

Chinese Bulletin of Botany ›› 2022, Vol. 57 ›› Issue (2): 227-235.DOI: 10.11983/CBB21069 cstr: 32102.14.CBB21069
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Churan Li1,2, Ling Fu3, Yun Liu1,3, Xiaoqin Yang1, Guolei Zhu1, Sida Xie1, Huancheng Ma2, Ping Zhao1,3,*( )
)
Received:2021-04-27
Accepted:2021-11-24
Online:2022-03-01
Published:2022-03-24
Contact:
Ping Zhao
Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum[J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235.
| Code | Factors | Levels | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | Sucrose concentration (g·L-1) | 30 | 40 | 50 |
| B | pH value | 4.5 | 5 | 5.5 |
| C | Cultivation medium volume (mL) | 40 | 45 | 50 |
| D | Initial inoculation size (g) | 2.0 | 2.5 | 3.0 |
| E | Shaking speed (r·min-1) | 120 | 150 | 180 |
Table 1 The factors and levels of response surface methodology
| Code | Factors | Levels | ||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| A | Sucrose concentration (g·L-1) | 30 | 40 | 50 |
| B | pH value | 4.5 | 5 | 5.5 |
| C | Cultivation medium volume (mL) | 40 | 45 | 50 |
| D | Initial inoculation size (g) | 2.0 | 2.5 | 3.0 |
| E | Shaking speed (r·min-1) | 120 | 150 | 180 |
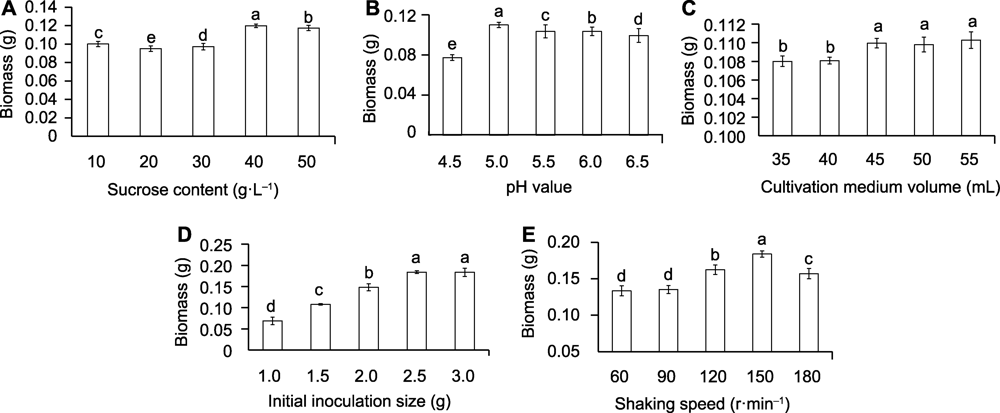
Figure 1 Effects of sucrose concentration (A), pH value (B), medium volume (C), initial inoculation size (D), and shaking speed (E) on the biomass of suspension culture cells of Vaccinium dunalianum Different lowercase letters indicate significant differences (P<0.05).
