

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (2): 204-216.DOI: 10.11983/CBB23109 cstr: 32102.14.CBB23109
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Heping Wang1,2, Zhen Sun2, Yuchen Liu2, Yanlong Su2, Jinyu Du2,5, Yan Zhao5, Hongbo Zhao1, Zhaoming Wang6, Feng Yuan6, Yaling Liu6, Zhenying Wu2,3,4, Feng He2,3,4,*( ), Chunxiang Fu2,3,4,*(
), Chunxiang Fu2,3,4,*( )
)
Received:2023-08-09
Accepted:2023-12-19
Online:2024-03-10
Published:2024-03-10
Contact:
* E-mail: Heping Wang, Zhen Sun, Yuchen Liu, Yanlong Su, Jinyu Du, Yan Zhao, Hongbo Zhao, Zhaoming Wang, Feng Yuan, Yaling Liu, Zhenying Wu, Feng He, Chunxiang Fu. Sequence Identification and Functional Analysis of Cinnamyl Alcohol Dehydrogenase Gene from Agropyron mongolicum[J]. Chinese Bulletin of Botany, 2024, 59(2): 204-216.
| Sequence ID | Number of amino acid | Molecular weight (Da) | Theoretical pI | Instability index | Aliphatic index | Grand average of hydropathicity |
|---|---|---|---|---|---|---|
| PB.70108.1 | 360 | 38591.48 | 5.87 | 23.05 | 90.33 | 0.025 |
| PB.82665.1 | 354 | 38437.86 | 6.66 | 24.94 | 84.75 | -0.098 |
| PB.80567.1 | 358 | 38685.52 | 6.60 | 30.79 | 89.27 | 0.012 |
| PB.80906.1 | 355 | 38256.89 | 5.68 | 31.84 | 88.42 | 0.037 |
| PB.62308.1 | 269 | 28625.35 | 6.31 | 31.41 | 92.45 | 0.166 |
| PB.17482.1 | 344 | 36802.76 | 5.86 | 31.22 | 86.13 | 0.103 |
