

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (4): 600-612.DOI: 10.11983/CBB24012 cstr: 32102.14.CBB24012
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Yuchen Li1,2, Haixia Zhao2, Xiping Jiang2, Xintian Huang1, Yaling Liu3, Zhenying Wu2, Yan Zhao1,*( ), Chunxiang Fu2,*(
), Chunxiang Fu2,*( )
)
Received:2024-01-24
Accepted:2024-04-25
Online:2024-07-10
Published:2024-07-10
Contact:
*E-mail: zhaoyannmg@163.com; fucx@qibebt.ac.cn
Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum[J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612.

Figure 1 Schematic diagram of pANIC6B empty vector (refer to Mann et al., 2012) LB: Left border of T-DNA; OsAct1: Rice Act1 promoter and intron; hph: Screening marker gene for hygromycin resistance; 35S T: 35S terminator; PvUbi1: Switchgrass Ubi1 promoter and intron; GUSplus: β-glucuronidase plus reporter gene; NOS T: Agrobacterium tumefaciens carmine synthase gene terminator; ZmUbi1: Maize Ubi1 promoter and intron; Gateway cassette: attR1-CmR/ccdb-attR2; OCS T: Octopine synthase terminator; RB: Right border of T-DNA
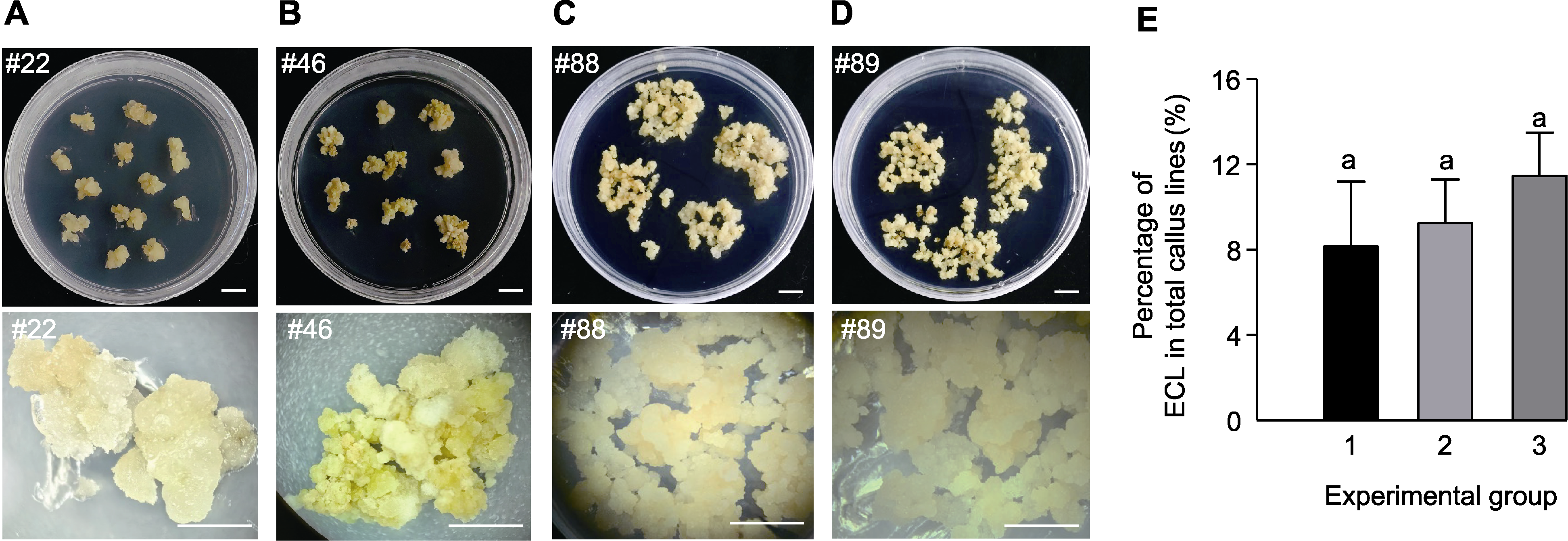
Figure 2 Phenotypes of callus lines induced from Mengnong No.1 seeds (A)-(D) Phenotypes of different callus lines (the up photos are calli grown on M5 medium, the down photos are calli under the microscope. Bars=1 cm); (E) Percentage of embryogenic callus lines (ECL) in total callus lines (One-way analysis of variance, Duncan’s test, P<0.05).