| No. | Sucrose concentration (g·L-1) | pH value | Cultivation medium volume (mL) | Initial inocul- ation size (g) | Shaking speed (r·min-1) | Biomass (g) |
|---|---|---|---|---|---|---|
| 1 | 30 | 4.5 | 45 | 2.5 | 150 | 0.0936 |
| 2 | 50 | 4.5 | 45 | 2.5 | 150 | 0.1319 |
| 3 | 30 | 5.5 | 45 | 2.5 | 150 | 0.0921 |
| 4 | 50 | 5.5 | 45 | 2.5 | 150 | 0.1487 |
| 5 | 40 | 5.0 | 40 | 2.0 | 150 | 0.1066 |
| 6 | 40 | 5.0 | 50 | 2.0 | 150 | 0.1109 |
| 7 | 40 | 5.0 | 40 | 3.0 | 150 | 0.1306 |
| 8 | 40 | 5.0 | 50 | 3.0 | 150 | 0.1752 |
| 9 | 40 | 4.5 | 45 | 2.5 | 120 | 0.1277 |
| 10 | 40 | 5.5 | 45 | 2.5 | 120 | 0.1240 |
| 11 | 40 | 4.5 | 45 | 2.5 | 180 | 0.0986 |
| 12 | 40 | 5.5 | 45 | 2.5 | 180 | 0.0999 |
| 13 | 30 | 5.0 | 40 | 2.5 | 150 | 0.1021 |
| 14 | 50 | 5.0 | 40 | 2.5 | 150 | 0.1106 |
| 15 | 30 | 5.0 | 50 | 2.5 | 150 | 0.1068 |
| 16 | 50 | 5.0 | 50 | 2.5 | 150 | 0.1806 |
| 17 | 40 | 5.0 | 45 | 2.0 | 120 | 0.0969 |
| 18 | 40 | 5.0 | 45 | 3.0 | 120 | 0.1289 |
| 19 | 40 | 5.0 | 45 | 2.0 | 180 | 0.0942 |
| 20 | 40 | 5.0 | 45 | 3.0 | 180 | 0.1171 |
| 21 | 40 | 4.5 | 40 | 2.5 | 150 | 0.1123 |
| 22 | 40 | 5.5 | 40 | 2.5 | 150 | 0.1362 |
| 23 | 40 | 4.5 | 50 | 2.5 | 150 | 0.1368 |
| 24 | 40 | 5.5 | 50 | 2.5 | 150 | 0.1304 |
| 25 | 30 | 5.0 | 45 | 2.0 | 150 | 0.1123 |
| 26 | 50 | 5.0 | 45 | 2.0 | 150 | 0.1085 |
| 27 | 30 | 5.0 | 45 | 3.0 | 150 | 0.0988 |
| 28 | 50 | 5.0 | 45 | 3.0 | 150 | 0.1223 |
| 29 | 40 | 5.0 | 40 | 2.5 | 120 | 0.1119 |
| 30 | 40 | 5.0 | 50 | 2.5 | 120 | 0.1080 |
| 31 | 40 | 5.0 | 40 | 2.5 | 180 | 0.1136 |
| 32 | 40 | 5.0 | 50 | 2.5 | 180 | 0.1099 |
| 33 | 30 | 5.0 | 45 | 2.5 | 120 | 0.0846 |
| 34 | 50 | 5.0 | 45 | 2.5 | 120 | 0.1359 |
| 35 | 30 | 5.0 | 45 | 2.5 | 180 | 0.0811 |
| 36 | 50 | 5.0 | 45 | 2.5 | 180 | 0.1203 |