| PB.52032.1 | 373 | 38747.35 | 6.53 | 24.05 | 91.58 | 0.136 |
| PB.47633.2 | 421 | 44353.00 | 6.42 | 31.04 | 84.28 | 0.092 |
| PB.55683.1 | 421 | 44338.97 | 6.42 | 29.60 | 83.80 | 0.088 |
| PB.83678.1 | 356 | 37359.82 | 5.40 | 25.49 | 92.25 | 0.146 |
| PB.84043.1 | 356 | 37036.55 | 5.84 | 26.83 | 93.88 | 0.211 |
| PB.84184.1 | 356 | 37019.73 | 6.08 | 23.61 | 92.25 | 0.195 |
Table 1 Analysis of physicochemical properties of AmCAD proteins from Agropyron mongolicum
| Sequence ID | Number of amino acid | Molecular weight (Da) | Theoretical pI | Instability index | Aliphatic index | Grand average of hydropathicity |
|---|---|---|---|---|---|---|
| PB.70108.1 | 360 | 38591.48 | 5.87 | 23.05 | 90.33 | 0.025 |
| PB.82665.1 | 354 | 38437.86 | 6.66 | 24.94 | 84.75 | -0.098 |
| PB.80567.1 | 358 | 38685.52 | 6.60 | 30.79 | 89.27 | 0.012 |
| PB.80906.1 | 355 | 38256.89 | 5.68 | 31.84 | 88.42 | 0.037 |
| PB.62308.1 | 269 | 28625.35 | 6.31 | 31.41 | 92.45 | 0.166 |
| PB.17482.1 | 344 | 36802.76 | 5.86 | 31.22 | 86.13 | 0.103 |
| PB.52032.1 | 373 | 38747.35 | 6.53 | 24.05 | 91.58 | 0.136 |
| PB.47633.2 | 421 | 44353.00 | 6.42 | 31.04 | 84.28 | 0.092 |
| PB.55683.1 | 421 | 44338.97 | 6.42 | 29.60 | 83.80 | 0.088 |
| PB.83678.1 | 356 | 37359.82 | 5.40 | 25.49 | 92.25 | 0.146 |
| PB.84043.1 | 356 | 37036.55 | 5.84 | 26.83 | 93.88 | 0.211 |