Figure 3 Regeneration analyses of embryogenic callus lines (ECL) induced from Mengnong No.1 seeds (A) Regeneration of embryogenic callus lines #88 and #89 with different genotypes (bars=1 cm); (B) Percentage of embryogenic callus lines with more than 30% regeneration efficiency in total embryogenic callus lines. Different lowercase letters indicate significant differences among three experimental groups (One-way analysis of variance, Duncan’s test, P<0.05).
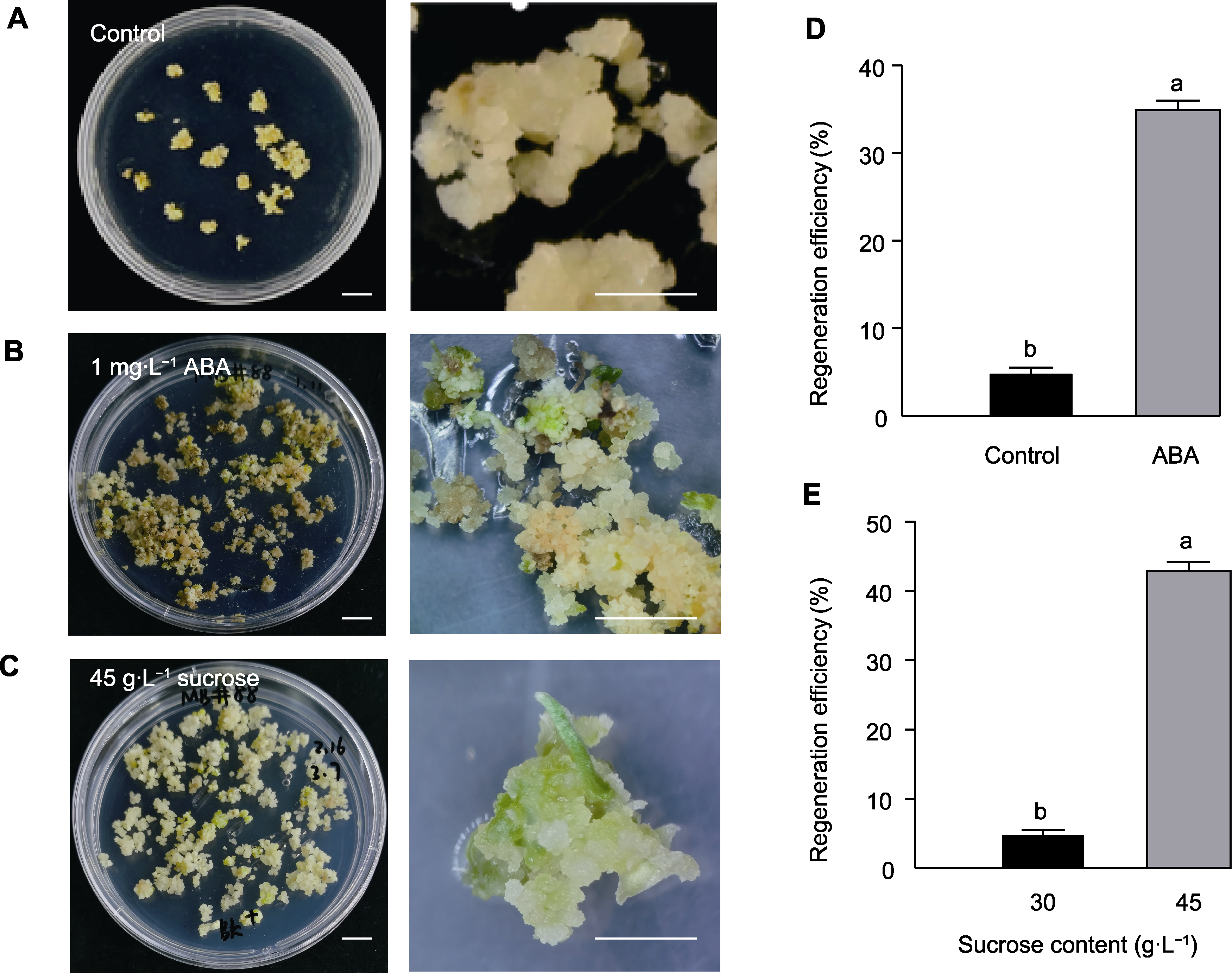
Figure 4 Effects of ABA and high concentration sucrose on the regeneration capacity of deteriorated embryogenic callus line #89 (A) Differentiation of deteriorated embryogenic callus line #89 grown in MSBK medium; (B) Differentiation of deteriorated embryogenic callus line #89 grown in MSBKA medium supplemented with 1 mg·L-1 ABA; (C) Differentiation of deteriorated embryogenic callus line #89 grown in MSBKS45 medium supplemented with 45 g·L-1 sucrose; (D) Regeneration efficiency of deteriorated embryogenic callus line #89 grown in MSBKA medium with ABA treatment; (E) Regeneration efficiency of deteriorated embryogenic callus line #89 grown in MSBKS45 medium with 30 g·L-1 and 