70±0.24 f |
| 3 | 0.5 | 0.5 | 2.17±0.27 c | 2.39±0.05 g | 2.91±0.29 ef |
| 4 | 1 | 0 | 2.38±0.04 c | 4.46±0.12 e | 7.85±0.33 a |
| 5 | 1 | 0.1 | 2.17±0.62 c | 4.99±0.11 c | 5.57±0.20 cd |
| 6 | 1 | 0.5 | 3.13±0.05 ab | 4.64±0.08 d | 6.30±0.14 b |
| 7 | 2 | 0 | 2.61±0.07 bc | 5.39±0.05 a | 5.26±0.27 d |
| 8 | 2 | 0.1 | 3.07±0.06 b | 5.18±0.19 b | 5.82±0.19 c |
| 9 | 2 | 0.5 | 3.53±0.04 a | 4.73±0.08 d | 3.38±0.15 e |
| Average | 2.22±1.12 C | 4.19±1.10 B | 4.79±1.73 A | ||
Table 4 Effects of different plant hormones on Cerasus serrulata adventitious bud proliferation of different explants (means±SE)
| No. | 6-BA (mg·L-1) | NAA (mg·L-1) | Proliferation coefficient | ||
|---|---|---|---|---|---|
| Annual grafted seedling leaflets | Axillary buds induce leaflets | Proliferate one generation of leaflets | |||
| 1 | 0.5 | 0 | 0 e | 2.42±0.04 g | 3.32±0.46 e |
| 2 | 0.5 | 0.1 | 0.88±0.77 d | 3.52±0.07 f | 2.70±0.24 f |
| 3 | 0.5 | 0.5 | 2.17±0.27 c | 2.39±0.05 g | 2.91±0.29 ef |
| 4 | 1 | 0 | 2.38±0.04 c | 4.46±0.12 e | 7.85±0.33 a |
| 5 | 1 | 0.1 | 2.17±0.62 c | 4.99±0.11 c | 5.57±0.20 cd |
| 6 | 1 | 0.5 | 3.13±0.05 ab | 4.64±0.08 d | 6.30±0.14 b |
| 7 | 2 | 0 | 2.61±0.07 bc | 5.39±0.05 a | 5.26±0.27 d |
| 8 | 2 | 0.1 | 3.07±0.06 b | 5.18±0.19 b | 5.82±0.19 c |
| 9 | 2 | 0.5 | 3.53±0.04 a | 4.73±0.08 d | 3.38±0.15 e |
| Average | 2.22±1.12 C | 4.19±1.10 B | 4.79±1.73 A | ||
| IBA (mg·L-1) | NAA (mg·L-1) | Rooting rate (%) | Number of roots (strips) |
|---|---|---|---|
| 0 | 0 | 100 a | 12.82±0.54 a |
| 0 | 0.5 | 86.72±1.62 b | 9.24±1.24 b |
| 0 | 1 | 0 e | 0 e |
