

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (6): 760-767.DOI: 10.11983/CBB20079 cstr: 32102.14.CBB20079
Previous Articles Next Articles
Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang*( )
)
Received:2020-05-12
Accepted:2020-08-26
Online:2020-11-01
Published:2020-11-11
Contact:
Ying Chang
Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans[J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767.
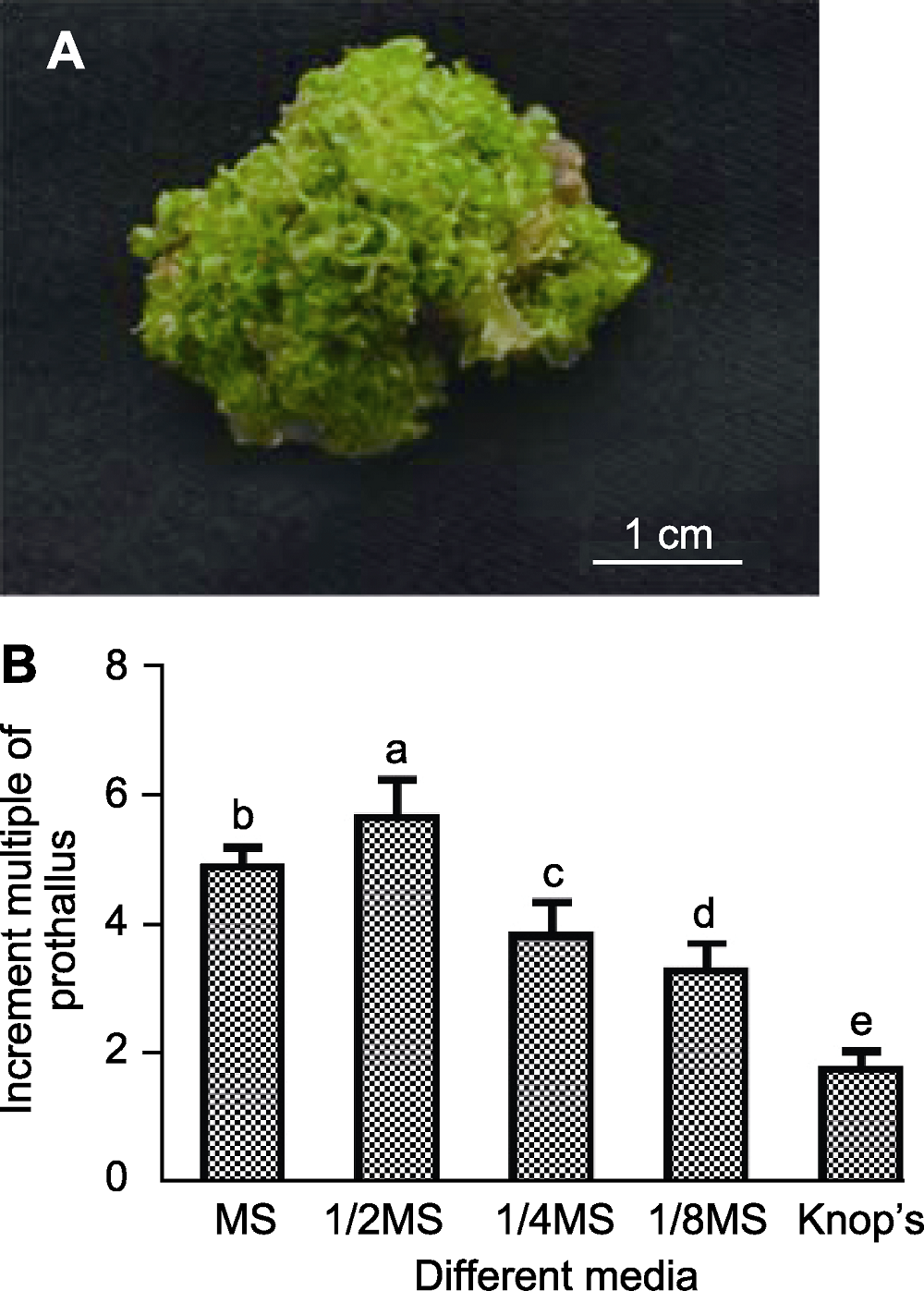
Figure 1 Dryopteris fragrans prothallus (A) and its prolifera-tion on different media (B) Different lowercase letters indicate significant differences (P< 0.05).