45 g·L-1 sucrose. Different lowercase letters indicate significant differences between treatments (One-way analysis of variance, Duncan’s test, P<0.05). The callus depicted in (A), (B), and (C) on the right side represents the microscopic observation of the corresponding structures on the left side. Bars=1 cm

Figure 5 Infection analyses of embryogenic callus lines (ECL) induced from Mengnong No.1 seeds (A) The infection efficiency of EHA105 on embryogenic callus lines through GUS staining; (B) The percentage of embryogenic callus lines with high infection efficiency (>40%) in total embryogenic callus lines. Different lowercase letters indicated significant differences among three experimental groups (One-way analysis of variance, Duncan’s test, P<0.05).
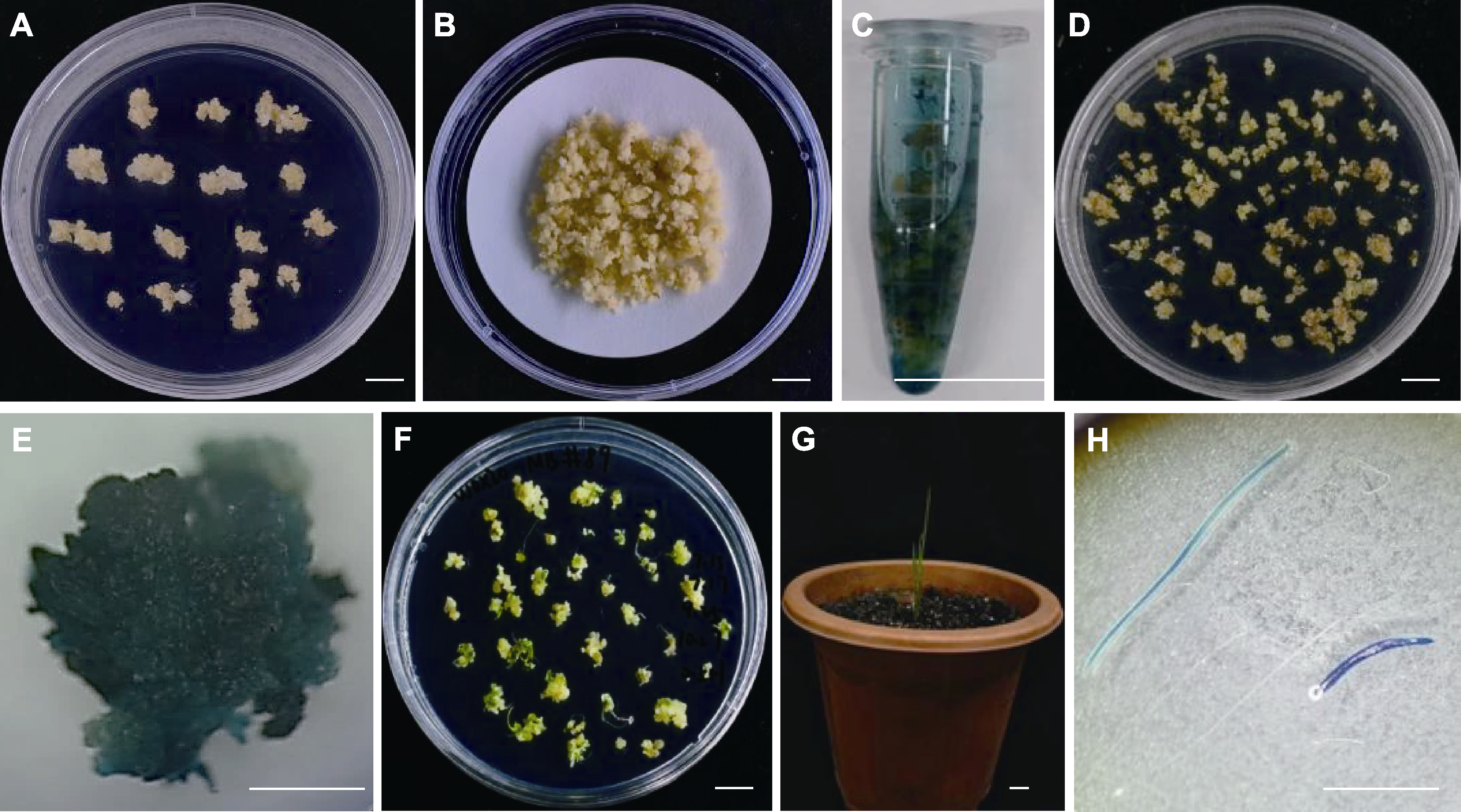
Figure 6 Establishment of the Agrobacterium-mediated transformation in Mengnong No.1 (A) #89 embryogenic callus line induced from Mengnong No.1 seeds grown on M2 medium; (B) Co-cultivation of #89 calli with Agrobacterium strain EHA105; (C) Agrobacterium infection analysis of #89 embryogenic calli by GUS staining; (D) Selection of hygromycin resistant calli through M2H30 medium; (E) GUS