| 0.5 | 0 | 68.32±2.95 c | 5.64±0.26 c |
| 0.5 | 0.5 | 44.09±1.74 d | 3.24±0.48 d |
Table 5 Effects of plant hormones on rooting of Cerasus serrulata (means±SE)
| IBA (mg·L-1) | NAA (mg·L-1) | Rooting rate (%) | Number of roots (strips) |
|---|---|---|---|
| 0 | 0 | 100 a | 12.82±0.54 a |
| 0 | 0.5 | 86.72±1.62 b | 9.24±1.24 b |
| 0 | 1 | 0 e | 0 e |
| 0.5 | 0 | 68.32±2.95 c | 5.64±0.26 c |
| 0.5 | 0.5 | 44.09±1.74 d | 3.24±0.48 d |
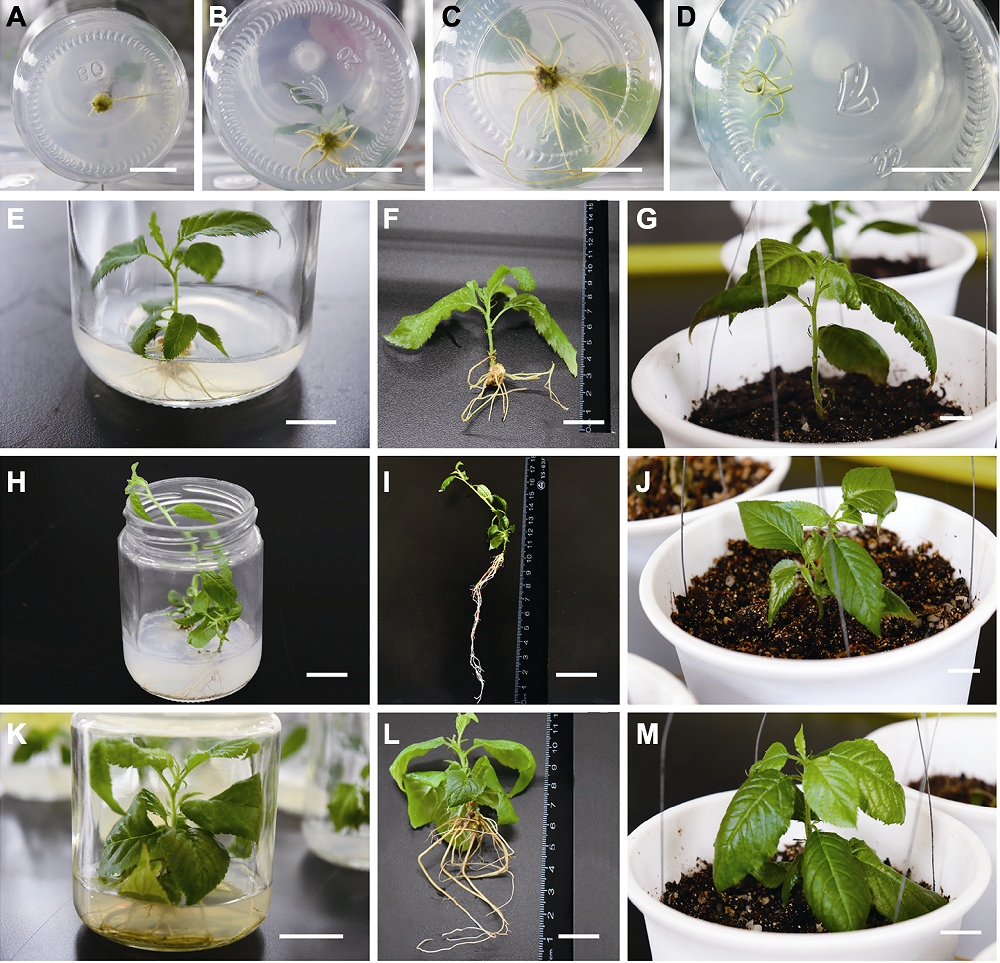
Figure 4 Rooting and transplantation of regenerated plants induced by different explants of Cerasus serrulata (A) Adventitious roots protuberant; (B) Fleshy taproots; (C) Lateral roots on the taproot; (D) Directly grow fibrous roots; (E)-(G) Leaflet regeneration of annual grafted seedlings, rooting and transplanting; (H)-(J) The axillary buds induced the rooting and transplanting of regenerated leaflets; (K)-(M) Proliferation of a generation of regenerated leaflets, rooting and transplanting. Bars=2 cm
| [1] | 陈雪, 张金柱, 潘兵兵, 桑成瑾, 马雪, 杨涛, 车代弟 (2011). 月季愈伤组织的诱导及植株再生. 植物学报 46, 569-574. |
| [2] | 房洪舟, 鲁敏, 安华明 (2019). 刺梨叶片愈伤组织培养体系建立及其主要活性物质分析. 植物生理学报 55, 1147-1155. |
| [3] | 郭希梅, 丛日晨, 张常青, 古润泽, 高俊平 (2011). 古油松衰弱衰老诊断的生理指标. 林业科学 47(4), 43-48. |
| [4] | 和凤美, 李璇, 邵琬珊, 朱永平, 杨晓红 (2010). 冬樱花愈伤组织诱导和抑制褐化初探. 中国农学通报 26(12), 130-134. |
| [5] | 黄守印, 池井存, 苏淑欣, 尚文艳, 任艳平 (2003). 雾灵山地区野生樱花的组织培养与快速繁殖. 植物生理学通讯 (3), 228. |
| [6] | 蒋冬月, 邹宜含, 柳新红, 程亚平, 王平, 沈鑫 (2019). 樱花粉红及黄绿色系品种苗期生长特性及适应性. 江西农业大学学报 41, 673-682. |
| [7] | 雷巾茗 (2020). 樱花组培快繁与扦插繁殖研究. 硕士论文. 北京: 北京林业大学. pp. 1-92. |
| [8] | 李水根, 李秀芬, 殷丽青, 高晨, 朱建军 (2020). 喜马拉雅樱花嫩茎离体快繁体系优化. 分子植物育种 18, 8217-8222. |