| Media | Prothallus color | Young sporophytes | The growth of prothallus |
|---|---|---|---|
| MS | Verdant | Abundant | +++ |
| 1/2MS | Verdant | Massive | ++++ |
| 1/4MS | Verdant with some pale | A small amount | ++ |
| 1/8MS | Pale green | A small amount | + |
| Knop’s | Pale green | Few | ? |
Table 1 The effect of different media on the prothallus of Dryopteris fragrans
| Media | Prothallus color | Young sporophytes | The growth of prothallus |
|---|---|---|---|
| MS | Verdant | Abundant | +++ |
| 1/2MS | Verdant | Massive | ++++ |
| 1/4MS | Verdant with some pale | A small amount | ++ |
| 1/8MS | Pale green | A small amount | + |
| Knop’s | Pale green | Few | ? |
| Media | The number of inoculation | Cultured days (d) | Sporozoites induction rate (%) |
|---|---|---|---|
| MS | 80 | 90 | 29.69±3.12 b |
| 1/2MS | 80 | 90 | 37.50±2.04 a |
| 1/4MS | 80 | 90 | 14.06±1.88 c |
| 1/8MS | 80 | 90 | 7.81±2.77 d |
| Knop’s | 80 | 90 | 1.88±1.25 e |
Table 2 The effect of different media on the sporophytes of Dryopteris fragrans
| Media | The number of inoculation | Cultured days (d) | Sporozoites induction rate (%) |
|---|---|---|---|
| MS | 80 | 90 | 29.69±3.12 b |
| 1/2MS | 80 | 90 | 37.50±2.04 a |
| 1/4MS | 80 | 90 | 14.06±1.88 c |
| 1/8MS | 80 | 90 | 7.81±2.77 d |
| Knop’s | 80 | 90 | 1.88±1.25 e |
| Treatments | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Induction period (d) | Induction rate (%) | Browning rate (%) | Callus growth |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 30 | 0 | 0 | |
| 2 | 3.0 | 0.5 | 30 | 43.33±3.33 | 38.61±2.98 | ++ |
| 3 | 3.0 | 1.0 | 30 | 33.33±3.33 | 36.94±7.24 | ++ |
| 4 | 3.0 | 1.5 | 30 | 17.78±1.92 | 68.89±10.18 | + |
| 5 | 2.0 | 0.5 | 30 | 96.67±5.77 | 3.33±5.77 | ++++ |
| 6 | 2.0 | 1.0 | 30 | 81.11±1.92 | 28.67±6.35 | +++ |
| 7 | 2.0 | 1.5 | 30 | 76.67±5.77 | 48.21±9.94 | ++ |
| 8 | 1.5 | 0.5 | 30 | 83.33±5.77 | 31.94±6.36 | +++ |
| 9 | 1.5 | 1.0 | 30 | 80.00±0.00 | 45.83±7.22 | ++ |
| 10 | 1.5 | 1.5 | 30 | 76.67±5.77 | 52.38±4.12 | ++ |
| 11 | 1.0 | 0.5 | 30 | 86.67±5.77 | 54.17±9.11 | ++ |
| 12 | 1.0 | 1.0 | 30 | 86.67±5.77 | 57.87±4.01 | ++ |
| 13 | 1.0 | 1.5 | 30 | 73.33±5.77 | 58.92±3.09 | ++ |
Table 3 Callus induction of Dryopteris fragrans with various concentrations of hormones from the shoot tip apex
| Treatments | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Induction period (d) | Induction rate (%) | Browning rate (%) | Callus growth |
|---|---|---|---|---|---|---|
| 1 | 0 | 0 | 30 | 0 | 0 | |
| 2 | 3.0 | 0.5 | 30 | 43.33±3.33 | 38.61±2.98 | ++ |
| 3 | 3.0 | 1.0 | 30 | 33.33±3.33 | 36.94±7.24 | ++ |
| 4 | 3.0 | 1.5 | 30 | 17.78±1.92 | 68.89±10.18 | + |
| 5 | 2.0 | 0.5 | 30 | 96.67±5.77 | 3.33±5.77 | ++++ |
| 6 | 2.0 | 1.0 | 30 | 81.11±1.92 | 28.67±6.35 | +++ |
