

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (3): 449-460.DOI: 10.11983/CBB22135 cstr: 32102.14.CBB22135
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Minling Liao1, Ya Pu1, Xiaoyun Wu1, Chaofeng Ma1, Wenkui Wang2, Silan Dai1( )
)
Received:2022-06-28
Accepted:2022-09-26
Online:2023-05-01
Published:2023-05-17
Contact:
*E-mail: silandai@sina.com
Minling Liao, Ya Pu, Xiaoyun Wu, Chaofeng Ma, Wenkui Wang, Silan Dai. Establishment of Regeneration System of Chrysanthemum indicum in Pingtan with Various Ligulate Floret Form[J]. Chinese Bulletin of Botany, 2023, 58(3): 449-460.
| No. | Basal medium | 6-BA (mg?L-1) | NAA (mg?L-1) |
|---|---|---|---|
| Y1 | MS | 1.0 | 0.5 |
| Y2 | MS | 1.0 | 1.0 |
| Y3 | MS | 1.0 | 2.0 |
| Y4 | MS | 2.0 | 0.5 |
| Y5 | MS | 2.0 | 1.0 |
| Y6 | MS | 2.0 | 2.0 |
| Y7 | MS | 3.0 | 0.5 |
| Y8 | MS | 3.0 | 1.0 |
| Y9 | MS | 3.0 | 2.0 |
| CK | MS | 0 | 0 |
Table 1 The composition of the medium for callus induction and adventitious buds differentiation
| No. | Basal medium | 6-BA (mg?L-1) | NAA (mg?L-1) |
|---|---|---|---|
| Y1 | MS | 1.0 | 0.5 |
| Y2 | MS | 1.0 | 1.0 |
| Y3 | MS | 1.0 | 2.0 |
| Y4 | MS | 2.0 | 0.5 |
| Y5 | MS | 2.0 | 1.0 |
| Y6 | MS | 2.0 | 2.0 |
| Y7 | MS | 3.0 | 0.5 |
| Y8 | MS | 3.0 | 1.0 |
| Y9 | MS | 3.0 | 2.0 |
| CK | MS | 0 | 0 |
| No. | Basal medium | NAA (mg?L-1 ) | 6-BA (mg?L-1 ) | TDZ (mg?L-1 ) |
|---|---|---|---|---|
| T1 | MS | 2.0 | 1.0 | 0.1 |
| T2 | MS | 2.0 | 1.0 | 0.2 |
| T3 | MS | 2.0 | 1.0 | 0.4 |
| T4 | MS | 2.0 | 2.0 | 0.1 |
| T5 | MS | 2.0 | 2.0 | 0.2 |
| T6 | MS | 2.0 | 2.0 | 0.4 |
| T7 | MS | 2.0 | 3.0 | 0.1 |
| T8 | MS | 2.0 | 3.0 | 0.2 |
| T9 | MS | 2.0 | 3.0 | 0.4 |
Table 2 The composition of the medium for callus induction with different concentrations of TDZ
| No. | Basal medium | NAA (mg?L-1 ) | 6-BA (mg?L-1 ) | TDZ (mg?L-1 ) |
|---|---|---|---|---|
| T1 | MS | 2.0 | 1.0 | 0.1 |
| T2 | MS | 2.0 | 1.0 | 0.2 |
| T3 | MS | 2.0 | 1.0 | 0.4 |
| T4 | MS | 2.0 | 2.0 | 0.1 |
| T5 | MS | 2.0 | 2.0 | 0.2 |
