

Chinese Bulletin of Botany ›› 2021, Vol. 56 ›› Issue (1): 71-79.DOI: 10.11983/CBB20119 cstr: 32102.14.CBB20119
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Lan Yang, Ya Liu, Yang Xiang, Xiujuan Sun, Jingwei Yan, Aying Zhang*( )
)
Received:2020-07-02
Accepted:2020-10-14
Online:2021-01-01
Published:2021-01-15
Contact:
Aying Zhang
Lan Yang, Ya Liu, Yang Xiang, Xiujuan Sun, Jingwei Yan, Aying Zhang. Establishment and Optimization of a Shoot Tip-based Genetic Transformation System for Foxtail Millet[J]. Chinese Bulletin of Botany, 2021, 56(1): 71-79.

Figure 1 The T-DNA region of plasmid pCUN-NHF LB: LB T-DNA repeat; Ubi promoter: Zea mays Ubiquitin1 promoter; HA: HA tag; MCS: Multiple cloning site; 35S promoter: CaMV35S promoter; Bar: Herbicide bialaphos-resistance gene; RB: RB T-DNA repeat
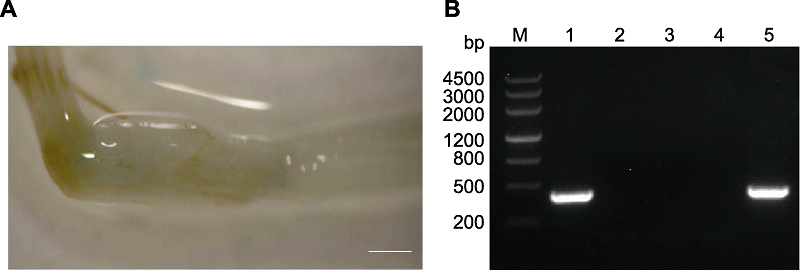
Figure 2 Identification of transgenic foxtail millet (A) Image from GUS staining of foxtail millet seedlings (bar=0.1 cm); (B) PCR identification (1: Positive control; 2: Negative control; 3: A leaf with one wound cut; 4: A leaf with two wounds cut; 5: A leaf without wound)
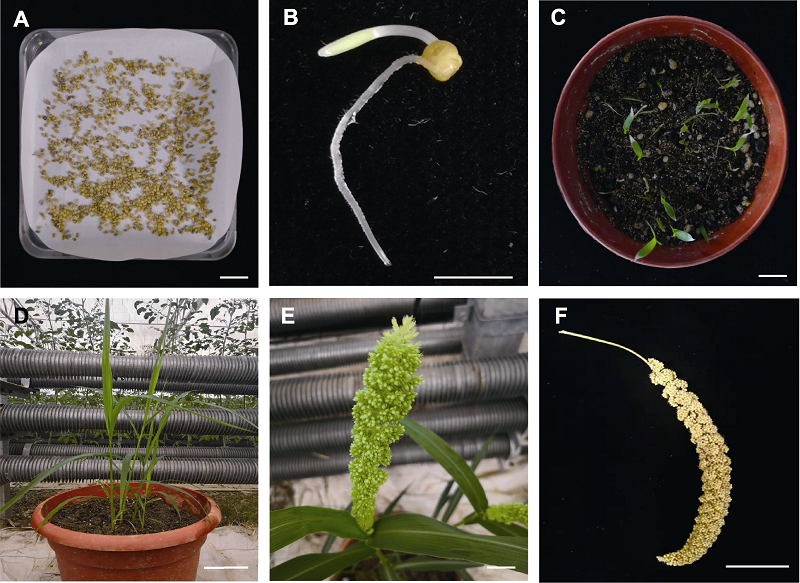
Figure 3 Establishment of the genetic transformation system for foxtail millet using shoot tip (A) Seeds to be germinated; (B) Seeds after germination for 3 d; (C) Seedlings after transformation for 5 d; (D) Plants at jointing stage; (E) Plants at heading stage; (F) Mature seeds. (A), (C), (E) Bars=1.0 cm; (B) Bar=0.5 cm; (D) Bar=10.0 cm; (F) Bar=5.0 cm
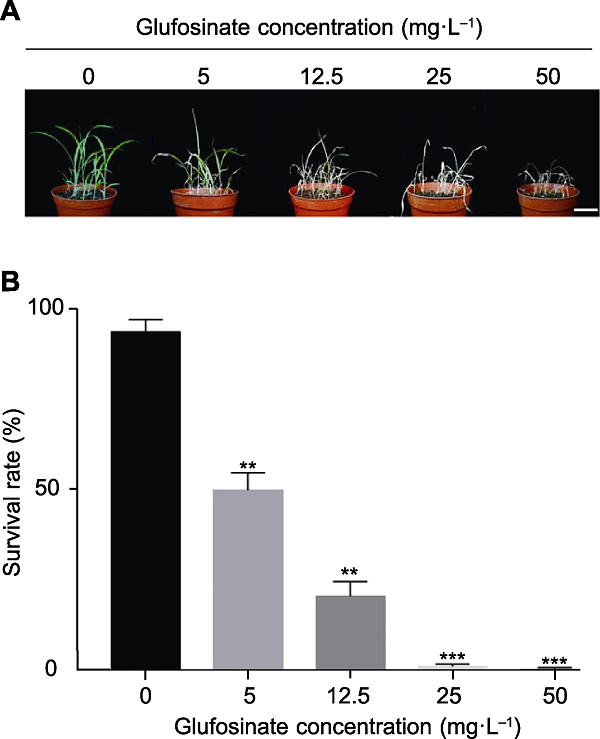
Figure 4 Effects of glufosinate concentrations on survival rate of foxtail millet seedlings (A) Phenotypes of foxtail millet seedlings treated with glufosinate at different concentrations (bar=5.0 cm); (B) The survival rate of foxtail millet seedlings treated with different concentrations of glufosinate. Data are means±SD (n=3). Student’s t-test, ** P<0.01, *** P<0.001
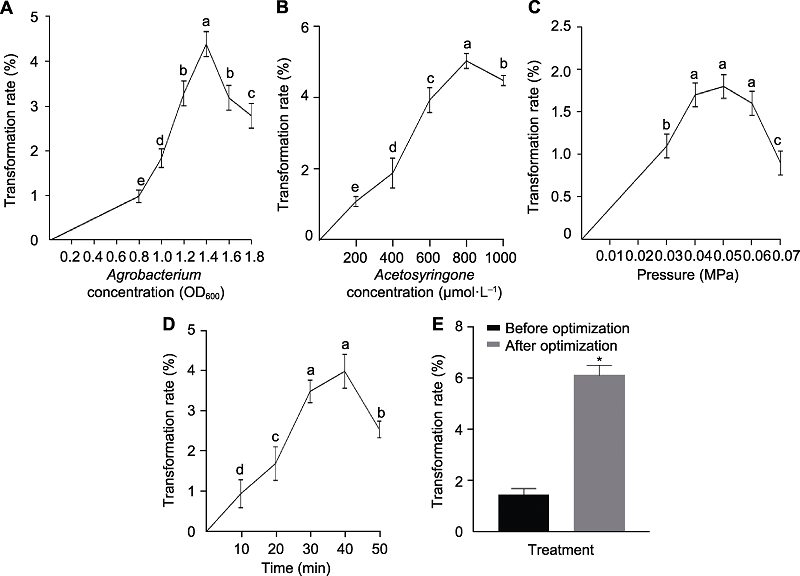
Figure 6 Effects of Agrobacterium concentration (A), acetosyringone concentration (B), infecting pressure (C), infecting time (D) and changing four variables (E) on transformation rates for foxtail millet using shoot tip Data are means±SD (n=3). Student’s t-test, * P<0.05. Different lowercase letters indicat significant differences (P<0.05).
| Agrobacterium concentration (OD600) | Acetosyringone concentration (μmol?L-1) | Pressure (MPa) | Time (min) | |