| 7 | 2.0 | 1.5 | 30 | 76.67±5.77 | 48.21±9.94 | ++ |
| 8 | 1.5 | 0.5 | 30 | 83.33±5.77 | 31.94±6.36 | +++ |
| 9 | 1.5 | 1.0 | 30 | 80.00±0.00 | 45.83±7.22 | ++ |
| 10 | 1.5 | 1.5 | 30 | 76.67±5.77 | 52.38±4.12 | ++ |
| 11 | 1.0 | 0.5 | 30 | 86.67±5.77 | 54.17±9.11 | ++ |
| 12 | 1.0 | 1.0 | 30 | 86.67±5.77 | 57.87±4.01 | ++ |
| 13 | 1.0 | 1.5 | 30 | 73.33±5.77 | 58.92±3.09 | ++ |
| Treatments | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Proliferation days (d) | Proliferation rate (%) |
|---|---|---|---|---|
| 1 | 3.0 | 0.5 | 60 | 3.71 |
| 2 | 3.0 | 1.0 | 60 | 3.19 |
| 3 | 3.0 | 1.5 | 60 | 3.16 |
| 4 | 2.0 | 0.5 | 60 | 11.50 |
| 5 | 2.0 | 1.0 | 60 | 7.59 |
| 6 | 2.0 | 1.5 | 60 | 5.92 |
| 7 | 1.5 | 0.5 | 60 | 10.37 |
| 8 | 1.5 | 1.0 | 60 | 6.41 |
| 9 | 1.5 | 1.5 | 60 | 6.07 |
| 10 | 1.0 | 0.5 | 60 | 13.30 |
| 11 | 1.0 | 1.0 | 60 | 9.75 |
| 12 | 1.0 | 1.5 | 60 | 4.97 |
Table 4 The effect of different hormones on Dryopteris fragrans callus proliferation
| Treatments | 6-BA (mg·L-1) | 2,4-D (mg·L-1) | Proliferation days (d) | Proliferation rate (%) |
|---|---|---|---|---|
| 1 | 3.0 | 0.5 | 60 | 3.71 |
| 2 | 3.0 | 1.0 | 60 | 3.19 |
| 3 | 3.0 | 1.5 | 60 | 3.16 |
| 4 | 2.0 | 0.5 | 60 | 11.50 |
| 5 | 2.0 | 1.0 | 60 | 7.59 |
| 6 | 2.0 | 1.5 | 60 | 5.92 |
| 7 | 1.5 | 0.5 | 60 | 10.37 |
| 8 | 1.5 | 1.0 | 60 | 6.41 |
| 9 | 1.5 | 1.5 | 60 | 6.07 |
| 10 | 1.0 | 0.5 | 60 | 13.30 |
| 11 | 1.0 | 1.0 | 60 | 9.75 |
| 12 | 1.0 | 1.5 | 60 | 4.97 |
| Treat- ments | Media | KT (mg·L-1) | NAA (mg·L-1) | Induction period (d) | Buds induction rate (%) |
|---|---|---|---|---|---|
| 1 | 1/2MS | 0 | 0 | 60 | 53.33±3.33 a |
| 2 | 1/2MS | 0.2 | 0 | 60 | 43.33±6.67 b |
| 3 | 1/2MS | 0.5 | 0 | 60 | 33.33±5.77 bc |
| 4 | 1/2MS | 0 | 0.2 | 60 | 26.67±6.67 c |
| 5 | 1/2MS | 0 | 0.5 | 60 | 30.00±8.82 bc |
| 6 | 1/2MS | 0 | 1.0 | 60 | 11.11±5.09 d |
Table 5 The effects of hormones on buds’ differentiation of Dryopteris fragrans
| Treat- ments | Media | KT (mg·L-1) | NAA (mg·L-1) | Induction period (d) | Buds induction rate (%) |
|---|---|---|---|---|---|
| 1 | 1/2MS | 0 | 0 | 60 | 53.33±3.33 a |
| 2 | 1/2MS | 0.2 | 0 | 60 | 43.33±6.67 b |
| 3 | 1/2MS | 0.5 | 0 | 60 | 33.33±5.77 bc |
| 4 | 1/2MS | 0 | 0.2 | 60 | 26.67±6.67 c |
| 5 | 1/2MS | 0 | 0.5 | 60 | 30.00±8.82 bc |
| 6 | 1/2MS | 0 | 1.0 | 60 | 11.11±5.09 d |

Figure 2 Proliferation, differentiation and transplanting of Dryopteris fragrans callus (A) Callus of Dryopteris fragrans before proliferation; (B) Callus of D. fragrans after 60 d of proliferation; (C) Differentiation of callus and formation of bud of D. fragrans; (D)-(H) Spore body of D. fragrans with bud differentiation; (I) The sporophyte of D. fragrans after domestication. Bars=1 cm
| Treatments | Media | NAA (mg·L-1) | Induction period (d) | Number of roots |