| T6 | MS | 2.0 | 2.0 | 0.4 |
| T7 | MS | 2.0 | 3.0 | 0.1 |
| T8 | MS | 2.0 | 3.0 | 0.2 |
| T9 | MS | 2.0 | 3.0 | 0.4 |
| No. | Basal medium | NAA (mg?L-1 ) |
|---|---|---|
| G1 | 1/2MS | 0.2 |
| G2 | 1/2MS | 0.5 |
| G3 | 1/2MS | 1.0 |
| 1/2MS | 1/2MS | 0 |
| MS | MS | 0 |
Table 3 Formula of rooting medium
| No. | Basal medium | NAA (mg?L-1 ) |
|---|---|---|
| G1 | 1/2MS | 0.2 |
| G2 | 1/2MS | 0.5 |
| G3 | 1/2MS | 1.0 |
| 1/2MS | 1/2MS | 0 |
| MS | MS | 0 |

Figure 1 Ray floret variations in Chrysanthemum indicum in Pingtan (A1) Flat type; (A2) Spoon type; (A3) Tubular type; (A4) Mix type; (B1), (B2) Chenille-like; (C1), (C2) Swallowtail-like; (D1) Evaginable; (D2) Incurvate; (E1) Difference in ray floret length; (E2), (E3) Difference in degree of curvature; (E4) Difference in cracking degree; (F1)-(F4) Difference in ray floret number; (G1)-(G4) Difference in proportion of each petal type in mix type; (H) Difference in diameter of capitulum. Bars=1 cm

Figure 2 Chrysanthemum indicum in Pingtan stains with various ligulate floret form (A) Plant (bar=3.5 cm); (B) Leaf shape (bar=1 cm); (C) Capitulum (bar=1 cm)
| Treat-ments | Callus formation rate on the 14th day (%) | Differentiation rate on the 40th day (%) | Treatments | Callus formation rate on the 14th day (%) | ||
|---|---|---|---|---|---|---|
| Leaves | tTCLs | Leaves | tTCLs | |||
| Y1 | 100.00±0.00 a | 100.00±0.00 a | 0 | 82.00±3.46 a | T1 | 100.00±0.00 a |
| Y2 | 100.00±0.00 a | 100.00±0.00 a | 0 | 53.50±4.95 c | T2 | 100.00±0.00 a |
| Y3 | 100.00±0.00 a | 90.50±4.95 b | 3.71±0.19 b | 33.50±6.36 d | T3 | 97.00±0.04 ab |
| Y4 | 100.00±0.00 a | 100.00±0.00 a | 0 | 63.50±4.95 bc | T4 | 83.50±0.06 ab |
| Y5 | 100.00±0.00 a | 100.00±0.00 a | 0 | 67.00±5.66 b | T5 | 97.50±0.03 ab |
| Y6 | 100.00±0.00 a | 98.50±2.12 a | 9.39±3.35 b | 57.50±0.71 bc | T6 | 87.50±0.09 ab |
| Y7 | 100.00±0.00 a | 100.00±0.00 a | 0 | 61.00±1.41 bc | T7 | 81.50±0.05 b |
| Y8 | 100.00±0.00 a | 93.50±0.71 b | 0 | 16.50±3.54 e | T8 | 85.00±0.14 ab |