| PB.84184.1 | 356 | 37019.73 | 6.08 | 23.61 | 92.25 | 0.195 |
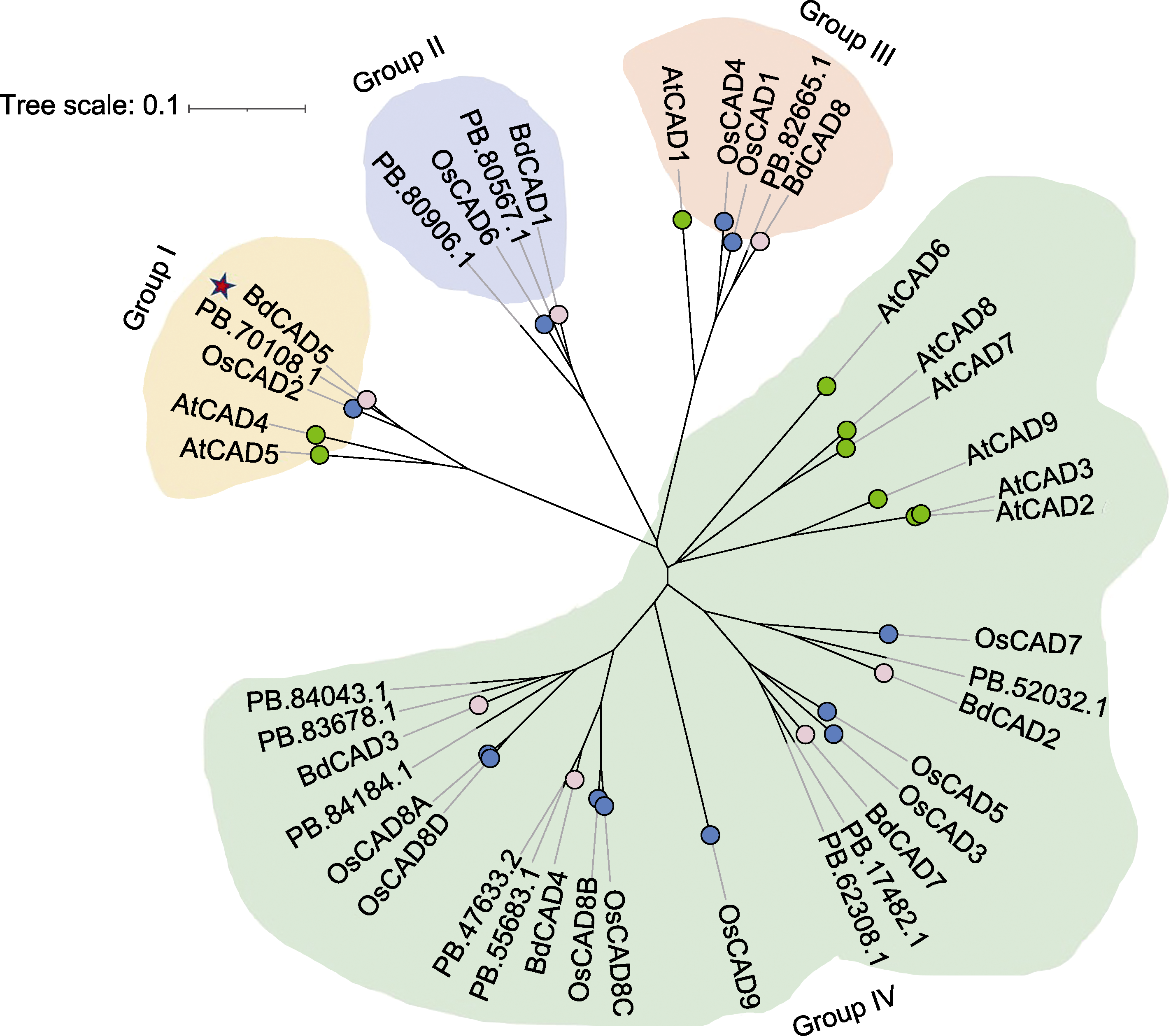
Figure 2 Phylogenetic tree of CADs in Agropyron mongolicum, Arabidopsis thaliana, Oryza sativa, and Brachypodium distachyon The red pentagram represents the functional CAD protein of A. mongolicum that may be involved in lignin synthesis; the pink dots represent the CAD protein of B. distachyon; the green dots represent the CAD protein of A. thaliana; and the blue dots represent the CAD protein of O. sativa.
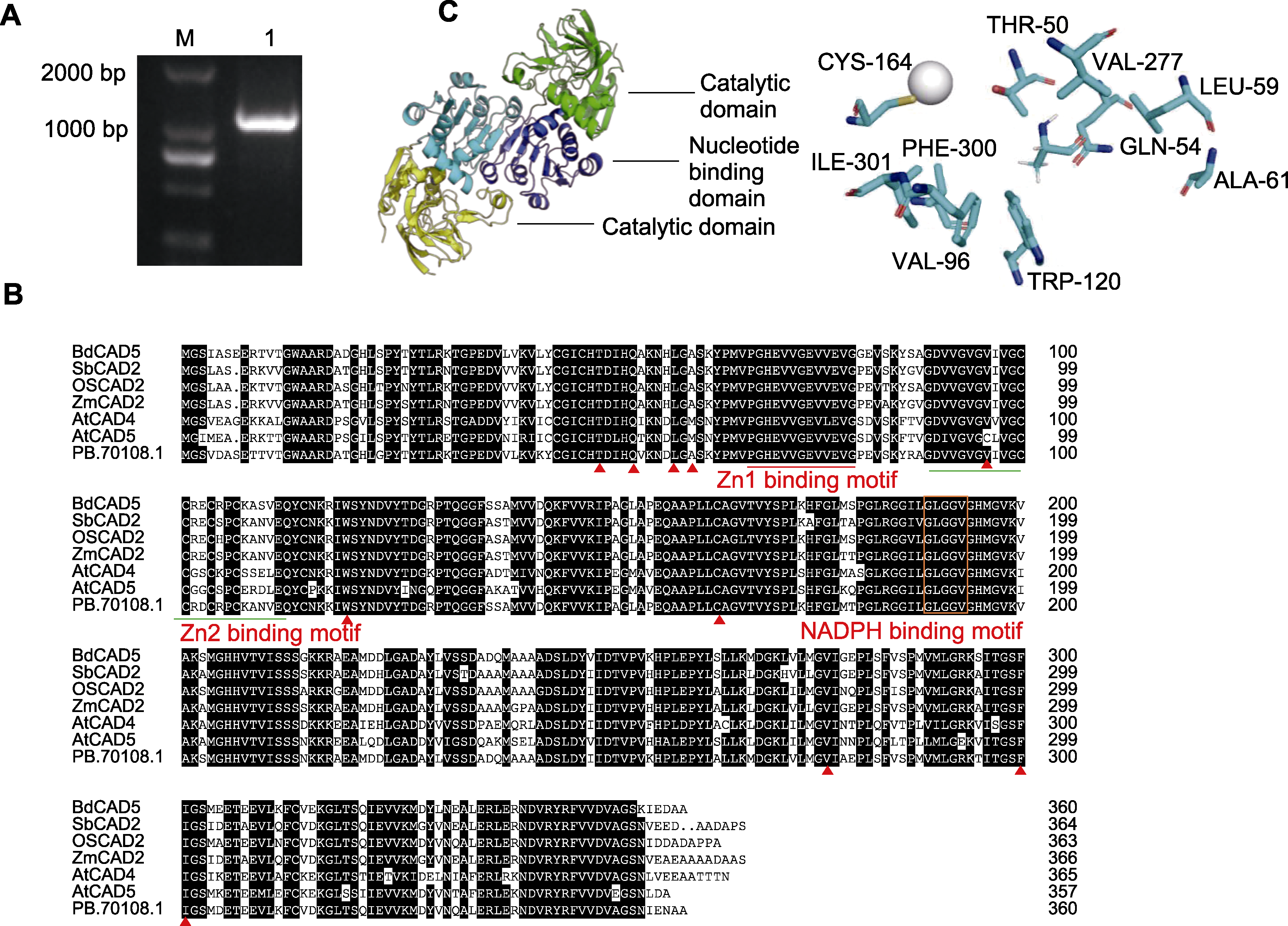
Figure 3 Amplification of Agropyron mongolicum AmCAD and protein structure analysis (A) AmCAD gel map (M: DNA ladder; 1: Amplification sequence); (B) Comparison of CAD amino acid sequences among A. mongolicum and other species (the residue forming the substrate binding pocket is marked with a triangle (▲)); (C) Three-dimensional protein structure model and substrate binding pocket of AmCAD (the nucleotide binding and catalytic domains of the bottom subunit are colored yellow and blue, respectively, the corresponding compounds in the upper subunit are colored green and dark blue, while the zinc ions are indicated by gray spheres)
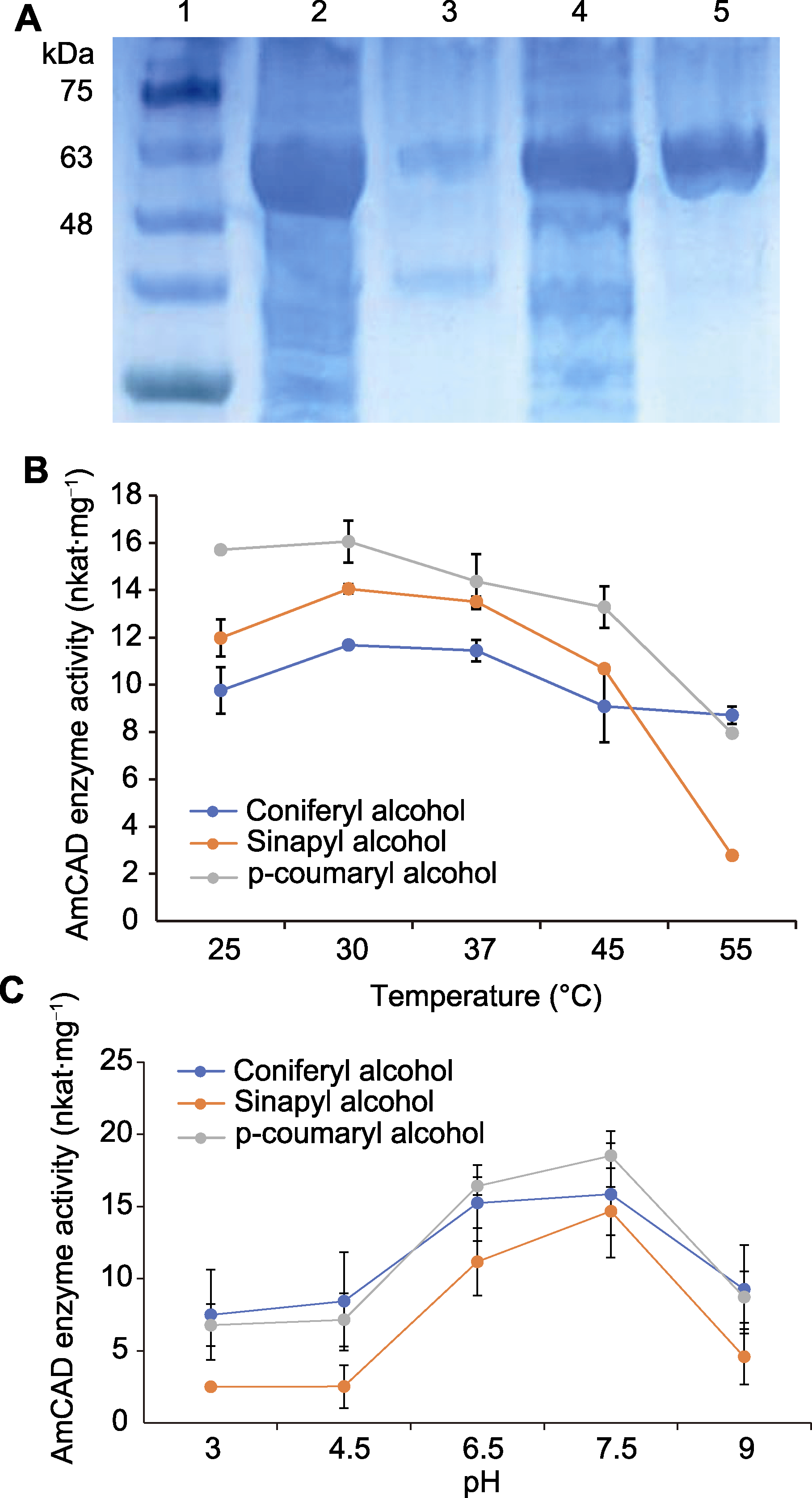
Figure 4 Heterologous expression (A) and enzymatic properties of AmCAD recombinant protein in Escherichia coli at different temperatures (B) and pH (C) 1: Marker; 2: Supernatant; 3: Precipitant; 4: Effluent; 5: Purified protein