|---|---|---|---|---|
| 1 | 1/2MS | 0 | 30 | 3.33±0.57 b |
| 2 | 1/2MS | 0.2 | 30 | 5.33±0.57 a |
| 3 | 1/2MS | 0.5 | 30 | 3.67±1.53 ab |
| 4 | 1/2MS | 1.0 | 30 | 2.67±1.15 b |
Table 6 The effects of different media on rooting of Dryop-teris fragrans
| Treatments | Media | NAA (mg·L-1) | Induction period (d) | Number of roots |
|---|---|---|---|---|
| 1 | 1/2MS | 0 | 30 | 3.33±0.57 b |
| 2 | 1/2MS | 0.2 | 30 | 5.33±0.57 a |
| 3 | 1/2MS | 0.5 | 30 | 3.67±1.53 ab |
| 4 | 1/2MS | 1.0 | 30 | 2.67±1.15 b |
| [1] | 包日双, 尹培培, 郭斌, 尉亚辉 (2012). 蛇足石杉原叶体的培养及孢子体的诱导. 植物生理学报 48, 393-396. |
| [2] | 包文美, 敖志文, 陈发生 (1985). 东北蕨类植物配子体发育的研究. I. 水龙骨科. 植物研究 5(4), 101-114. |
| [3] | 常缨 (2009). 香鳞毛蕨国内外研究进展. 北方园艺 (4), 113-115. |
| [4] | 丁恒山 (2010). 中外药用孢子植物资源志要 贵州: 贵州科技出版社. pp. 1-3. |
| [5] | 杜文钊, 宋国强, 刘海燕, 冯淡开, 沈志滨 (2016). 香鳞毛蕨中间苯三酚类化合物的研究. 广东药学院学报 32, 22-24. |
| [6] | 关丽霞, 白禹萱, 李阳, 蒲东祥 (2019). 过山蕨组织培养技术研究. 黑龙江农业科学 (7), 39-42. |
| [7] | 韩喜国, 任英, 聂振波, 高静, 王庆军, 王晓慧 (2017). 蕨菜组织培养技术研究现状及发展趋势. 长江蔬菜 (16), 30-33. |
| [8] | 黄笛, 冯玉兰, 董丽 (2009). 银粉背蕨的配子体发育及孢子繁殖技术的研究. 园艺学报 36, 1345-1352. |
| [9] | 黄庆阳, 樊锐锋, 袁强, 常缨 (2007). 香鳞毛蕨配子体发育及快速繁殖的研究. 中草药 38, 1573-1576. |
| [10] | 黄执缨 (2004). 二歧鹿角蕨组织培养的研究. 生物学杂志 21(5), 22-24. |
| [11] | 姜长阳, 宁淑香, 于淼, 王宇, 宋立秀 (2003). 蕨菜愈伤组织高效再生体系的建立. 园艺学报 30, 343-345. |
| [12] | 李静, 夏桂春, 龚慧, 曾宪锋 (2008). 狭眼凤尾蕨的形态发生及组织培养. 热带作物学报 29, 626-631. |
| [13] | 彭晓明, 曾宋君 (2004). 铁线蕨的组织培养及植株再生. 植物生理学通讯 40, 575. |
| [14] | 孙莉莉 (2015). 香鳞毛蕨中黄酮代谢途径关键酶基因的克隆与功能验证. 博士论文. 哈尔滨: 东北农业大学. pp. 1-128. |
| [15] | 王辉, 秦建蓉, 植爽, 田娜, 肖小君 (2013). 桫椤原叶体增殖及幼孢子体形成试验. 林业科技开发 27(5), 86-88. |
| [16] | 王淼, 孙丽娜, 吴春华, 石元亮 (2016). 东北蕨菜的GGB组培移栽技术及驯化. 园艺与种苗 (1), 27-29. |
| [17] | 王晓倩, 张婷婷, 孟卓, 董丽 (2014). 阔鳞鳞毛蕨孢子的无菌繁殖. 植物生理学报 50, 159-163. |
| [18] | 王一诺, 韦莹, 李翠, 李林轩, 肖冬, 王晓峰, 韦坤华 (2016). 植物生长调节素对西南凤尾蕨组织培养的影响. 湖北农业科学 55, 2117-2119. |
| [19] | 韦莹, 张占江, 李翠, 黄宝优, 韦坤华, 黄雪彦 (2013). 植物生长调节剂对槲蕨离体培养的效应. 湖北农业科学 52, 5909-5911. |
| [20] | 吴芹 (2007). 几种观赏蕨类植物的繁殖技术研究. 硕士论文. 南京: 南京林业大学. pp. 71. |
| [21] | 吴小凤, 胡若洋, 李学东 (2013). 仙鹤藓的孢子萌发及愈伤组织诱导. 植物学报 48, 651-657. |
| [22] | 邢福武 (2011). 无花之美——蕨类植物在园林造景中的应用. 中国花卉园艺 (3), 14-15. |
| [23] | 徐文杰 (2007). 北京地区蕨类植物引种、栽培及繁殖技术的研究. 硕士论文. 北京: 北京林业大学. pp. 1-77. |
| [24] | 袁玲 (2007). 两种观赏蕨类植物离体快繁体系的建立. 硕士论文. 武汉: 华中农业大学. pp. 1-53. |
| [25] | 赵怀宝, 殷寿延 (2019). 红树植物卤蕨的组织培养. 海南热带海洋学院学报 26(5), 34-39. |
| [26] | Fernández H, Revilla MA (2003). In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult 73, 1-13. |
| [27] |
Gao R, Wang WZ, Huang QY, Fan RF, Wang X, Feng P, Zhao GM, Bian S, Ren HL, Chang Y (2018). Complete chloroplast genome sequence of Dryopteris fragrans (L.) Schott and the repeat structures against the thermal environment. Sci Rep 8, 16635.
URL PMID |
| [28] |