staining of hygromycin resistant callus; (F) Differentiation of the hygromycin resistant calli on MSBKH2 medium; (G) Growth of the hygromycin resistant plant in soil; (H) GUS staining of leaves from the hygromycin resistant plants. Bars=1 cm
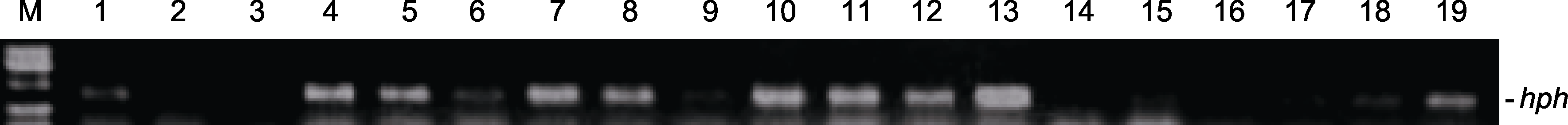
Figure 7 PCR analysis of hph in Mengnong No.1 Agropyron mongolicum transgenic plants M: 2000 bp DNA marker; 1-19: PCR template (1: pANIC6B empty vector; 2: ddH2O; 3: Non-transgenic plant regenerated from embryogenic callus line #89; 4-19: Transgenic plants regenerated from embryogenic callus #89. The sizes of PCR products are 375 bp for hph).
| [1] |
Armstrong CL, Green CE (1985). Establishment and maintenance of friable, embryogenic maize callus and the involvement of L-proline. Planta 164, 207-214.
DOI PMID |
| [2] | Chen H, Zhao Y, Chong K (2008). Improved high-efficiency system for rice transformation using mature embryoderived calli. Chin Bull Bot 25, 322-331. (in Chinese) |
| 陈惠, 赵原, 种康 (2008). 一种改进的水稻成熟胚愈伤组织高效基因转化系统. 植物学通报 25, 322-331. | |
| [3] |
Chen ZL, Debernardi JM, Dubcovsky J, Gallavotti A (2022). Recent advances in crop transformation technologies. Nat Plants 8, 1343-1351.