|---|---|---|---|---|
| Before optimization | 1.0 | 400 | 0.04 | 20 |
| After optimization | 1.4 | 800 | 0.05 | 40 |
Table 1 Comparison of the conditions of the initially established system and the optimized system
| Agrobacterium concentration (OD600) | Acetosyringone concentration (μmol?L-1) | Pressure (MPa) | Time (min) | |
|---|---|---|---|---|
| Before optimization | 1.0 | 400 | 0.04 | 20 |
| After optimization | 1.4 | 800 | 0.05 | 40 |
| [1] | 陈倩楠, 王轲, 汤沙, 杜丽璞, 智慧, 贾冠清, 赵宝华, 叶兴国, 刁现民 (2018). 以抗除草剂Bar基因稳定转化谷子技术研究. 作物学报 44, 1423-1432. |
| [2] | 刁现民, 程汝宏 (2017). 十五年区试数据分析展示谷子糜子育种现状. 中国农业科学 50, 4469-4474. |
| [3] | 贺美林 (2018). 谷子成熟胚再生体系建立及EPSPS基因遗传转化研究. 硕士论文. 晋中: 山西农业大学. pp. 26-27. |
| [4] | 冷秋思, 屈燕, 刘伟, 区智 (2019). 绿绒蒿属植物不同RNA提取方式的比较分析. 分子植物育种 17, 4643-4647. |
| [5] | 李明浩, 陈炜, 邢莉萍, 肖进, 王海燕, 曹爱忠, 王秀娥 (2010). 普通小麦品种Alondra's遗传转化体系的建立. 植物学报 45, 466-471. |
| [6] | 李顺国, 刘斐, 刘猛, 刁现民 (2018). 新时期中国谷子产业发展技术需求与展望. 农学学报 8(6), 96-100. |
| [7] | 李顺国, 刘斐, 刘猛, 赵宇, 王慧军 (2014). 我国谷子产业现状、发展趋势及对策建议. 农业现代化研究 35, 531-535. |
| [8] | 李顺国, 刘猛, 赵宇, 刘斐, 王慧军 (2012). 河北省谷子产业现状和技术需求及发展对策. 农业现代化研究 33, 286-289. |
| [9] | 李颜方, 杜艳伟, 张正, 王高鸿, 赵根有, 赵晋锋, 余爱丽 (2019). 农杆菌介导谷子成熟胚遗传转化体系的建立与优化. 作物杂志 ( 3), 73-79. |
| [10] | 李臻, 刘炜, 管延安, 王庆国, 潘教文 (2015). 谷子遗传转化体系研究进展. 山东农业科学 47(4), 134-138. |
| [11] | 刘宝玲, 张莉, 孙岩, 薛金爱, 高昌勇, 苑丽霞, 王计平, 贾小云, 李润植 (2016). 谷子bZIP转录因子的全基因组鉴定及其在干旱和盐胁迫下的表达分析. 植物学报 51, 473-487. |
| [12] | 刘颖慧, 于静娟, 赵倩, 朱登云, 敖光明 (2005). 根癌农杆菌介导谷子的遗传转化. 农业生物技术学报 13, 32-37. |
| [13] | 宋利军 (2019). 谷子高产栽培技术分析. 农业技术与装备 ( 7), 87-88. |
| [14] | 王永芳, 李伟, 刁现民 (2003). 根癌农杆菌共培养转化谷子技术体系的建立. 河北农业科学 7(4), 1-6. |
| [15] | 张明洲, 崔海瑞, 舒庆尧, 夏英武 (2006). 高粱茎尖再生体系及其遗传转化影响因子的研究. 核农学报 20, 23-26. |
| [16] | 张笑寒, 仇志浪, 赵德刚 (2016). 农杆菌介导McCHIT1基因遗传转化水稻茎尖研究. 中国农学通报 32(27), 114-120. |
| [17] | 张园, 刘正杰, 林春, 闫亚泽, 袁加红, 王入, 毛自朝, 杨焕文 (2020). 芦笋茎尖遗传转化体系的建立与优化. 西北农业学报 29, 109-116. |
| [18] |
Arockiasamy S, Ignacimuthu S (2007). Regeneration of transgenic plants from two indica rice (Oryza sativa L.) cultivars using shoot apex explants. Plant Cell Rep 26, 1745-1753.
DOI URL PMID |
| [19] | Dellaporta SL, Wood J, Hicks JB (1983). A plant DNA minipreparation: version II. Plant Mol Biol Rep 1, 19-21. |
| [20] | Jeong JY, Yim HS, Ryu JY, Lee HS, Lee JH, Seen DS, Kang SG (2012). One-step sequence- and ligation-in- dependent cloning as a rapid and versatile cloning method for functional genomics studies. Appl Environ Microbiol 78, 5440-5443. |