| 37 | 40 | 4.5 | 45 | 2.0 | 150 | 0.1196 |
| 38 | 40 | 5.5 | 45 | 2.0 | 150 | 0.0999 |
| 39 | 40 | 4.5 | 45 | 3.0 | 150 | 0.1211 |
| 40 | 40 | 5.5 | 45 | 3.0 | 150 | 0.1263 |
| 41 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1819 |
| 42 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1809 |
| 43 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1816 |
| 44 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1820 |
| 45 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1823 |
| 46 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1806 |
Table 2 Design and results of single factor experiment on the biomass of Vaccinium dunalianum
| No. | Sucrose concentration (g·L-1) | pH value | Cultivation medium volume (mL) | Initial inocul- ation size (g) | Shaking speed (r·min-1) | Biomass (g) |
|---|---|---|---|---|---|---|
| 1 | 30 | 4.5 | 45 | 2.5 | 150 | 0.0936 |
| 2 | 50 | 4.5 | 45 | 2.5 | 150 | 0.1319 |
| 3 | 30 | 5.5 | 45 | 2.5 | 150 | 0.0921 |
| 4 | 50 | 5.5 | 45 | 2.5 | 150 | 0.1487 |
| 5 | 40 | 5.0 | 40 | 2.0 | 150 | 0.1066 |
| 6 | 40 | 5.0 | 50 | 2.0 | 150 | 0.1109 |
| 7 | 40 | 5.0 | 40 | 3.0 | 150 | 0.1306 |
| 8 | 40 | 5.0 | 50 | 3.0 | 150 | 0.1752 |
| 9 | 40 | 4.5 | 45 | 2.5 | 120 | 0.1277 |
| 10 | 40 | 5.5 | 45 | 2.5 | 120 | 0.1240 |
| 11 | 40 | 4.5 | 45 | 2.5 | 180 | 0.0986 |
| 12 | 40 | 5.5 | 45 | 2.5 | 180 | 0.0999 |
| 13 | 30 | 5.0 | 40 | 2.5 | 150 | 0.1021 |
| 14 | 50 | 5.0 | 40 | 2.5 | 150 | 0.1106 |
| 15 | 30 | 5.0 | 50 | 2.5 | 150 | 0.1068 |
| 16 | 50 | 5.0 | 50 | 2.5 | 150 | 0.1806 |
| 17 | 40 | 5.0 | 45 | 2.0 | 120 | 0.0969 |
| 18 | 40 | 5.0 | 45 | 3.0 | 120 | 0.1289 |
| 19 | 40 | 5.0 | 45 | 2.0 | 180 | 0.0942 |
| 20 | 40 | 5.0 | 45 | 3.0 | 180 | 0.1171 |