DOI PMID |
| [4] | Doyle JJ, Doyle JL (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19, 11-15. |
| [5] | Fu SB, Li H, Zhao J, Fu XJ, Zhu SL, Zhang JF (2017). Investigation of browning factors in embryogenic callus culture of Pinus tabulaeformis and optimization of proliferation medium. J South China Agric Univ 38(5), 91-96. (in Chinese) |
| 付双彬, 李慧, 赵健, 符学军, 朱松林, 张金凤 (2017). 油松胚性愈伤组织培养中褐化因素研究及增殖培养基的优化. 华南农业大学学报 38(5), 91-96. | |
| [6] | Gao SM, Li FL (2002). Advances in researches on effects of ABA on somatic embryogenesis. J Beijing For Univ 24(4), 122-129. (in Chinese) |
| 高述民, 李凤兰 (2002). ABA对植物体细胞胚胎发育影响的研究进展. 北京林业大学学报 24(4), 122-129. | |
| [7] | Gu AL, Yun JF (1994). Establishment of different Agropyron species & cultivars in arid and semi-arid grasslands of Inner Mongolia. Grassland China 16(3), 37-41. (in Chinese) |
| 谷安琳, 云锦凤 (1994). 冰草属植物在内蒙古干旱草原的建植试验. 中国草地 16(3), 37-41. | |
| [8] | Guo L, Cheng YH, Wang ZY, He B, Zhang JJ, Liu SQ, Liu MH, Chen ZL, Qu LJ, Gu HY (2006). Primary study on gene expression during root differentiation from rice calli. Acta Sci Nat Univ Pek 42, 175-179. (in Chinese) |
| 郭蕾, 程英豪, 王紫祎, 何斌, 张军军, 柳世庆, 刘美华, 陈章良, 瞿礼嘉, 顾红雅 (2006). 水稻愈伤组织根分化过程相关基因的初步筛选. 北京大学学报(自然科学版) 42, 175-179. | |
| [9] | Hu ZH, Chen HQ, Wu GT, Jin W, Zhang XL, Lang CX, Huang RZ, Chen XY, Liu ZH, Xia ZH, Chen JQ (2003). Tissue culture and green plant regeneration from mature embryos of bermudagrass. Acta Prata Sin 12(1), 85-89. (in Chinese) |
| 胡张华, 陈火庆, 吴关庭, 金卫, 章锡良, 郎春秀, 黄锐之, 陈笑云, 刘智宏, 夏正红, 陈锦清 (2003). 百慕大成熟胚的组织培养及植株再生. 草业学报 12(1), 85-89. | |
| [10] | Huo XW, Yun JF, Mi FG, Wei JH (2005). Study on culture and regeneration of wheatgrass and its genetic transformation for drought tolerance. Acta Agre Sin 13, 172-173. (in Chinese) |
|
霍秀文, 云锦凤, 米福贵, 魏建华 (2005). 冰草组织培养再生体系建立及耐旱转基因研究. 草地学报 13, 172-173.
DOI |
|
| [11] | Lan BX, Li LH, Wang H (2005). Genetic diversity of Agropyron mongolicum Keng populations. Sci Agric Sin 38, 468-473. (in Chinese) |
| 兰保祥, 李立会, 王辉 (2005). 蒙古冰草居群遗传多样性研究. 中国农业科学 38, 468-473. | |
| [12] | Li HC, Hu DF, Wang H (1990). Study on the factors affecting mature embryo culture of wheat. Acta Agric Boreali-Sin 5(1), 22-27. (in Chinese) |
|
李宏潮, 胡道芬, 王虹 (1990). 影响小麦成熟胚培养因素的研究. 华北农学报 5(1), 22-27.
DOI |
|
| [13] | Li LH, Dong YC (1993). Progress in the studies of Agropyron Gaertn. Heredity 15(1), 45-48. (in Chinese) |
| 李立会, 董玉琛 (1993). 冰草属研究进展. 遗传 15(1), 45-48. | |
| [14] | Ling DH, Yoshida S (1987). The study of some factors affecting somatic embryogenesis in IR lines of rice. Acta Bot Sin 29, 1-8. (in Chinese) |
| 凌定厚, 吉田昌一 (1987). 影响籼稻体细胞胚胎发生几个因素的研究. 植物学报 29, 1-8. | |
| [15] | Liu WL (2022). Community Characteristics and Population Distribution Patterns of Agropyron mongolicum in Desert Steppe in Heterogeneous Habitats. Master’s thesis. Yinchuan: Ningxia University. pp. 1-51. (in Chinese) |
| 刘万龙 (2022). 异质生境下荒漠草原蒙古冰草群落特征及种群分布格局. 硕士论文. 银川: 宁夏大学. pp. 1-51. | |
| [16] | Liu YF, Zhao HX, Jiang XP, Qiu R, Zhou XY, Zhao Y, Fu CX (2023). Establishment of highly efficient tissue culture and Agrobacterium-mediated callus infection systems for Hordeum brevisubulatum. Chin Bull Bot 58, 440-448. (in Chinese) |
|
刘叶飞, 赵海霞, 姜希萍, 邱锐, 周昕越, 赵彦, 付春祥 (2023). 野大麦高效组培快繁及农杆菌介导的愈伤侵染体系建立. 植物学报 58, 440-448.