| Substract | Km (μmol·L-1) | Vmax (μmol·L-1·s-1) | Kcat (·s−1) | Kcat/Km (μmol·L-1·s-1) |
|---|---|---|---|---|
| Coniferylaldehyde | 8.94±0.15 | 6.87±0.008 | 441.37±0.53 | 49.35±0.90 |
| Sinapaldehyde | 10.35±2.14 | 7.18±0.43 | 461.51±27.94 | 45.26±6.69 |
| p-coumaraldehyde | 35.35±3.74 | 15.21±1.60 | 977.60±102.97 | 27.65±0.01 |
Table 2 Enzyme kinetic characteristics of different substrates catalyzed by recombinant AmCAD
| Substract | Km (μmol·L-1) | Vmax (μmol·L-1·s-1) | Kcat (·s−1) | Kcat/Km (μmol·L-1·s-1) |
|---|---|---|---|---|
| Coniferylaldehyde | 8.94±0.15 | 6.87±0.008 | 441.37±0.53 | 49.35±0.90 |
| Sinapaldehyde | 10.35±2.14 | 7.18±0.43 | 461.51±27.94 | 45.26±6.69 |
| p-coumaraldehyde | 35.35±3.74 | 15.21±1.60 | 977.60±102.97 | 27.65±0.01 |
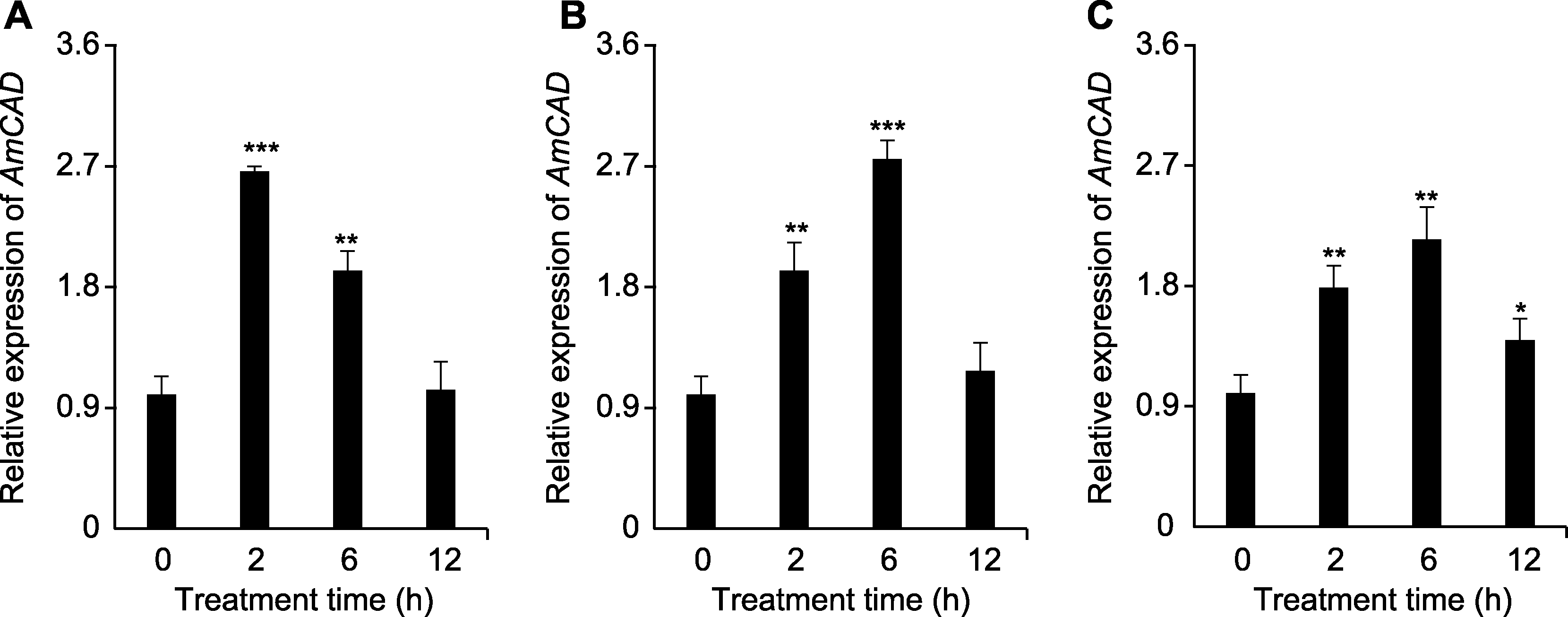
Figure 6 Expression analysis of AmCAD gene under drought stress at different time points and mannitol concentrations (A) 75 mmol∙L-1 mannitol treatment; (B) 150 mmol∙L-1 mannitol treatment; (C) 250 mmol∙L-1 mannitol treatment. * P<0.05; ** P<0.01; *** P<0.001
| [1] |
Barakat A, Bagniewska-Zadworna A, Choi A, Plakkat U, DiLoreto DS, Yellanki P, Carlson JE (2009). The cinnamyl alcohol dehydrogenase gene family in Populus: phylogeny, organization, and expression. BMC Plant Biol 9, 26.