| 21 | 40 | 4.5 | 40 | 2.5 | 150 | 0.1123 |
| 22 | 40 | 5.5 | 40 | 2.5 | 150 | 0.1362 |
| 23 | 40 | 4.5 | 50 | 2.5 | 150 | 0.1368 |
| 24 | 40 | 5.5 | 50 | 2.5 | 150 | 0.1304 |
| 25 | 30 | 5.0 | 45 | 2.0 | 150 | 0.1123 |
| 26 | 50 | 5.0 | 45 | 2.0 | 150 | 0.1085 |
| 27 | 30 | 5.0 | 45 | 3.0 | 150 | 0.0988 |
| 28 | 50 | 5.0 | 45 | 3.0 | 150 | 0.1223 |
| 29 | 40 | 5.0 | 40 | 2.5 | 120 | 0.1119 |
| 30 | 40 | 5.0 | 50 | 2.5 | 120 | 0.1080 |
| 31 | 40 | 5.0 | 40 | 2.5 | 180 | 0.1136 |
| 32 | 40 | 5.0 | 50 | 2.5 | 180 | 0.1099 |
| 33 | 30 | 5.0 | 45 | 2.5 | 120 | 0.0846 |
| 34 | 50 | 5.0 | 45 | 2.5 | 120 | 0.1359 |
| 35 | 30 | 5.0 | 45 | 2.5 | 180 | 0.0811 |
| 36 | 50 | 5.0 | 45 | 2.5 | 180 | 0.1203 |
| 37 | 40 | 4.5 | 45 | 2.0 | 150 | 0.1196 |
| 38 | 40 | 5.5 | 45 | 2.0 | 150 | 0.0999 |
| 39 | 40 | 4.5 | 45 | 3.0 | 150 | 0.1211 |
| 40 | 40 | 5.5 | 45 | 3.0 | 150 | 0.1263 |
| 41 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1819 |
| 42 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1809 |
| 43 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1816 |
| 44 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1820 |
| 45 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1823 |
| 46 | 40 | 5.0 | 45 | 2.5 | 150 | 0.1806 |
| Source | Sum of squares | Degree of freedom | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Model | 0.0351 | 20 | 0.0018 | 11.1033 | <0.0001** |
| A | 0.0052 | 1 | 0.0052 | 32.6506 | <0.0001** |
| B | 1.58E-05 | 1 | 1.58E-05 | 0.0999 | 0.7545 |
| C | 0.0011 | 1 | 0.0011 | 6.9608 | 0.0141* |
| D | 0.0019 | 1 | 0.0019 | 11.8855 | 0.0020** |
| E | 0.0004 | 1 | 0.0004 | 2.7363 | 0.1106 |
| AB | 8.37E-05 | 1 | 8.37E-05 | 0.5295 | 0.4736 |
| AC | 0.0011 | 1 | 0.0011 | 6.7422 | 0.0155* |