| [9] | 李艳敏, 孟月娥, 张玉, 赵秀山, 王利民, 王慧娟 (2012). 新优彩叶植物红叶樱花外植体采集及离体培养技术研究. 河南农业科学 41(9), 127-130, 142. |
| [10] | 李艳敏, 孟月娥, 赵秀山, 王慧娟, 张强, 王利民 (2008). ‘红叶樱花’的组织培养和快速繁殖. 植物生理学通讯 44, 1163-1164. |
| [11] | 刘莉莉, 卢淑波, 徐佳萍, 张庆田, 李昌禹 (2015). 以黄花乌头发根为外植体的再生培养体系建立. 植物学报 50, 623-627. |
| [12] | 刘晓莉 (2012). 14个樱花品种观赏性状综合评价和樱花园林应用研究. 硕士论文. 杭州: 浙江农林大学. pp. 1-89. |
| [13] | 吕月良, 陈璋, 施季森, 黄宇翔, 刘金燕, 谢建丽 (2006). 福建山樱花不定芽诱导和植株再生规模化繁殖试验. 南京林业大学学报(自然科学版) (3), 105-108. |
| [14] | 任如意, 薛巨坤, 国会艳, 魏继承 (2017). 北玄参毛状根诱导及其植株再生. 植物学报 52, 783-787. |
| [15] | 史港影, 南程慧, 伊贤贵, 张开文, 王贤荣 (2014). 雪落樱再生体系的建立. 南京林业大学学报(自然科学版) 38, 20-24. |
| [16] | 宋斯妤 (2018). 迎春樱和华中樱优良品系组培快繁技术的研究. 硕士论文. 杭州: 浙江农林大学. pp. 1-62. |
| [17] | 徐晨捷, 欧静 (2020). 染井吉野樱的茎段培养与胚培养比较. 北方园艺 (23), 65-71. |
| [18] | 闫国华, 周宇, 张晓明, 张开春 (2002). 植物离体培养中的顽拗现象及其生理和遗传基础. 植物生理学通讯 38, 481-486. |
| [19] |
燕丽萍, 李丽, 刘翠兰, 吴德军, 王因花, 任飞, 赵梁军 (2016). 绒毛白蜡体胚诱导和植株再生. 植物学报 51, 807-816.
DOI |
| [20] | 杨小燕, 欧静, 张凤泉, 翁钰舟, 于瀚, 曹时波 (2019). 贵阳市樱花资源及其园林应用研究. 山地农业生物学报 38(6), 14-20. |
| [21] | 于波, 黄丽丽, 朱玉, 朱根发, 孙映波 (2020). 朱顶红幼嫩花梗胚性愈伤组织诱导和高效植株再生. 园艺学报 47, 907-915. |
| [22] | 张灵灵, 蒋细旺 (2015). 2个日本晚樱品种组织培养和快繁技术研究. 西南林业大学学报 35(4), 27-32. |
| [23] | 张旭红, 王頔, 梁振旭, 孙美玉, 张金政, 石雷 (2018). 欧洲百合愈伤组织诱导及植株再生体系的建立. 植物学报 53, 840-847. |
| [24] | 朱继军, 奉树成, 陈必胜 (2015). 晚樱花品种的引种与筛选. 中国园艺文摘 31(5), 1-3, 24. |
| [25] | 邹娜, 陈璋, 林思祖, 林庆良 (2013). 福建山樱花愈伤组织的诱导及植株再生. 核农学报 27, 1417-1423. |
| [26] |
Bartos PMC, Gomes HT, do Amaral LIV, Teixeira JB, Scherwinski-Pereira JE (2018). Biochemical events during somatic embryogenesis in Coffea arabica L. 3 Biotech 8, 209.