DOI PMID |
| [2] |
Bukh C, Nord-Larsen PH, Rasmussen SK (2012). Phylogeny and structure of the cinnamyl alcohol dehydrogenase gene family in Brachypodium distachyon. J Exp Bot 63, 6223-6236.
DOI PMID |
| [3] |
Che YH, Li LH (2007). Genetic diversity of prolamines in Agropyron mongolicum Keng indigenous to northern China. Genet Resour Crop Evol 54, 1145-1151.
DOI URL |
| [4] | Cherney JH, Cherney DJR, Akin DE, Axtell JD (1991). Potential of brown-midrib, low-lignin mutants for improving forage quality. Adv Agron 46, 157-198. |
| [5] |
Eudes A, Pollet B, Sibout R, Do CT, Séguin A, Lapierre C, Jouanin L (2006). Evidence for a role of AtCAD 1 in lignification of elongating stems of Arabidopsis thaliana. Planta 225, 23-39.
DOI PMID |
| [6] | Fan BB, Zhang XF, Yu Z, Zhao Y, Ma YH (2021). Bioinformatics and expression analysis of NAC transcription factors related to drought resistance of Agropyron mongolicum Keng. Acta Agre Sin 29, 1183-1192. (in Chinese) |
|
范菠菠, 张学峰, 于卓, 赵彦, 马艳红 (2021). 与蒙古冰草抗旱相关的NAC转录因子生物信息学及其表达分析. 草地学报 29, 1183-1192.
DOI |
|
| [7] | Fu CX, Xiao XR, Xi YJ, Ge YX, Chen F, Bouton J, Dixon RA, Wang ZY (2011). Downregulation of cinnamyl alcohol dehydrogenase (CAD) leads to improved saccharification efficiency in switchgrass. BioEnergy Res 4, 153-164. |
| [8] |
Halpin C, Holt K, Chojecki J, Oliver D, Chabbert B, Monties B, Edwards K, Barakate A, Foxon GA (1998). Brown-midrib maize (bm1)—a mutation affecting the cinnamyl alcohol dehydrogenase gene. Plant J 14, 545-553.
DOI PMID |
| [9] | Huang WH (2014). Selection of Control Gene in Quantitative PCR and Analysis of Differential Expression of P5CS Gene in Agropyron mongolicum Keng Under Drought Stress. Master’s thesis. Hohhot: Inner Mongolia Agricultural University. pp. 11-17. (in Chinese) |
| 黄文华 (2014). 蒙古冰草干旱胁迫下内参基因的筛选及P5CS基因定量表达分析. 硕士论文. 呼和浩特: 内蒙古农业大学. pp. 11-17. | |
| [10] | Ibrahim W, Zhu YM, Chen Y, Qiu CW, Zhu SJ, Wu FB (2019). Genotypic differences in leaf secondary metabolism, plant hormones and yield under alone and combined stress of drought and salinity in cotton genotypes. Physiol Plant 165, 343-355. |
| [11] |
Janiak A, Kwaśniewski M, Szarejko I (2016). Gene expression regulation in roots under drought. J Exp Bot 67, 1003-1014.
DOI PMID |
| [12] | Jin YZ, Zhang C, Liu W, Qi HY, Chen H, Cao SX (2014). The cinnamyl alcohol dehydrogenase gene family in melon (Cucumis melo L.): bioinformatic analysis and expression patterns. PLoS One 9, e101730. |
| [13] | Kim SJ, Kim MR, Bedgar DL, Moinuddin SGA, Cardenas CL, Davin LB, Kang C, Lewis NG (2004). Functional reclassification of the putative cinnamyl alcohol dehydrogenase multigene family in Arabidopsis. Proc Natl Acad Sci USA 101, 1455-1460. |
| [14] | Lange BM, Lapierre C, Sandermann Jr H (1995). Elicitor-induced spruce stress lignin (structural similarity to early developmental lignins). Plant Physiol 108, 1277-1287. |
| [15] |
Lee CJ, Kim SE, Park SU, Lim YH, Choi HY, Kim WG, Ji CY, Kim HS, Kwak SS (2021). Tuberous roots of transgenic sweetpotato overexpressing IbCAD1 have enhanced low-temperature storage phenotypes. Plant Physiol Biochem 166, 549-557.