DOI |
|
| [17] | Mann DGJ, Lafayette PR, Abercrombie LL, King ZR, Mazarei M, Halter MC, Poovaiah CR, Baxter H, Shen H, Dixon RA, Parrott WA, Neal Stewart C Jr (2012). Gateway-compatible vectors for high-throughput gene functional analysis in switchgrass (Panicum virgatum L). and other monocot species. Plant Biotechnol J 10, 226- 236. |
| [18] |
Ning Z, Lin X (2021). Pluripotency acquisition in the middle cell layer of callus is required for organ regeneration. Nat Plants 7, 1453-1460.
DOI PMID |
| [19] | Praveena M, Giri CC (2012). Plant regeneration from immature inflorescence derived callus cultures of salt tolerant kallar grass (Leptochloa fusca L). Physiol Mol Biol Plants 18, 345-356. |
| [20] | Sun Q, Hu JJ (2006). Research Technology of Plant Physiology. Yangling: Northwest A&F University Press. pp. 209. (in Chinese) |
| 孙群, 胡景江 (2006). 植物生理学研究技术. 杨凌: 西北农林科技大学出版社. pp. 209. | |
| [21] |
Suo JQ, Zhou CL, Zeng ZH, Li XP, Bian HW, Wang JH, Zhu MY, Han N (2021). Identification of regulatory factors promoting embryogenic callus formation in barley through transcriptome analysis. BMC Plant Biol 21, 145.
DOI PMID |
| [22] | Tang F, Xu B, Shi FL, Zheng WY, Zhao Y, Yang ZY (2021). Establishment of efficient tissue culture and regeneration system of Agropyron mongolicum Keng root tip. Chin J Grassland 43(3), 113-118. (in Chinese) |
| 唐芳, 徐舶, 石凤翎, 郑文洋, 赵彦, 杨紫贻 (2021). 蒙古冰草根尖高效组培再生体系的建立. 中国草地学报 43(3), 113-118. | |
| [23] |
Vasil IK (1994). Molecular improvement of cereals. Plant Mol Biol 25, 925-937.
DOI PMID |
| [24] | Wang HF (2011). Research on Regeneration Properties of Culture System from Immature Embryos and Genetic Transformation of Wheat by Biolistic Particle. Master’s thesis. Yangling: Northwest A&F University. pp. 1-47. (in Chinese) |
| 王海凤 (2011). 不同品种小麦幼胚组织培养再生性能及基因枪介导的遗传转化研究. 硕士论文. 杨凌: 西北农林科技大学. pp. 1-47. | |
| [25] | Wang SY, Huang YZ, Li ZY, Huang HH, Lin EP (2022). Research progress in plant somatic embryogenesis and its molecular regulation mechanism. J Zhejiang A&F Univ 39, 223-232. (in Chinese) |
| 王诗忆, 黄奕孜, 李舟阳, 黄华宏, 林二培 (2022). 植物体细胞胚胎发生及其分子调控机制研究进展. 浙江农林大学学报 39, 223-232. | |
| [26] | Wang XC, Gao T, Yang WD, Wang C, Chen CJ (2023). Study on embryogenic callus induction and embryoid differentiation of alfalfa. Crops (4), 98-103. (in Chinese) |
| 王晓春, 高婷, 杨炜迪, 王川, 陈彩锦 (2023). 紫花苜蓿胚性愈伤组织诱导及胚状体分化研究. 作物杂志 (4), 98-103. | |
| [27] | Wang YZ, Xu B, Zhang L, Mou SL, Xu AK (2023). Study on high-frequency regeneration system of embryonal tissue culture of Leymus chinensis seeds. Acta Agre Sin 31, 3706-3714. (in Chinese) |
|
王英哲, 徐博, 张玲, 牟书靓, 徐安凯 (2023). 羊草种子胚性组织培养高频再生体系的研究. 草地学报 31, 3706- 3714.