| [21] | Jha P, Shashi, Rustagi A, Agnihotri PK, Kulkarni VM, Bhat V (2011). Efficient Agrobacterium-mediated transformation of Pennisetum glaucum (L.) R. Br. using shoot apices as explant source. Plant Cell Tissue Organ Cult 107, 501-512. |
| [22] | Li WB, Masilamany P, Kasha KJ, Pauls KP (2002). Developmental, tissue culture, and genotypic factors affecting plant regeneration from shoot apical meristems of germinated Zea mays L. seedlings. In Vitro Cell Dev Biol Plant 38, 285-292. |
| [23] | Ma HZ, Liu C, Li ZX, Ran QJ, Xie GN, Wang BM, Fang S, Chu JF, Zhang JR (2018). ZmbZIP4 contributes to stress resistance in maize by regulating ABA synthesis and root development. Plant Physiol 178, 753-770. |
| [24] | Sood P, Singh RK, Prasad M (2020). An efficient Agrobacterium-mediated genetic transformation method for foxtail millet (Setaria italica L.). Plant Cell Rep 39, 511-525. |
| [25] | Yan JW, Fang L, Yang L, He H, Huang Y, Liu Y, Zhang AY (2020). Abscisic acid positively regulates L-arabinose metabolism to inhibit seed germination through ABSCISIC ACID INSENSITIVE4-mediated transcriptional promotions of MUR4 in Arabidopsis thaliana. New Phytol 225, 823-834. |
| [26] | Yellisetty V, Reddy LA, Mandapaka M (2015). In planta transformation of sorghum (Sorghum bicolor (L.) Moench) using TPS1 gene for enhancing tolerance to abiotic stresses. J Genet 94, 425-434. |
| [27] | Zhao MC, Tang S, Zhang HS, He MM, Liu JH, Zhi H, Sui Y, Liu XT, Jia GQ, Zhao ZY, Yan JJ, Zhang BC, Zhou YH, Chu JF, Wang XC, Zhao BH, Tang WQ, Li JY, Wu CY, Liu XG, Diao XM (2020). DROOPY LEAF 1 controls leaf architecture by orchestrating early brassinosteroid signaling. Proc Natl Acad Sci USA 117, 21766-21774. |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zeng Wendan, Yan Huabing, Wu Zhengdan, Shang Xiaohong, Cao Sheng, Lu Liuying, Xiao Liang, Shi Pingli, Cheng Dong, Long Ziyuan, Li Jieyu. Agrobacterium rhizogenes-mediated Transformation System of Pueraria lobata Hairy Roots [J]. Chinese Bulletin of Botany, 2025, 60(3): 425-434. |
| [3] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [4] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [5] | Yu Xiaomin, Wang Yaqin, Liu Yuhan, Yi Qingping, Cheng Wenhan, Zhu Yu, Duan Feng, Zhang Lixue, He Yanhong. Establishment of Agrobacterium tumefaciens-mediated Genetic Transformation System of Marigold (Tagetes erecta) [J]. Chinese Bulletin of Botany, 2023, 58(5): 760-769. |