| Y9 | 75.48±4.30 b | 98.50±2.12 a | 19.09±1.29 a | 41.00±4.24 d | T9 | 90.00±0.06 ab |
| CK | 0.00±0.00 c | 0.00±0.00 c | 0 | 0 | - | - |
Table 4 Effects of different media on callus induction and adventitious buds differentiation for leaves and transverse thin cell layers (tTCLs) of Chrysanthemum indicum in Pingtan
| Treat-ments | Callus formation rate on the 14th day (%) | Differentiation rate on the 40th day (%) | Treatments | Callus formation rate on the 14th day (%) | ||
|---|---|---|---|---|---|---|
| Leaves | tTCLs | Leaves | tTCLs | |||
| Y1 | 100.00±0.00 a | 100.00±0.00 a | 0 | 82.00±3.46 a | T1 | 100.00±0.00 a |
| Y2 | 100.00±0.00 a | 100.00±0.00 a | 0 | 53.50±4.95 c | T2 | 100.00±0.00 a |
| Y3 | 100.00±0.00 a | 90.50±4.95 b | 3.71±0.19 b | 33.50±6.36 d | T3 | 97.00±0.04 ab |
| Y4 | 100.00±0.00 a | 100.00±0.00 a | 0 | 63.50±4.95 bc | T4 | 83.50±0.06 ab |
| Y5 | 100.00±0.00 a | 100.00±0.00 a | 0 | 67.00±5.66 b | T5 | 97.50±0.03 ab |
| Y6 | 100.00±0.00 a | 98.50±2.12 a | 9.39±3.35 b | 57.50±0.71 bc | T6 | 87.50±0.09 ab |
| Y7 | 100.00±0.00 a | 100.00±0.00 a | 0 | 61.00±1.41 bc | T7 | 81.50±0.05 b |
| Y8 | 100.00±0.00 a | 93.50±0.71 b | 0 | 16.50±3.54 e | T8 | 85.00±0.14 ab |
| Y9 | 75.48±4.30 b | 98.50±2.12 a | 19.09±1.29 a | 41.00±4.24 d | T9 | 90.00±0.06 ab |
| CK | 0.00±0.00 c | 0.00±0.00 c | 0 | 0 | - | - |
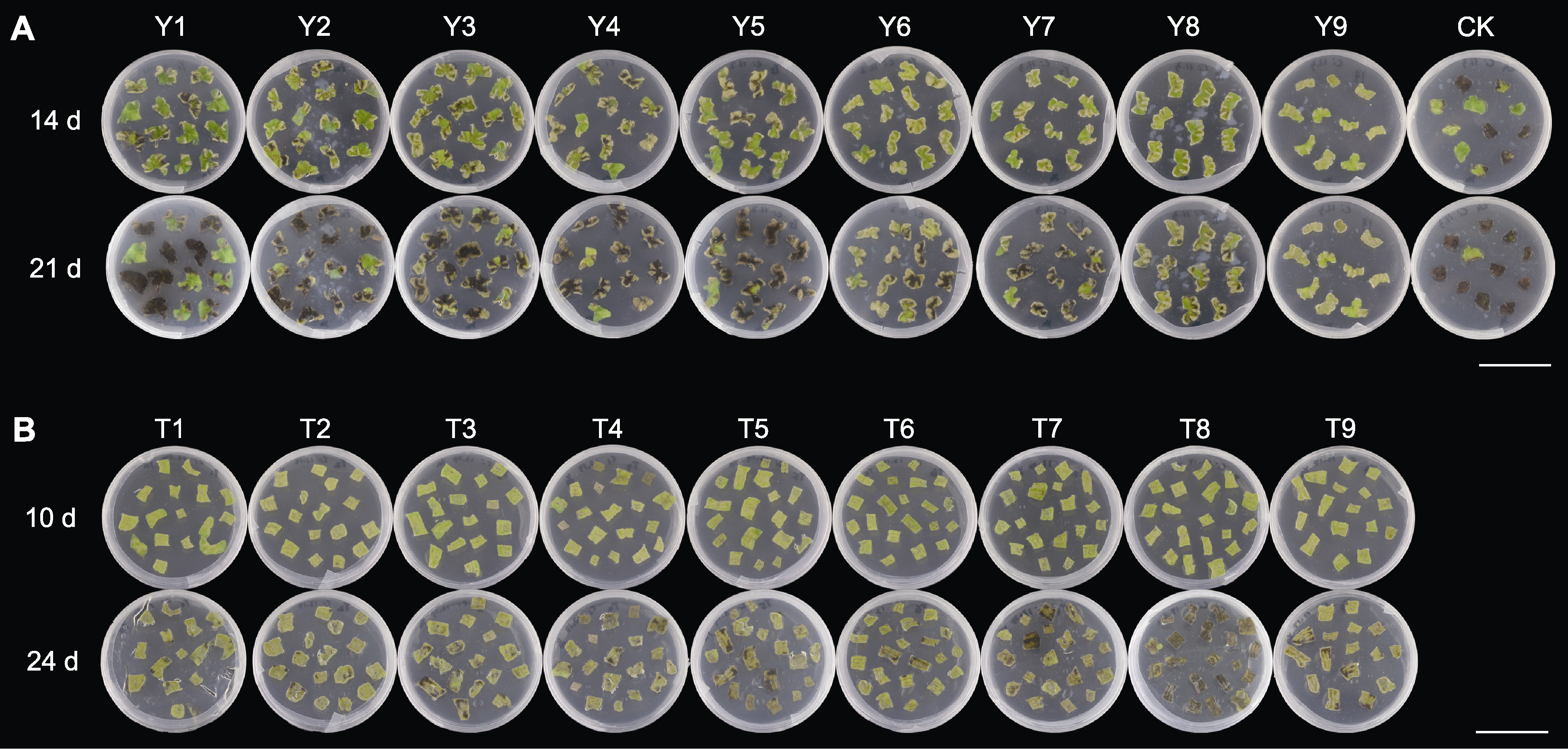
Figure 3 Regeneration of Chrysanthemum indicum in Pingtan leaves in different media (A) Leaves in different media; (B) Leaves in media with TDZ. Y1-Y9, and CK are the same as in Table 1; T1-T9 are the same as in Table 2. Bars=4.5 cm

Figure 4 Adventitious bud differentiation for leaves of Chrysanthemum indicum in Pingtan in different media (A) Y3; (B) Y6; (C) Y9. Y3, Y6 and Y9 are the same as in Table 1. From left to right: Adventitious bud differentiation of leaves in different media at 21, 28, 35, 42 and 49 d. Bar=1 cm
| Treatments | Browning rate on the 21th day (%) | Treat- ments | Browning rate on the 24th day (%) |