DOI |
|
| [28] | Wei KF, Liu YP, Lin ZY, Yang YF, Zhang ZH, Jia WS (2008). Problems and Solutions in Agrobacterium tumefaciens-mediated genetic transformation of monocotyledons. Chin Bull Bot 25, 491-496. (in Chinese) |
| 魏开发, 刘逸萍, 林子英, 杨雅芳, 张泽宏, 贾文锁 (2008). 农杆菌介导单子叶植物遗传转化问题与对策. 植物学通报 25, 491-496. | |
| [29] | Xie JH, Yun JF, Yang B, Hou JH (2006). Influence of 2,4-D and BAP on callus induction and growth of immature embryo of wheatgrass. Chin J Grassland 28(2), 44-47. (in Chinese) |
| 解继红, 云锦凤, 杨斌, 侯建华 (2006). 2,4-D和BAP对蒙古冰草幼胚愈伤组织诱导及生长的影响. 中国草地学报 28(2), 44-47. | |
| [30] | Yu GR, Yin J, Guo TC, Niu JS (2003). Selection of the optimum genotype for immature embryo culture of wheat. J Tritic Crops 23(2), 14-18. (in Chinese) |
| 余桂荣, 尹钧, 郭天财, 牛吉山 (2003). 小麦幼胚培养基因型的筛选. 麦类作物学报 23(2), 14-18. | |
| [31] | Yuan JC, Liu YH, Dong ZP (2013). Evaluation and optimization on callus induction and regeneration of mature embryo in millet. Biotechnol Bull (3), 77-82. (in Chinese) |
| 袁进成, 刘颖慧, 董志平 (2013). 谷子成熟胚诱导愈伤组织及植株再生的研究和条件的优化. 生物技术通报 (3), 77-82. | |
| [32] | Zhang W, Ma YT, Zhu ZD, Huang LJ, Ali A, Luo X, Zhou Y, Li Y, Xu PZ, Yang J, Li Z, Shi HR, Wang JS, Gong WZ, Zou Q, Tao LR, Kang ZM, Tang R, Zhao ZJ, Li Z, Guo SX, Fu SH (2021). Maternal karyogene and cytoplasmic genotype affect the induction efficiency of doubled haploid inducer in Brassica napus. BMC Plant Biol 21, 207. |
| [33] | Zhang WJ, Guan QL, Fu YP, Su J (2014). Antisense suppression expression of rice (Oryza sativa) sucrose transporter gene (OsSUT5) leads to reducing callus induction and plantlet regeneration. J Agricul Biotechnol 22, 825- 831. (in Chinese) |
| 张武君, 管其龙, 付艳萍, 苏军 (2014). 反义抑制水稻蔗糖转运蛋白基因(OsSUT5)的表达降低其愈伤组织诱导和植株再生频率. 农业生物技术学报 22, 825-831. | |
| [34] | Zhang Z, Shi WG, Lu XP (2004). Advances in study on Agropyron mongolicum Keng. In:Second Session of the Sixth Session of the Chinese Grass Society and International Academic Workshop (International Symposium on Pratacultural Science and Technological Innovation). Hohhot: Chinese Grass Society. pp. 479-485. (in Chinese) |
| 张众, 师文贵, 逯晓萍 (2004). 蒙古冰草研究概况. 见:中国草学会六届二次会议暨国际学术研讨会(草业科学与技术创新国际学术研讨会). 呼和浩特: 中国草学会. pp. 479- 485. | |
| [35] | Zhao Y, Chen XY, Yun JF, Liu XP (2016). Induction of callus and plant regeneration from shoot tip in Agropyron mongolicum Keng. J Northern Agric 44(2), 18-22. (in Chinese) |
| 赵彦, 陈雪英, 云锦凤, 刘湘萍 (2016). 蒙古冰草茎尖愈伤组织及其再生植株诱导. 北方农业学报 44(2), 18-22. |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zeng Wendan, Yan Huabing, Wu Zhengdan, Shang Xiaohong, Cao Sheng, Lu Liuying, Xiao Liang, Shi Pingli, Cheng Dong, Long Ziyuan, Li Jieyu. Agrobacterium rhizogenes-mediated Transformation System of Pueraria lobata Hairy Roots [J]. Chinese Bulletin of Botany, 2025, 60(3): 425-434. |
| [3] | Jinyu Du, Zhen Sun, Yanlong Su, Heping Wang, Yaling Liu, Zhenying Wu, Feng He, Yan Zhao, Chunxiang Fu. Identification and Functional Analysis of an Agropyron mongolicum Caffeic Acid 3-O-methyltransferase Gene AmCOMT1 [J]. Chinese Bulletin of Botany, 2024, 59(3): 383-396. |