DOI URL |
| [16] |
Li LG, Cheng XF, Leshkevich J, Umezawa T, Harding SA, Chiang VL (2001). The last step of syringyl monolignol biosynthesis in angiosperms is regulated by a novel gene encoding sinapyl alcohol dehydrogenase. Plant Cell 13, 1567-1586.
PMID |
| [17] | Li X, Ma DM, Chen JL, Pu GB, Ji YP, Lei CY, Du ZG, Liu BY, Ye HC, Wang H (2012). Biochemical characterization and identification of a cinnamyl alcohol dehydrogenase from Artemisia annua. Plant Sci 193-194, 85-95. |
| [18] | Li Z, Wang HZ, Li RF, Wei JH (2009). Lignin biosynthesis and manipulation in plants and utilization of biomass energy. Chin Bull Bot 44, 262-272. (in Chinese) |
|
李桢, 王宏芝, 李瑞芬, 魏建华 (2009). 植物木质素合成调控与生物质能源利用. 植物学报 44, 262-272.
DOI |
|
| [19] | Lin KJ, Liu ZP, Luo D, Wu ZN (2023). The current status, problems and suggestions for the researchon forage germplasm resources. Chin Bull Bot 58, 241-247. (in Chinese) |
|
林克剑, 刘志鹏, 罗栋, 武自念 (2023). 饲草种质资源研究现状、存在问题与发展建议. 植物学报 58, 241-247.
DOI |
|
| [20] |
Liu W, Jiang Y, Wang CH, Zhao LL, Jin YZ, Xing QJ, Li M, Lv TH, Qi HY (2020). Lignin synthesized by CmCAD2 and CmCAD3 in oriental melon (Cucumis melo L.) seedlings contributes to drought tolerance. Plant Mol Biol 103, 689-704.
DOI PMID |
| [21] |
Livak KJ, Schmittgen TDL (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25, 402-408.
DOI PMID |
| [22] |
Ma QH (2010). Functional analysis of a cinnamyl alcohol dehydrogenase involved in lignin biosynthesis in wheat. J Exp Bot 61, 2735-2744.
DOI URL |
| [23] | Ma QH, Tian B (2005). Biochemical characterization of a cinnamoyl-CoA reductase from wheat. Biol Chem 386, 553-560. |
| [24] |
McKie JH, Jaouhari R, Douglas KT, Goffner D, Feuillet C, Grima-Pettenati J, Boudet AM, Baltas M, Gorrichon L (1993). A molecular model for cinnamyl alcohol dehydrogenase, a plant aromatic alcohol dehydrogenase involved in lignification. Biochim Biophys Acta 1202, 61-69.
PMID |
| [25] |
Pan HY, Zhou R, Louie GV, Mühlemann JK, Bomati EK, Bowman ME, Dudareva N, Dixon RA, Noel JP, Wang XQ (2014). Structural studies of cinnamoyl-CoA reductase and cinnamyl-alcohol dehydrogenase, key enzymes of monolignol biosynthesis. Plant Cell 26, 3709-3727.
DOI URL |
| [26] |
Pandey B, Pandey VP, Dwivedi UN (2011). Cloning, expression, functional validation and modeling of cinnamyl alcohol dehydrogenase isolated from xylem of Leucaena leucocephala. Protein Expres Purif 79, 197-203.
DOI URL |
| [27] |
Park HL, Kim TL, Bhoo SH, Lee TH, Lee SW, Cho MH (2018). Biochemical characterization of the rice cinnamyl alcohol dehydrogenase gene family. Molecules 23, 2659.
DOI URL |
| [28] | Qi KJ, Song XF, Yuan YZ, Bao JP, Gong X, Huang XS, Khanizadeh S, Zhang SL, Tao ST (2021). CAD genes: genome-wide identification, evolution, and their contribution to lignin biosynthesis in pear (Pyrus bretschneideri). Plants 10, 1444. |
| [29] | Qiu R, He F, Li R, Wang YM, Xing SN, Cao YP, Liu YF, Zhou XY, Zhao Y, Fu CX (2023). Highly efficient gene editing of lignin gene F5H in switchgrass. Chin Bull Bot 58, 298-307. (in Chinese) |
| 邱锐, 何峰, 李瑞, 王亚梅, 邢思年, 曹英萍, 刘叶飞, 周昕越, 赵彦, 付春祥 (2023). 柳枝稷木质素基因F5H的高效编辑. 植物学报 58, 298-307. | |
| [30] |
Rollins JA, Habte E, Templer SE, Colby T, Schmidt J, von Korff M (2013). Leaf proteome alterations in the context of physiological and morphological responses to drought and heat stress in barley (Hordeum vulgare L.). J Exp Bot 64, 3201-3212.