Li X, Han JD, Fang YH, Bai SN, Rao GY (2017). Expression analyses of embryogenesis-associated genes during somatic embryogenesis of Adiantum capillus-veneris L. in vitro: new insights into the evolution of reproductive organs in land plants. Front Plant Sci 8, 658.
URL PMID |
| [29] |
Lin HQ, Liu XP, Shen ZB, Cheng WQ, Zeng ZJ, Chen YF, Tang CP, Jiang T (2019). The effect of isoflavaspidic acid PB extracted from Dryopteris fragrans (L.) Schott on planktonic and biofilm growth of dermatophytes and the possible mechanism of antibiofilm. J Ethnopharmacol 241, 111956.
URL PMID |
| [30] | Liu ZD, Zhao DD, Jiang S, Xue B, Zhang YL, Yan XF (2018). Anticancer phenolics from Dryopteris fragrans (L.) Schott. Molecules 23, 680. |
| [31] |
Lu Z, Huang QY, Zhang T, Hu BZ, Chang Y (2018). Global transcriptome analysis and characterization of Dryopteris fragrans (L.) Schott sporangium in different developmental stages. BMC Genomics 19, 471.
DOI URL |
| [32] | Zhang T, Wang L, Duan DH, Zhang YH, Huang SX, Chang Y (2018). Cytotoxicity-guided isolation of two new phenolic derivatives from Dryopteris fragrans (L.) Schott. Molecules 23, 1652. |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper [J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255. |
| [3] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [4] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [5] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [6] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [7] | Xiaoyun Wu, Minling Liao, Xueru Li, Zichun Shu, Jiatong Xin, Bohan Zhang, Silan Dai. Establishment of Regeneration System of Chrysanthemum vestitum with Three Floret Forms [J]. Chinese Bulletin of Botany, 2024, 59(2): 245-256. |
| [8] | Zhaoxuan Zhong, Dongrui Zhang, Lu Li, Ying Su, Daining Wang, Zeran Wang, Yang Liu, Ying Chang. Bioinformatic and Expression Pattern Analysis of dfr-miR160a and Target Gene DfARF10 in Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2024, 59(1): 22-33. |
| [9] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [10] | Minling Liao, Ya Pu, Xiaoyun Wu, Chaofeng Ma, Wenkui Wang, Silan Dai. Establishment of Regeneration System of Chrysanthemum indicum in Pingtan with Various Ligulate Floret Form [J]. Chinese Bulletin of Botany, 2023, 58(3): 449-460. |
| [11] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [12] | Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum [J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235. |
| [13] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [14] | Yanmin Li, Hui Jiang, Zhenzhu Fu, Jing Zhang, Xin Yuan, Huijuan Wang, Jie Gao, Xiaoyu Dong, Limin Wang, Hechen Zhang. Callus Induction and Somatic Embryogenesis in Anther Culture of Paeonia lactiflora [J]. Chinese Bulletin of Botany, 2021, 56(4): 443-450. |
| [15] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||