|---|---|---|---|
| Y1 | 83.04±7.92 abcd | T1 | 52.78±3.93 f |
| Y2 | 96.43±5.05 a | T2 | 56.08±3.88 ef |
| Y3 | 80.36±6.84 bcde | T3 | 81.80±0.78 c |
| Y4 | 90.11±8.58 abc | T4 | 72.14±2.18 d |
| Y5 | 77.56±3.95 cde | T5 | 89.45±0.78 b |
| Y6 | 67.86±9.22 e | T6 | 61.51±0.56 e |
| Y7 | 74.22±2.49 de | T7 | 97.50±3.54 a |
| Y8 | 42.50±5.00 f | T8 | 100.00±0.00 a |
| Y9 | 34.17±1.18 f | T9 | 87.30±2.25 bc |
| CK | 92.73±9.50 ab |
Table 5 The browning rate for leaves of Chrysanthemum indicum in Pingtan in different media
| Treatments | Browning rate on the 21th day (%) | Treat- ments | Browning rate on the 24th day (%) |
|---|---|---|---|
| Y1 | 83.04±7.92 abcd | T1 | 52.78±3.93 f |
| Y2 | 96.43±5.05 a | T2 | 56.08±3.88 ef |
| Y3 | 80.36±6.84 bcde | T3 | 81.80±0.78 c |
| Y4 | 90.11±8.58 abc | T4 | 72.14±2.18 d |
| Y5 | 77.56±3.95 cde | T5 | 89.45±0.78 b |
| Y6 | 67.86±9.22 e | T6 | 61.51±0.56 e |
| Y7 | 74.22±2.49 de | T7 | 97.50±3.54 a |
| Y8 | 42.50±5.00 f | T8 | 100.00±0.00 a |
| Y9 | 34.17±1.18 f | T9 | 87.30±2.25 bc |
| CK | 92.73±9.50 ab |

Figure 5 Adventitious bud differentiation of transverse thin cell layers of Chrysanthemum indicum in Pingtan in different media (40 d) Y1-Y9 are the same as in Table 1. Bar=1 cm
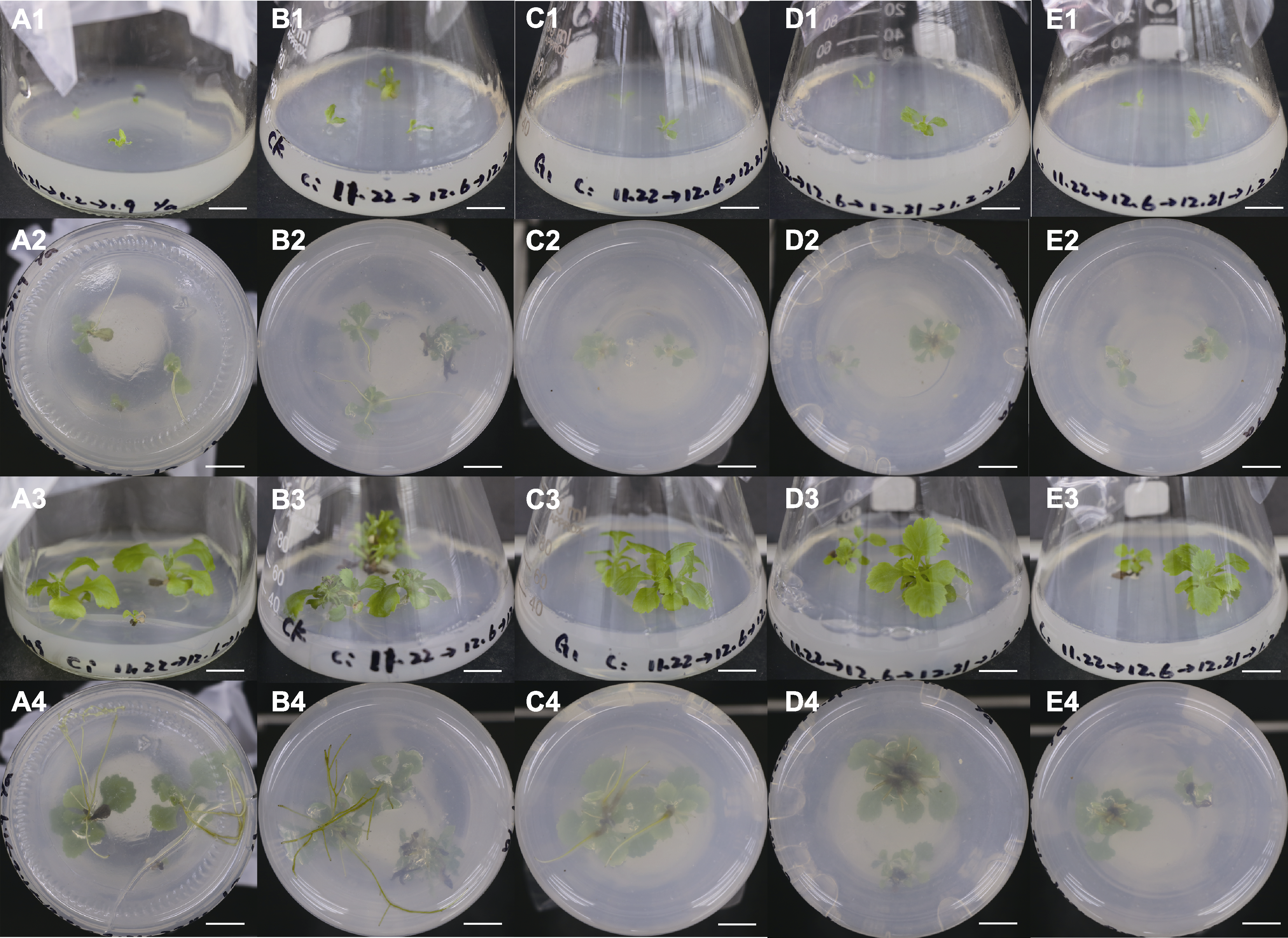
Figure 6 Rooting and plant status of Chrysanthemum indicum in Pingtan adventitious buds (A1)-(E1) Plant status (0 d); (A2)-(E2) Rooting condition (10 d); (A3)-(E3) Plant status (26 d); (A4)-(E4) Rooting condition (26 d). From left to right: MS, 1/2MS, G1, G2, and G3 (the same as in Table 3). Bars=1 cm
| Treat- ments | Time of rooting (d) | Rate of rooting (%) | Average root length (cm) | The situation of root and plant | Number of branches |
|---|---|---|---|---|---|
| MS | 6 | 66.7 | 12.59±2.60 a | The root system is dense, slender, and long; the plant is weak with branches and small leaves | 3±1 a |
| 1/2MS | 6 | 100 | 5.49±0.86 b | The root system is sparse and slender; the plant is weak with many branches and small leaves | 6±2 a |