| AD | 0.0002 | 1 | 0.0002 | 1.784 | 0.2880 |
| AE | 3.66E-05 | 1 | 3.66E-05 | 0.2315 | 0.6346 |
| BC | 0.0002 | 1 | 0.0002 | 1.4517 | 0.2395 |
| BD | 0.0001 | 1 | 0.0001 | 0.9803 | 0.3316 |
| BE | 6.25E-06 | 1 | 6.25E-06 | 0.0395 | 0.8440 |
| CD | 0.0004 | 1 | 0.0004 | 2.3193 | 0.1403 |
| CE | 1E-08 | 1 | 1E-08 | 6.32E-05 | 0.9937 |
| DE | 2.07E-05 | 1 | 2.07E-05 | 0.1309 | 0.7205 |
| A2 | 0.0110 | 1 | 0.0110 | 69.6601 | <0.0001** |
| B2 | 0.0077 | 1 | 0.0077 | 48.9624 | <0.0001** |
| C2 | 0.0049 | 1 | 0.0049 | 28.3590 | <0.0001** |
| D2 | 0.0089 | 1 | 0.0089 | 56.9051 | <0.0001** |
| E2 | 0.1539 | 1 | 0.1539 | 97.3289 | <0.0001** |
| Lack of fit | 0.0039 | 20 | 0.0002 | 0.0714 | 0.7925 |
| Pure error | 2.215E-06 | 5 | 4.43E-07 | ||
| Cor total | 0.0390 | 45 |
Table 3 Results of variance analysis for the biomass of Vaccinium dunalianum
| Source | Sum of squares | Degree of freedom | Mean square | F-value | P-value |
|---|---|---|---|---|---|
| Model | 0.0351 | 20 | 0.0018 | 11.1033 | <0.0001** |
| A | 0.0052 | 1 | 0.0052 | 32.6506 | <0.0001** |
| B | 1.58E-05 | 1 | 1.58E-05 | 0.0999 | 0.7545 |
| C | 0.0011 | 1 | 0.0011 | 6.9608 | 0.0141* |
| D | 0.0019 | 1 | 0.0019 | 11.8855 | 0.0020** |
| E | 0.0004 | 1 | 0.0004 | 2.7363 | 0.1106 |
| AB | 8.37E-05 | 1 | 8.37E-05 | 0.5295 | 0.4736 |
| AC | 0.0011 | 1 | 0.0011 | 6.7422 | 0.0155* |
| AD | 0.0002 | 1 | 0.0002 | 1.784 | 0.2880 |
| AE | 3.66E-05 | 1 | 3.66E-05 | 0.2315 | 0.6346 |
| BC | 0.0002 | 1 | 0.0002 | 1.4517 | 0.2395 |
| BD | 0.0001 | 1 | 0.0001 | 0.9803 | 0.3316 |
| BE | 6.25E-06 | 1 | 6.25E-06 | 0.0395 | 0.8440 |
| CD | 0.0004 | 1 | 0.0004 | 2.3193 | 0.1403 |
| CE | 1E-08 | 1 | 1E-08 | 6.32E-05 | 0.9937 |
| DE | 2.07E-05 | 1 | 2.07E-05 | 0.1309 | 0.7205 |