DOI URL |
| [27] |
Ben Mahmoud K, Jedidi E, Delporte F, Muhovski Y, Jemmali A, Druart P (2017). Molecular investigations of the somatic embryogenesis recalcitrance in the cherry ( Prunus cerasus L.) rootstock CAB 6P. Turk J Biol 41, 158-165.
DOI URL |
| [28] |
Bernula D, Benkő P, Kaszler N, Domonkos I, Szőllősi R, Ferenc G, Ayaydin F, Fehér A, Gémes K (2020). Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell Tissue Organ Cult 140, 327-339.
DOI URL |
| [29] | Chen BH, Li JM, Zhang J, Wu ZX, Fan HH, Li QZ (2016). Optimizing the rapid technique for propagation of Cerasus campanulata by tissue culture. Pak J Bot 48, 305-309. |
| [30] |
Correia S, Lopes ML, Canhoto JM (2011). Somatic embryogenesis induction system for cloning an adult Cyphomandra betacea (Cav.) Sendt. (tamarillo). Trees 25, 1009-1020.
DOI URL |
| [31] |
Díaz-Sala C (2019). Molecular dissection of the regenerative capacity of forest tree species: special focus on conifers. Front Plant Sci 9, 1943.
DOI URL |
| [32] |
Hu RY, Sun YH, Wu B, Duan HJ, Zheng HQ, Hu DL, Lin HZ, Tong ZK, Xu JL, Li Y (2017). Somatic embryogenesis of immature Cunninghamia lanceolata (Lamb.) hook zygotic embryos. Sci Rep 7, 56.
DOI URL |
| [33] |
Martínez MT, San José MC, Vieitez AM, Cernadas MJ, Ballester A, Corredoira E (2017). Propagation of mature Quercus ilex L. (holm oak) trees by somatic embryogenesis. Plant Cell Tissue Organ Cult 131, 321-333.
DOI URL |
| [34] |
McCown BH (2000). Special symposium: in vitro plant recalcitrance recalcitrance of woody and herbaceous perennial plants: dealing with genetic predeterminism. In Vitro Cell Dev Biol Plant 36, 149-154.
DOI URL |
| [35] |
Ming NJ, Mostafiz SB, Johon NS, Zulkifli NSA, Wagiran A (2019). Combination of plant growth regulators, maltose, and partial desiccation treatment enhance somatic embryogenesis in selected Malaysian rice cultivar. Plants 8, 144.
DOI URL |
| [36] |
Singh R, Rai MK, Kumari N (2015). Somatic embryogenesis and plant regeneration in Sapindus mukorossi gaertn. from leaf-derived callus induced with 6-benzylaminopurine. Appl Biochem Biotechnol 177, 498-510.
DOI URL |
| [37] |
Wu GY, Wei XL, Wang X, Wei Y (2020). Induction of somatic embryogenesis in different explants from Ormosia henryi Prain. Plant Cell Tissue Organ Cult 142, 229-240.
DOI URL |
| [38] |
Wu H, Chen BJ, Fiers M, Wróbel-Marek J, Kodde J, Groot SPC, Angenent G, Feng H, Bentsink L, Boutilier K (2019). Seed maturation and post-harvest ripening negatively affect Arabidopsis somatic embryogenesis. Plant Cell Tissue Organ Cult 139, 17-27.
DOI URL |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper [J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255. |
| [3] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [4] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [5] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [6] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [7] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [8] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [9] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [10] | Liu Xiaofei, Sun Yingbo, Huang Lili, Yang Yuchai, Zhu Genfa, Yu Bo. Efficient Plant Regeneration via Somatic Embryogenesis in Alocasia reginula cv. ‘Black Velvet’ [J]. Chinese Bulletin of Botany, 2023, 58(5): 750-759. |
| [11] | Jiming Cheng, Huimin He, Hongyu Niu, Hongmao Zhang. Research progress on the effect of intraspecific personality differences on seed dispersal in rodents [J]. Biodiv Sci, 2023, 31(4): 22446-. |
| [12] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [13] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [14] | Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum [J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235. |
| [15] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||