| G1 | 14 | 100 | 4.41±0.80 b | The root system is crude and short, with many root hairs; the plant is weak with branches | 4±2 a |
| G2 | 10 | 100 | 3.21±2.62 b | The root system is crude and short, with many root hairs; the plant is robust and higher, with larger leaves | 2±1 a |
| G3 | 24 | 100 | 3.80±2.22 b | The root system is crude and short, with many root hairs; the plant is robust and highest, with largest leaves | 2±1 a |
Table 6 Rooting and plant status of Chrysanthemum indicum in Pingtan adventitious buds
| Treat- ments | Time of rooting (d) | Rate of rooting (%) | Average root length (cm) | The situation of root and plant | Number of branches |
|---|---|---|---|---|---|
| MS | 6 | 66.7 | 12.59±2.60 a | The root system is dense, slender, and long; the plant is weak with branches and small leaves | 3±1 a |
| 1/2MS | 6 | 100 | 5.49±0.86 b | The root system is sparse and slender; the plant is weak with many branches and small leaves | 6±2 a |
| G1 | 14 | 100 | 4.41±0.80 b | The root system is crude and short, with many root hairs; the plant is weak with branches | 4±2 a |
| G2 | 10 | 100 | 3.21±2.62 b | The root system is crude and short, with many root hairs; the plant is robust and higher, with larger leaves | 2±1 a |
| G3 | 24 | 100 | 3.80±2.22 b | The root system is crude and short, with many root hairs; the plant is robust and highest, with largest leaves | 2±1 a |
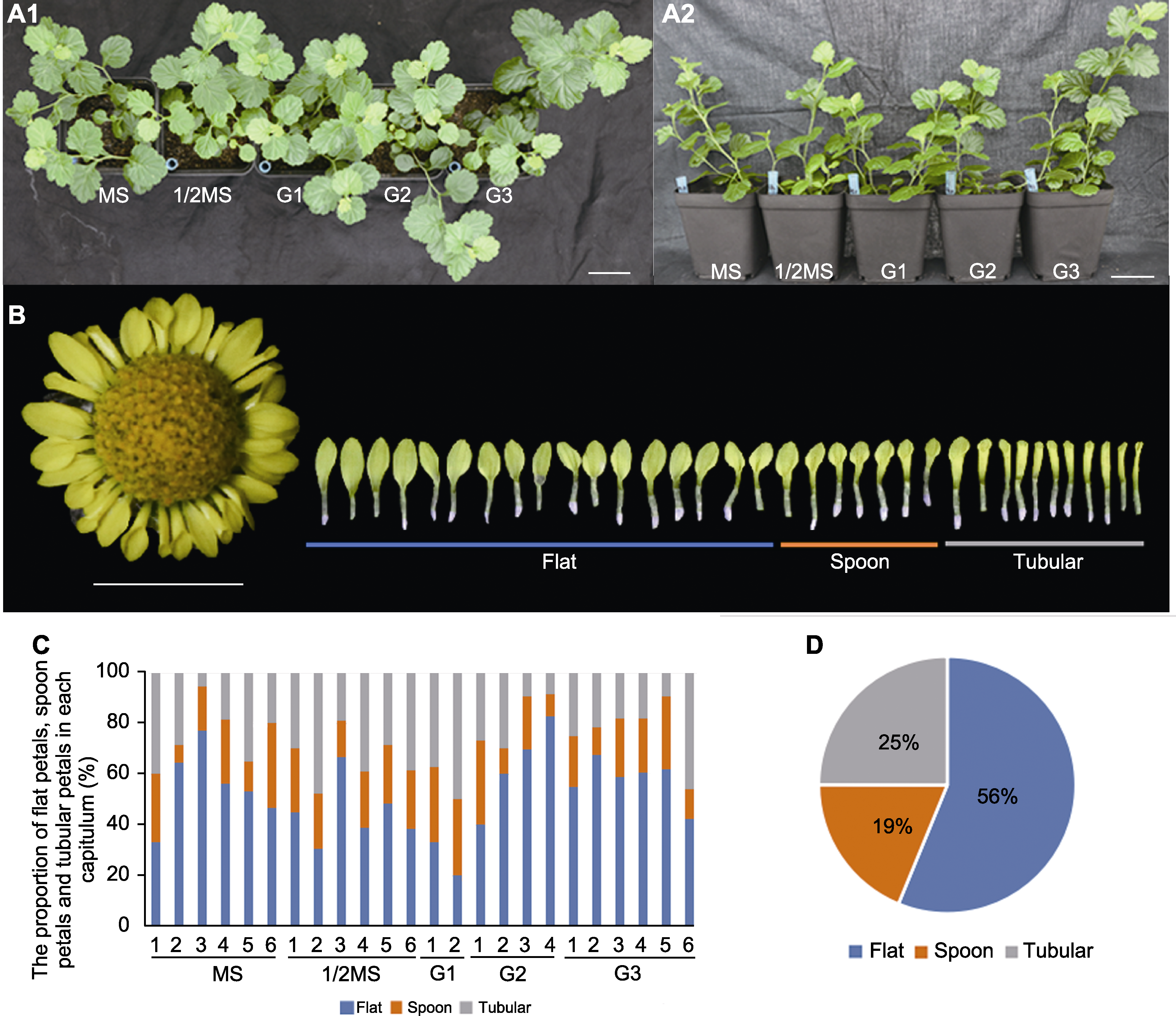
Figure 7 The capitulum of regenerated plants and the proportion of each petal type in Chrysanthemum indicum in Pingtan (A1), (A2) Plant status after transplanting for 60 days, from left to right, their rooting media were MS, 1/2MS, G1, G2, and G3, respectively (bars=3.5 cm); (B) The capitulum of regenerated plants (bar=1 cm); (C) The proportion of flat petals, spoon petals and tubular petals in each capitulum; (D) Average proportion of each petal type in all capitulums. MS, 1/2MS, G1, G2, and G3 are the same as in Table 3.