| [6] | Rong Sun, Yulu Yang, Yajun Li, Hui Zhang, Xukai Li. Genome-wide Identification and Analysis of PLATZ Transcription Factor Gene Family in Foxtail Millet [J]. Chinese Bulletin of Botany, 2023, 58(4): 548-559. |
| [7] | Qi Wang, Yanli Xu, Peng Yan, Haosheng Dong, Wei Zhang, Lin Lu, Zhiqiang Dong. Effects of PAC on Soil Nitrogen Supply and Leaf Antioxidant Properties in Foxtail Millet at Anthesis Stage [J]. Chinese Bulletin of Botany, 2023, 58(1): 90-107. |
| [8] | Hui Zhang, Hongkai Liang, Hui Zhi, Linlin Zhang, Xianmin Diao, Guanqing Jia. Analyses on the Transcription and Structure Variation of β-carotene Isomerase Gene Family in Foxtail Millet [J]. Chinese Bulletin of Botany, 2023, 58(1): 34-50. |
| [9] | Huang Junwen, Feng Qiyi, Zheng Kaiyong, Huang Junjie, Wang Linbo, Lai Jianbin Lai Ruiqiang, Yang Chengwei. An Effective in Vitro SUMOylation Detection System for Plant Proteins [J]. Chinese Bulletin of Botany, 2022, 57(4): 490-499. |
| [10] | Qingzhen Zhao,Lijing Liu,Qi Xie,Feifei Yu. In Vitro Ubiquitination Assay for Plant Proteins [J]. Chinese Bulletin of Botany, 2019, 54(6): 764-772. |
| [11] | Junhua Li,Shiyu Liu,Chenglong Li,Linlin Han,Yahui Dong,Xiaoli Zhang,Xiting Zhao,Mingjun Li. Establishment of a Genetic Transformation System for Dioscorea opposita Using Microtuber [J]. Chinese Bulletin of Botany, 2019, 54(1): 72-80. |
| [12] | Yan Wang, Haojie Mou, Yongping Lü, Haiying Li, Yiting Wang, Jianping Chen. In vitro Regeneration and Industrial Micropropagation of Haworthia retusa × cooperi cv. ‘Variegata’ [J]. Chinese Bulletin of Botany, 2017, 52(3): 331-336. |
| [13] | Baoling Liu, Li Zhang, Yan Sun,Jinai Xue, Changyong Gao, Lixia Yuan, Jiping Wang, Xiaoyun Jia, Runzhi Li. Genome-wide Characterization of bZIP Transcription Factors in Foxtail Millet and Their Expression Profiles in Response to Drought and Salt Stresses [J]. Chinese Bulletin of Botany, 2016, 51(4): 473-487. |
| [14] | Lijun Guo, Bingshan Zeng, Ying Liu. Agrobacterium-mediated High-efficient Transformation of Eucalyptus grandis Clone Eg5 [J]. Chinese Bulletin of Botany, 2013, 48(1): 87-93. |
| [15] | Guimei Cui, Yi Sun, Yaoshan Hao, Jianzhong Du, Yixue Wang. The Improvement of Maize Pollen In Vitro Germination Method and Its Role in Pollen-mediated Plant Genetic Transformation [J]. Chinese Bulletin of Botany, 2012, 47(2): 155-161. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||