| A2 | 0.0110 | 1 | 0.0110 | 69.6601 | <0.0001** |
| B2 | 0.0077 | 1 | 0.0077 | 48.9624 | <0.0001** |
| C2 | 0.0049 | 1 | 0.0049 | 28.3590 | <0.0001** |
| D2 | 0.0089 | 1 | 0.0089 | 56.9051 | <0.0001** |
| E2 | 0.1539 | 1 | 0.1539 | 97.3289 | <0.0001** |
| Lack of fit | 0.0039 | 20 | 0.0002 | 0.0714 | 0.7925 |
| Pure error | 2.215E-06 | 5 | 4.43E-07 | ||
| Cor total | 0.0390 | 45 |
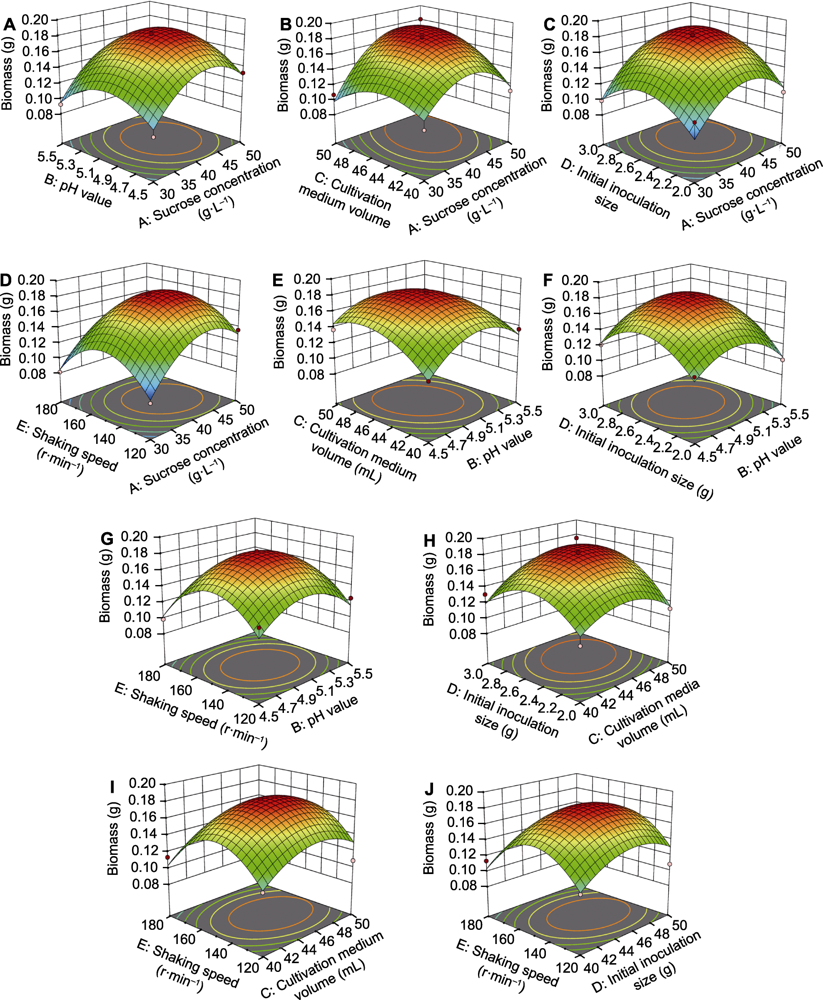
Figure 2 3D response surface analysis for the interactive effects of two different factors on the biomass of suspension culture cells of Vaccinium dunalianum (A) Sucrose concentration and pH value; (B) Sucrose concentration and cultivation medium volume; (C) Sucrose concentration and initial inoculation size; (D) Sucrose concentration and shaking speed; (E) pH value and cultivation medium volume; (F) pH value and initial inoculation size; (G) pH value and shaking speed; (H) Cultivation medium volume and initial inoculation size; (I) Cultivation medium volume and shaking speed; (J) Initial inoculation size and shaking speed
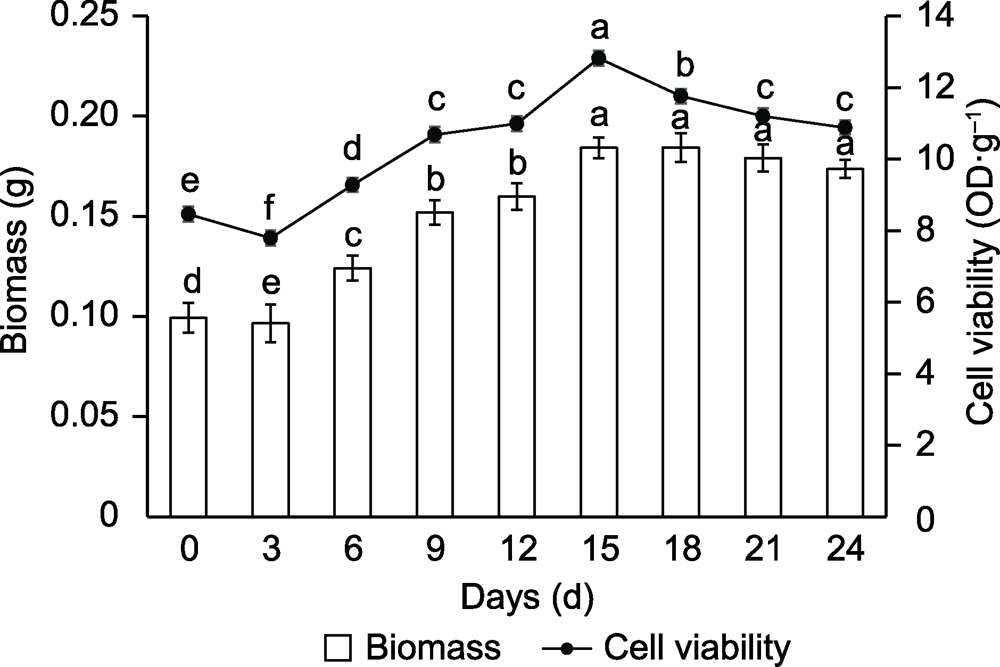
Figure 3 Curve of biomass and cell viability of suspension culture in Vaccinium dunalianum Different lowercase letters indicated significant differences (P<0.05).
| [1] | 陈继光, 上官新晨, 尹忠平, 任民红, 付晓 (2014). 青钱柳悬浮细胞的培养及其基质消耗的规律. 现代食品科技 30(1), 44-49, 107. |
| [2] | 董燕, 刁玲武, 周联 (2011). 药用植物细胞悬浮培养的影响因素. 中医药信息 28(3), 36-40. |
| [3] | 付羚, 张訸, 罗旭璐, 尚俊可, 唐军荣, 赵平 (2019). 樟叶越桔愈伤组织诱导及其细胞悬浮培养体系建立. 西部林业科学 48, 119-124, 130. |
| [4] | 谷贵章, 殷晓敏 (2006). 金丝桃细胞悬浮培养条件的优化. 食品与药品 8(11), 31-34. |
| [5] | 郭紫娟 (2015). 白背三七悬浮细胞培养体系的建立. 农技服务 32(10), 90-92. |
| [6] | 江苏省植物研究所 (1991). 新华本草纲要(第二册). 上海: 上海科学技术出版社. pp. 358-360. |
| [7] |
李萍, 董亚辉, 李成龙, 何雨龙, 李明军 (2020). 怀牛膝细胞悬浮培养条件的优化. 植物学报 55, 90-95.