| [4] | Heping Wang, Zhen Sun, Yuchen Liu, Yanlong Su, Jinyu Du, Yan Zhao, Hongbo Zhao, Zhaoming Wang, Feng Yuan, Yaling Liu, Zhenying Wu, Feng He, Chunxiang Fu. Sequence Identification and Functional Analysis of Cinnamyl Alcohol Dehydrogenase Gene from Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(2): 204-216. |
| [5] | Yu Xiaomin, Wang Yaqin, Liu Yuhan, Yi Qingping, Cheng Wenhan, Zhu Yu, Duan Feng, Zhang Lixue, He Yanhong. Establishment of Agrobacterium tumefaciens-mediated Genetic Transformation System of Marigold (Tagetes erecta) [J]. Chinese Bulletin of Botany, 2023, 58(5): 760-769. |
| [6] | Lan Yang, Ya Liu, Yang Xiang, Xiujuan Sun, Jingwei Yan, Aying Zhang. Establishment and Optimization of a Shoot Tip-based Genetic Transformation System for Foxtail Millet [J]. Chinese Bulletin of Botany, 2021, 56(1): 71-79. |
| [7] | Jianfei Liu, Yan Liu, Kejian Liu, Yang Chi, Zhifa Huo, Yonghong Huo, Xiangling You. Optimization of the Regeneration System from Somatic Embryogenesis in Larix olgensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 605-612. |
| [8] | Junhua Li,Shiyu Liu,Chenglong Li,Linlin Han,Yahui Dong,Xiaoli Zhang,Xiting Zhao,Mingjun Li. Establishment of a Genetic Transformation System for Dioscorea opposita Using Microtuber [J]. Chinese Bulletin of Botany, 2019, 54(1): 72-80. |
| [9] | Lin Liu, Bin Yu, Pengyan Huang, Jun Jia, Hua Zhao, Junhua Peng, Peng Chen, Liangcai Peng. Frequency of Callus Induction and Plant Regeneration Among Eight Genotypes in Miscanthus sinensis Species [J]. Chinese Bulletin of Botany, 2013, 48(2): 192-198. |
| [10] | Lijun Guo, Bingshan Zeng, Ying Liu. Agrobacterium-mediated High-efficient Transformation of Eucalyptus grandis Clone Eg5 [J]. Chinese Bulletin of Botany, 2013, 48(1): 87-93. |
| [11] | Guimei Cui, Yi Sun, Yaoshan Hao, Jianzhong Du, Yixue Wang. The Improvement of Maize Pollen In Vitro Germination Method and Its Role in Pollen-mediated Plant Genetic Transformation [J]. Chinese Bulletin of Botany, 2012, 47(2): 155-161. |
| [12] | Xuanyu Liu, Qingyun Wang, Shujun Liu, Songquan Song. Advances in the Genetic Transformation of Sorghum bicolor [J]. Chinese Bulletin of Botany, 2011, 46(2): 216-223. |
| [13] | Shuan Chen, Xiaodong Wang, Bing Zhao, Yuchun Wang. Regulating the Cell Growth and Shoot Induction of Crocus sativus Embryogenic Callus by Rare Earth Elements [J]. Chinese Bulletin of Botany, 2010, 45(05): 609-614. |
| [14] | Hongbing Fu;Chongshi Cui;Xi Zhao;Qi Liu. Establishment of Cucurbita moschata Genetic Transformation System by Agrobacterium tumefaciens Transfection [J]. Chinese Bulletin of Botany, 2010, 45(04): 472-478. |
| [15] | Daojie Wang, Cuiling Yang, Ming Lu. Transformation of Brassica napus by Vacuum Infiltration [J]. Chinese Bulletin of Botany, 2009, 44(02): 216-222. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||