DOI PMID |
| [31] | Rong W, Luo MY, Shan TL, Wei XN, Du LP, Xu HJ, Zhang ZY (2016). A wheat cinnamyl alcohol dehydrogenase TaCAD12 contributes to host resistance to the sharp eyespot disease. Front Plant Sci 7, 1723. |
| [32] |
Saballos A, Ejeta G, Sanchez E, Kang C, Vermerris W (2009). A genomewide analysis of the cinnamyl alcohol dehydrogenase family in sorghum [Sorghum bicolor (L.) Moench] identifies SbCAD2 as the Brown midrib6 gene. Genetics 181, 783-795.
DOI PMID |
| [33] |
Sattler SE, Saathoff AJ, Haas EJ, Palmer NA, Funnell-Harris DL, Sarath G, Pedersen JF (2009). A nonsense mutation in a cinnamyl alcohol dehydrogenase gene is responsible for the sorghum brown midrib6 phenotype. Plant Physiol 150, 584-595.
DOI PMID |
| [34] |
Shafiei R, Hooper M, McClellan C, Oakey H, Stephens J, Lapierre C, Tsuji Y, Goeminne G, Vanholme R, Boerjan W, Ralph J, Halpin C (2023). Downregulation of barley ferulate 5-hydroxylase dramatically alters straw lignin structure without impact on mechanical properties. Front Plant Sci 13, 1125003.
DOI URL |
| [35] |
Sibout R, Eudes A, Pollet B, Goujon T, Mila I, Granier F, Séguin A, Lapierre C, Jouanin L (2003). Expression pattern of two paralogs encoding cinnamyl alcohol dehydrogenases in Arabidopsis. Isolation and characterization of the corresponding mutants. Plant Physiol 132, 848-860.
PMID |
| [36] | Tobias CM, Chow EK (2005). Structure of the cinnamyl-alcohol dehydrogenase gene family in rice and promoter activity of a member associated with lignification. Planta 220, 678-688. |
| [37] |
Tsuruta SI, Ebina M, Nakagawa H, Kawamura O, Akashi R (2007). Isolation and characterization of cDNA encoding cinnamyl alcohol dehydrogenase (CAD) in sorghum (Sorghum bicolor (L.) Moench). Grassl Sci 53, 103-109.
DOI URL |
| [38] |
Vasupalli N, Hou D, Singh RM, Wei HT, Zou LH, Yrjälä K, Wu AM, Lin XC (2021). Homo- and hetero-dimers of CAD enzymes regulate lignification and abiotic stress response in moso bamboo. Int J Mol Sci 22, 12917.
DOI URL |
| [39] |
Vermerris W, Thompson KJ, McIntyre LM (2002). The maize Brown midrib1 locus affects cell wall composition and plant development in a dose-dependent manner. Heredity 88, 450-457.
PMID |
| [40] | Wang RH, Shi L, Tang GG, Liang YC, Zhang CY (2003). Effect of osmotic stress on activities of protective enzymes system in Agropyron mongolicum seedling. Chin Bull Bot 20, 330-335. (in Chinese) |
| 王荣华, 石雷, 汤庚国, 梁寅初, 张称意 (2003). 渗透胁迫对蒙古冰草幼苗保护酶系统的影响. 植物学通报 20, 330-335. (in Chinese) | |
| [41] | Wang RH, Shi L, Tang GG, Liang YC, Zhang CY (2004). Effect of NaCl stress on growth and content of severalions of wheatgrass. Bull Botanical Res 24, 326-330. (in Chinese) |
| 王荣华, 石雷, 汤庚国, 梁寅初, 张称意 (2004). 盐胁迫下蒙古冰草幼苗生长和离子含量的变化. 植物研究 24, 326-330. | |
| [42] |
Xiao SH, Hu Q, Shen JL, Liu SM, Yang ZG, Chen K, Klosterman SJ, Javornik B, Zhang XL, Zhu LF (2021). GhMYB4 downregulates lignin biosynthesis and enhances cotton resistance to Verticillium dahliae. Plant Cell Rep 40, 735-751.
DOI PMID |
| [43] |
Xu L, Zhu LF, Tu LL, Liu LL, Yuan DJ, Jin L, Long L, Zhang XL (2011). Lignin metabolism has a central role in the resistance of cotton to the wilt fungus Verticillium dahliae as revealed by RNA-Seq-dependent transcriptional analysis and histochemistry. J Exp Bot 62, 5607-5621.
DOI PMID |
| [44] |
Youn B, Camacho R, Moinuddin SGA, Lee C, Davin LB, Lewis NG, Kang C (2006). Crystal structures and catalytic mechanism of the Arabidopsis cinnamyl alcohol dehydrogenases AtCAD5 and AtCAD4. Org Biomol Chem 4, 1687-1697.
PMID |
| [45] |
Yusuf CYL, Nabilah NS, Taufik NAAM, Seman IA, Abdullah MP (2022). Genome-wide analysis of the CAD gene family reveals two bona fide CAD genes in oil palm. 3 Biotech 12, 149.
DOI |
| [46] |
Zeng JJ, Helms GL, Gao X, Chen SL (2013). Quantification of wheat straw lignin structure by comprehensive NMR analysis. J Agric Food Chem 61, 10848-10857.
DOI URL |
| [47] |
Zhang KW, Qian Q, Huang ZJ, Wang YQ, Li M, Hong LL, Zeng DL, Gu MH, Chu CC, Cheng ZK (2006). GOLD HULL AND INTERNODE2 encodes a primarily multifunctional cinnamyl-alcohol dehydrogenase in rice. Plant Physiol 140, 972-983.