DOI |
| [8] | 李蕤, 谭晓芳, 陈群, 樊家荣 (2011). 霍山石斛细胞悬浮培养及条件优化. 中草药 42, 358-362. |
| [9] | 李宗艳 (2004). 石竹细胞悬浮培养研究. 广西植物 24, 266- 269. |
| [10] | 梁雪, 张佳薇, 赵兴堂, 何利明, 康雪, 詹亚光 (2018). 水曲柳松散愈伤组织的悬浮培养和超低温保存. 分子植物育种 16, 5376-5385. |
| [11] | 廖礼佳, 李祥会, 杨军, 伍春莲 (2013). 长春花悬浮培养条件的优化. 西华师范大学学报(自然科学版) 34, 252-255. |
| [12] | 刘思妤, 杨悦, 王鹰, 王丽娟, 吴秀菊 (2017). 北细辛悬浮培养体系的建立及优化. 草业科学 34, 2254-2260. |
| [13] | 栾林莉, 宋玉凤, 侯辛辛, 陈健妙 (2019). 巴西橡胶树体胚胚性愈伤组织悬浮系的建立和植株再生. 分子植物育种 17, 2614-2621. |
| [14] | 罗旭璐, 唐军荣, 李娜, 丁勇, 张德国, 马焕成, 赵平 (2014). 樟叶越桔的组织培养与快速繁殖. 植物生理学报 50, 1717- 1720. |
| [15] | 毛堂芬, 颜谦, 方周伯 (1994). 蔗糖和硝酸铵对黄连悬浮培养细胞生长和小檗碱含量的影响. 生物技术 4(3), 33-35. |
| [16] | 孙国政, 马江, 曹磊, 陆攀科, 罗俏俏, 张忠明 (2017). 天山雪莲细胞液体悬浮培养基的优化研究. 中国食品工业 (11), 68-71. |
| [17] |
王玲, 李琰, 代伟娜, 严静, 张朝红 (2018). 葡萄细胞悬浮培养体系的建立和优化. 生物技术通报 34(8), 80-86.
DOI |
| [18] | 吴燕燕, 李明, 马婷玉, 段修冉, 潘丽萍 (2018). 白木香悬浮细胞培养的研究. 中药材 41, 1044-1047. |
| [19] | 邢建民, 赵德修, 李茂寅, 叶和春, 李国凤, 李佐虎 (1999). 碳源和氮源对水母雪莲悬浮培养细胞生长和黄酮合成的影响. 生物工程学报 15, 230-234. |
| [20] | 徐志荣, 王婷, 娄佳兰, 魏赛金 (2019). 南方红豆杉细胞悬浮培养体系优化及动力学研究. 林业科学研究 32(1), 8-14. |
| [21] | 颜昌敬 (1990). 植物组织培养手册. 上海: 上海科学技术出版社. pp. 117-133. |
| [22] | 闫静辉, 张小兵, 李亚璞, 李春生, 吴萌, 陈英珠, 程华, 籍宝霞, 陈瑞琴 (2005). 西洋参悬浮细胞系的建立及其生长特性的研究. 河北省科学院学报 22(4), 23-26, 36. |
| [23] | 张进仁, 陈善春, 高峰 (1993). 柑桔细胞悬浮培养及再生植株的研究. 热带作物学报 14(2), 67-70. |
| [24] | 赵继鹏, 杨淑慎 (2014). 曼地亚红豆杉细胞悬浮培养体系的建立. 西北农林科技大学学报(自然科学版) 42(1), 189-195. |
| [25] | 中国科学院中国植物志编辑委员会 (1991). 中国植物志, 第57卷(第3分册). 北京: 科学出版社. pp. 93. |
| [26] | 邹璐, 何钢, 刘贤桂, 张虹, 席飞飞, 叶生宝 (2019). 仙茅愈伤组织诱导和细胞悬浮培养体系的建立. 分子植物育种 17, 5383-5389. |
| [27] |
Iborra JL, Guardiola J, Montaner S, Cánovas M, Manjón A (1992). 2,3,5-triphenyltetrazolium chloride as a viability assay for immobilized plant cells. Biotechnol Tech 6, 319- 322.