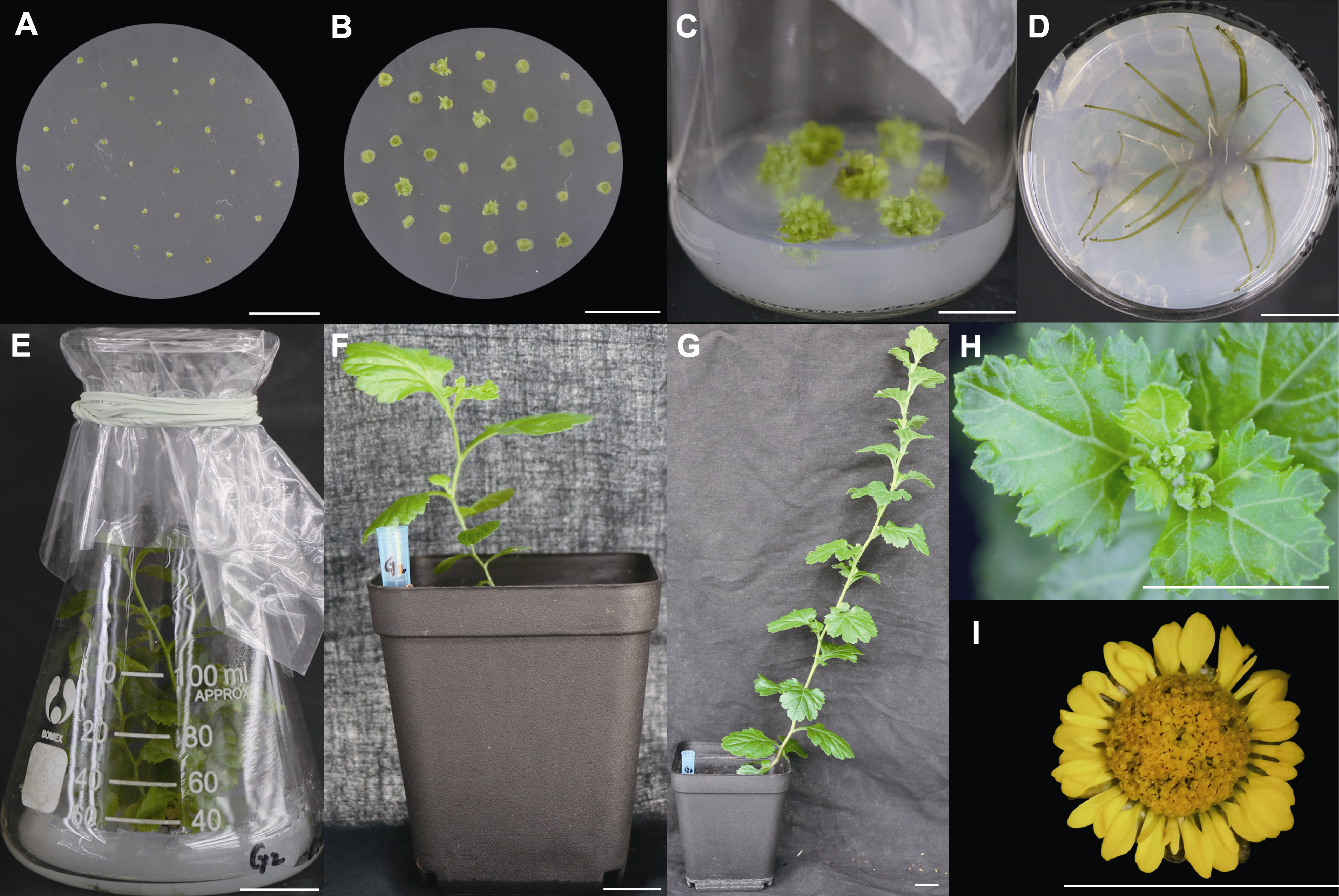
Figure 8 Regeneration system of transverse thin cell layers in Chrysanthemum indicum in Pingtan stains with various ligulate floret forms (A), (B) Callus induction and adventitious bud differentiation in Y1; (C) Adventitious buds grown to 0.8 cm; (D), (E) Rooting and plant status of adventitious buds in G2; (F), (G) Plant status after transplanting for 30 and 90 days; (H) Flower buds; (I) Capitulum of regenerated plant. Y1 is the same as in Table 1; G2 is the same as in Table 3. Bars=2 cm
| [1] | 晨卉, 王艳芳, 陈素梅, 刘思余, 滕年军, 兰伟, 陈发棣 (2009). 五种菊花近缘植物组织培养研究. 南京农业大学学报 32, 30-35. |
| [2] | 陈鹏彦 (2010). 朝鲜野菊再生系统建立的研究. 硕士论文. 大连: 辽宁师范大学. pp. 17-28. |
| [3] | 戴思兰, 陈俊愉, 李文彬 (1998). 菊花起源的RAPD分析. 植物学报 40, 76-82. |
| [4] | 戴思兰, 钟杨, 张晓艳 (1995). 中国菊属植物部分种的数量分类研究. 北京林业大学学报 17(4), 9-15. |
| [5] | 付建新, 张超, 王翊, 戴思兰 (2012). 甘菊下胚轴离体再生体系的建立. 北京林业大学学报 34, 91-96. |
| [6] | 宫明雪 (2019). 野菊CiMYB44基因调控植株垂枝性状的研究. 硕士论文. 哈尔滨: 东北林业大学. pp. 27-33. |
| [7] | 韩磊, 吕鑫, 张文静, 杨小霁 (2009). 野菊花蕾的组培与快繁技术研究. 北方园艺 (5), 103-105. |
| [8] | 韩正洲 (2017). 野菊资源研究与野菊花药材品质评价. 博士论文. 广州: 广州中医药大学. pp. 29-52. |
| [9] | 李梦雨 (2020). 神农香菊CiMYB4基因抗旱性功能验证及RNAi载体构建. 硕士论文. 哈尔滨: 东北林业大学. pp. 22-31. |
| [10] | 李娜 (2018). ChiMYB基因转化野菊的研究. 硕士论文. 佳木斯: 佳木斯大学. pp. 24-26. |
| [11] | 李亚军, 李悦, 黄河, 戴思兰 (2018). 切花菊‘粉贵人’高效再生体系的建立. 见: 中国观赏园艺研究进展2018. 北京: 中国林业出版社. pp. 427-434. |
| [12] | 林镕, 石铸, 傅国勋 (1983). 中国植物志, 第76卷第1分册. 北京: 科学出版社. pp. 29-42. |
| [13] | 刘倩倩 (2007). 中国大菊品种形态分类及细胞学研究. 硕士论文. 北京: 北京林业大学. pp. 15-41. |
| [14] |
罗虹, 温小蕙, 周圆圆, 戴思兰 (2020). 芳香堆心菊离体再生体系的建立. 植物学报 55, 318-328.
DOI |
| [15] | 马晓蓉 (2017). 大别山地区野菊野生资源调查及其活性成分研究. 硕士论文. 南京: 南京农业大学. pp. 31-37. |
| [16] | 曲晓慧 (2020). 几种菊属野生植物再生体系的建立. 硕士论文. 南京: 南京农业大学. pp. 11-42. |
| [17] | 任江珊, 顾雪琪, 蒲娅, 黄河 (2021). 葫芦岛野菊舌状花再生体系建立. 现代园艺 44(24), 3-4, 7. |
| [18] | 汤忠皓 (1963). 中国菊花品种分类的探讨. 园艺学报 2, 411-420. |
| [19] | 汪劲武, 杨继, 李懋学 (1993). 野菊和甘菊的形态变异及其核型特征. 植物分类学报 31, 140-146. |
| [20] | 王霁佳 (2019). 神农香菊CiMYB4基因的克隆及对野菊的遗传转化. 硕士论文. 哈尔滨: 东北林业大学. pp. 23-32. |
| [21] | 王文奎, 周春玲, 戴思兰 (1999). 毛华菊花朵形态变异. 北京林业大学学报 21(3), 92-95. |