DOI PMID |
| [48] | Zhao Y, Chen XY, Shi FM, Yun JF, Wang JJ (2015). Cloning and expression analysis of MwDREB3 from Mongolian wheatgrass. Acta Agre Sin 23, 377. (in Chinese) |
| 赵彦, 陈雪英, 石凤敏, 云锦凤, 王俊杰 (2015). 蒙古冰草MwDREB3基因的克隆及表达分析. 草地学报 23, 377. |
| [1] | DU Shu-Hui, CHU Jian-Min, DUAN Jun-Guang, XUE Jian-Guo, XU Lei, XU Xiao-Qing, WANG Qi-Bing, HUANG Jian-Hui, ZHANG Qian. Influence of lignin phenols on soil organic carbon in degraded grassland in Nei Mongol, China [J]. Chin J Plant Ecol, 2025, 49(1): 30-41. |
| [2] | Ziyang Wang, Shengxue Liu, Zhirui Yang, Feng Qin. Genetic Dissection of Drought Resistance in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 883-902. |
| [3] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [4] | Xingxin Liao, Yi Niu, Xingwu Duo, Akeyedeli Jumahazi, Marhaba Abdukuyum, Rizwangul Hufur, Haiyan Lan, Jing Cao. Heterologous Expression of Suaeda aralocaspica SaPEPC2 Gene Improves Drought Resistance and Photosynthesis in Transgenic Tobacco [J]. Chinese Bulletin of Botany, 2024, 59(4): 585-599. |
| [5] | Jinyu Du, Zhen Sun, Yanlong Su, Heping Wang, Yaling Liu, Zhenying Wu, Feng He, Yan Zhao, Chunxiang Fu. Identification and Functional Analysis of an Agropyron mongolicum Caffeic Acid 3-O-methyltransferase Gene AmCOMT1 [J]. Chinese Bulletin of Botany, 2024, 59(3): 383-396. |
| [6] | Lei Zhang, Pengfei Jiang, Yiming Wang, Ting Lan, Yanjing Liu, Qingyin Zeng. Comparative Study on the Drought Resistance of Young Seedling from Populus laurifolia × P. simonii F1 Progeny [J]. Chinese Bulletin of Botany, 2023, 58(4): 519-534. |
| [7] | Rui Qiu, Feng He, Rui Li, Yamei Wang, Sinian Xing, Yingping Cao, Yefei Liu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Highly Efficient Gene Editing of Lignin Gene F5H in Switchgrass [J]. Chinese Bulletin of Botany, 2023, 58(2): 298-307. |
| [8] | LUO Dan-Dan, WANG Chuan-Kuan, JIN Ying. Response mechanisms of hydraulic systems of woody plants to drought stress [J]. Chin J Plant Ecol, 2021, 45(9): 925-941. |
| [9] | Yigong Zhang, Yi Zhang, Ayibaiheremu Mutailifu, Daoyuan Zhang. Heterologous Overexpression of Desiccation-tolerance Moss ScABI3 Gene Changes Stomatal Phenotype and Improves Drought Resistance in Transgenic Arabidopsis [J]. Chinese Bulletin of Botany, 2021, 56(4): 414-421. |
| [10] | Chenyang Pan, Yue Zhang, Han Lin, Qianyu Chen, Kairu Yang, Jiaji Jiang, Mengjia Li, Tao Lu, Kexin Wang, Mei Lu, Sheng Wang, Hanfei Ye, Yuchun Rao, Haitao Hu. QTL Mapping and Candidate Gene Analysis on Rice Leaf Water Potential [J]. Chinese Bulletin of Botany, 2021, 56(3): 275-283. |
| [11] | Weitao Li, Min He, Xuewei Chen. Discovery of ZmFBL41 Chang7-2 as A Key Weapon against Banded Leaf and Sheath Blight Resistance in Maize [J]. Chinese Bulletin of Botany, 2019, 54(5): 547-549. |
| [12] | Huaifeng Gao,Yafei Zhang,Guodong Wang,Xiwu Sun,Yue He,Futian Peng,Yuansong Xiao. The Effect of Molybdenum on Drought Stress Response in Peach [J]. Chinese Bulletin of Botany, 2019, 54(2): 227-236. |
| [13] | Haitao Long, Limei Li, Zehong Xie, Shuai Liu, Xiaoyun Li, Bin Deng, Haiyan Liu, Ling Li. Relationship Between Drought Resistance Evaluated by Comprehensive Subordinate Function Values and AhNCED1 Expression in Peanut Varieties [J]. Chinese Bulletin of Botany, 2015, 50(6): 706-712. |
| [14] | AN Dong-Sheng,CAO Juan,HUANG Xiao-Hua,ZHOU Juan,DOU Mei-An. Application of Lake-model based indices from chlorophyll fluorescence on sugarcane seedling drought resistance study [J]. Chin J Plan Ecolo, 2015, 39(4): 398-406. |
| [15] | CHEN Hui-Ying, ZHANG Jing-Hui, HUANG Yong-Mei, GONG Ji-Rui. Traits related to carbon sequestration of common plant species in a Stipa grandis steppe in Nei Mongol under different land-uses [J]. Chin J Plant Ecol, 2014, 38(8): 821-832. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||