DOI URL |
| [28] |
Jeandet P, Clément C, Courot E (2014). Resveratrol production at large scale using plant cell suspensions. Eng Life Sci 14, 622-632.
DOI URL |
| [29] |
Li N, Zeng WL, Luo XL, Yang CR, Zhang YJ, Ding Y, Zhao P (2018). A new arbutin derivative from the leaves of Vaccinium dunalianum Wight. Nat Prod Res 32, 65-70.
DOI PMID |
| [30] |
Pérez-González MZ, Nieto-Trujillo A, Gutiérrez-Rebolledo GA, García-Martínez I, Estrada-Zúñiga ME, Bernabé-Antonio A, Jiménez-Arellanes MA, Cruz-Sosa F (2019). Lupeol acetate production and antioxidant activity of a cell suspension culture from Cnidoscolus chayamansa leaves. South Afr J Bot 125, 30-38.
DOI URL |
| [31] |
Shekhawat MS, Shekhawat NS (2011). Micropropagation of Arnebia hispidissima (Lehm). DC. and production of alkannin from callus and cell suspension culture. Acta Physiol Plant 33, 1445-1450.
DOI URL |
| [32] |
Verpoorte R, Contin A, Memelink J (2002). Biotechnology for the production of plant secondary metabolites. Phytochem Rev 1, 13-25.
DOI URL |
| [33] |
Xu M, Lao QC, Zhao P, Zhu XY, Zhu HT, Luo XL, Yang CR, He JH, Li CQ, Zhang YJ (2014). 6'-O-caffeoylarbutin inhibits melanogenesis in zebrafish. Nat Prod Res 28, 932- 934.
DOI URL |
| [34] |
Zhao P, Tanaka T, Hirabayashi K, Zhang YJ, Yang CR, Kouno I (2008). Caffeoyl arbutin and related compounds from the buds of Vaccinium dunalianum. Phytochemistry 69, 3087-3094.
DOI URL |
| [1] | Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper [J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255. |
| [2] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [3] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [4] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [5] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [6] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [7] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [8] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [9] | Yanmin Li, Hui Jiang, Zhenzhu Fu, Jing Zhang, Xin Yuan, Huijuan Wang, Jie Gao, Xiaoyu Dong, Limin Wang, Hechen Zhang. Callus Induction and Somatic Embryogenesis in Anther Culture of Paeonia lactiflora [J]. Chinese Bulletin of Botany, 2021, 56(4): 443-450. |
| [10] | Pengfei Du, Yu Wang, Yingping Cao, Song Yang, Zhichao Sun, Decai Mao, Jiajun Yan, Daxu Li, Meizhen Sun, Chunxiang Fu, Shiqie Bai. Establishment of Biolistic Mediated Transformation System for Elymus sibiricus [J]. Chinese Bulletin of Botany, 2021, 56(1): 62-70. |
| [11] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [12] | Jianfei Liu, Yan Liu, Kejian Liu, Yang Chi, Zhifa Huo, Yonghong Huo, Xiangling You. Optimization of the Regeneration System from Somatic Embryogenesis in Larix olgensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 605-612. |
| [13] | Yan Xiao,Zhenxing Wang,Dongming Li,Yanhua Qi, Enhebayaer. Optimization of Tissue Culture and Plant Regeneration System of Mature Embryo of Leymus chinensis [J]. Chinese Bulletin of Botany, 2020, 55(2): 192-198. |
| [14] | Ping Li,Yahui Dong,Chenglong Li,Yulong He,Mingjun Li. Optimization of Cell Suspension Culture Conditions of Achyranthes bidentata [J]. Chinese Bulletin of Botany, 2020, 55(1): 90-95. |
| [15] | Ying Feng,Lianwen Qian,Qingliang Lin. The Effect of Different Hormones on Explant Browning and Callus Browning in Cyclocarya paliurus [J]. Chinese Bulletin of Botany, 2019, 54(5): 634-641. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||