| [22] | 王想 (2018). 神农香菊单萜合酶基因的克隆及对野菊的遗传转化. 硕士论文. 哈尔滨: 东北林业大学. pp. 34. |
| [23] | 武晓云, 温小蕙, 马朝峰, 戴思兰 (2018). 快速诱导甘菊叶片再生植株的方法. 见: 中国观赏园艺研究进展2018. 北京: 中国林业出版社. pp. 461-468. |
| [24] | 吴志苹, 高亦珂, 范敏, 高耀辉 (2020). 菊花‘金不凋’再生及遗传转化体系的构建. 分子植物育种 18, 150-158. |
| [25] | 肖政 (2009). 菊花离体再生及根癌农杆菌介导CBF1基因遗传转化的研究. 硕士论文. 杨凌: 西北农林科技大学. pp. 16-19. |
| [26] | 肖政, 范崇辉, 金万梅 (2009). 生长调节物质对菊花‘小金黄’叶片再生不定芽的影响. 西北林学院学报 24(6), 50-53. |
| [27] | 徐晓帆 (2019). 切花菊叶、花形态多样性与关联性分析. 硕士论文. 南京: 南京农业大学. pp. 34-44. |
| [28] | 徐晓峰, 黄学林 (2003). TDZ: 一种有效的植物生长调节剂. 植物学通报 20, 227-237. |
| [29] | 许莹修 (2005). 菊花形态性状多样性和品种分类的研究. 硕士论文. 北京: 北京林业大学. pp. 23-36. |
| [30] | 张树林 (1965). 菊花品种分类的研究. 园艺学报 4, 35-46. |
| [31] | 张鲜艳, 张飞, 陈发棣, 郭慧敏, 陈素梅 (2011). 12份不同地理居群野菊的遗传多样性分析. 南京农业大学学报 34(3), 48-54. |
| [32] | 郑芳昊 (2012). 野菊花种子质量标准与种苗繁育技术的研究. 硕士论文. 广州: 广州中医药大学. pp. 33-41. |
| [33] | 钟声远, 罗宇婷, 赵勇, 王振兴, 管志勇, 房伟民, 陈发棣, 王海滨 (2021). 切花菊品种资源表型多样性分析. 植物资源与环境学报 30(5), 22-33. |
| [34] | 周杰 (2009). 关于中国菊花起源问题的若干实验研究. 博士论文. 北京: 北京林业大学. pp. 31-36, 109-113. |
| [35] | 周婷, 杨惠婷, 胡计红, 朱梦珠, 洪荣钦, 潘东明, 佘文琴, 陈桂信 (2019). 单头切花菊‘白扇’组培快繁技术. 热带作物学报 40, 715-723. |
| [36] |
Liu XW, Xia B, Purente N, Chen B, Zhou YW, He M (2021). Transgenic Chrysanthemum indicum overexpressing cin- miR396a exhibits altered plant development and reduced salt and drought tolerance. Plant Physiol Biochem 168, 17-26.
DOI URL |
| [37] |
Ma YP, Chen MM, Wei JX, Zhao L, Liu PL, Dai SL, Wen J (2016). Origin of Chrysanthemum cultivars—evidence from nuclear low-copy LFY gene sequences. Biochem Syst Ecol 65, 129-136.
DOI URL |
| [38] |
Ma YP, Zhao L, Zhang WJ, Zhang YH, Xing X, Duan XX, Hu J, Harris AJ, Liu PL, Dai SL, Wen J (2020). Origins of cultivars of Chrysanthemum—evidence from the chloroplast genome and nuclear LFY gene. J Syst Evol 58, 925-944.
DOI URL |
| [39] |
Song XB, Gao K, Fan GX, Zhao XG, Liu ZL, Dai SL (2018). Quantitative classification of the morphological traits of ray florets in large-flowered Chrysanthemum. HortScience 53, 1258-1265.
DOI URL |
| [40] |
Song XB, Xu YH, Gao K, Fan GX, Zhang F, Deng CY, Dai SL, Huang H, Xin HG, Li YY (2020). High-density genetic map construction and identification of loci controlling flower-type traits in Chrysanthemum (Chrysanthemum × morifolium Ramat.). Hortic Res 7, 108.
DOI |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [3] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [4] | Xiaoyun Wu, Minling Liao, Xueru Li, Zichun Shu, Jiatong Xin, Bohan Zhang, Silan Dai. Establishment of Regeneration System of Chrysanthemum vestitum with Three Floret Forms [J]. Chinese Bulletin of Botany, 2024, 59(2): 245-256. |
| [5] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [6] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [7] | Yue Xu,Yingping Cao,Yu Wang,Chunxiang Fu,Shaojun Dai. Agrobacterium rhizogenes-mediated Transformation System of Spinacia oleracea [J]. Chinese Bulletin of Botany, 2019, 54(4): 515-521. |
| [8] | Zhang Xuhong, Wang Di, Liang Zhenxu, Sun Meiyu, Zhang Jinzheng, Shi Lei. Callus Induction and Establishment of a Plant Regeneration System with Lilium martagon [J]. Chinese Bulletin of Botany, 2018, 53(6): 840-847. |
| [9] | Xiting Zhao, Liwei Jiang, Miao Wang, Yuting Zhu, Wenfang Zhang, Mingjun Li. Establishment of Transgenic Acceptor by Indirect Somatic Embryogenesis Regeneration and Transformation of CmTGA1 Gene in Chrysanthemum morifolium cv. ‘Huaihuang’ [J]. Chinese Bulletin of Botany, 2016, 51(4): 525-532. |
| [10] | Huan Feng, Shuli Yi, Jiaheng Xie, Mengqi Lei, Xuan Huang. Callus Induction and Plant Regeneration of Rosa hybrida [J]. Chinese Bulletin of Botany, 2014, 49(5): 595-602. |
| [11] | Liming Zhao;Shujun Liu;Songquan Song;. Efficient Induction of Callus and Plant Regeneration from Seeds and Mature Embryos of Sweet Sorghum [J]. Chinese Bulletin of Botany, 2008, 25(04): 465-468. |
| [12] | LIU Jun;ZHAO Lan-Yong;FENG Zhen;ZHANG Mei-Rong;WU Yin-Feng. Optimization Selection of Genetic Transformation Regeneration System from Leaves of Dendranthema morifolium [J]. Chinese Bulletin of Botany, 2004, 21(05): 556-558. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||