

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (3): 461-474.DOI: 10.11983/CBB22092 cstr: 32102.14.CBB22092
• SPECIAL TOPICS • Previous Articles Next Articles
Jia Zhang1,2†, Qidong Li2,3†, Cui Li2, Qinghai Wang2, Xincun Hou2, Chunqiao Zhao2, Shuhe Li1( ), Qiang Guo2(
), Qiang Guo2( )
)
Received:2022-04-29
Accepted:2022-08-25
Online:2023-05-01
Published:2023-05-17
Contact:
*E-mail: 710580225@qq.com; guoqiang@grass-env.com
About author:†These authors contributed equally to this paper
Jia Zhang, Qidong Li, Cui Li, Qinghai Wang, Xincun Hou, Chunqiao Zhao, Shuhe Li, Qiang Guo. Research Progress on MATE Transporters in Plants[J]. Chinese Bulletin of Botany, 2023, 58(3): 461-474.
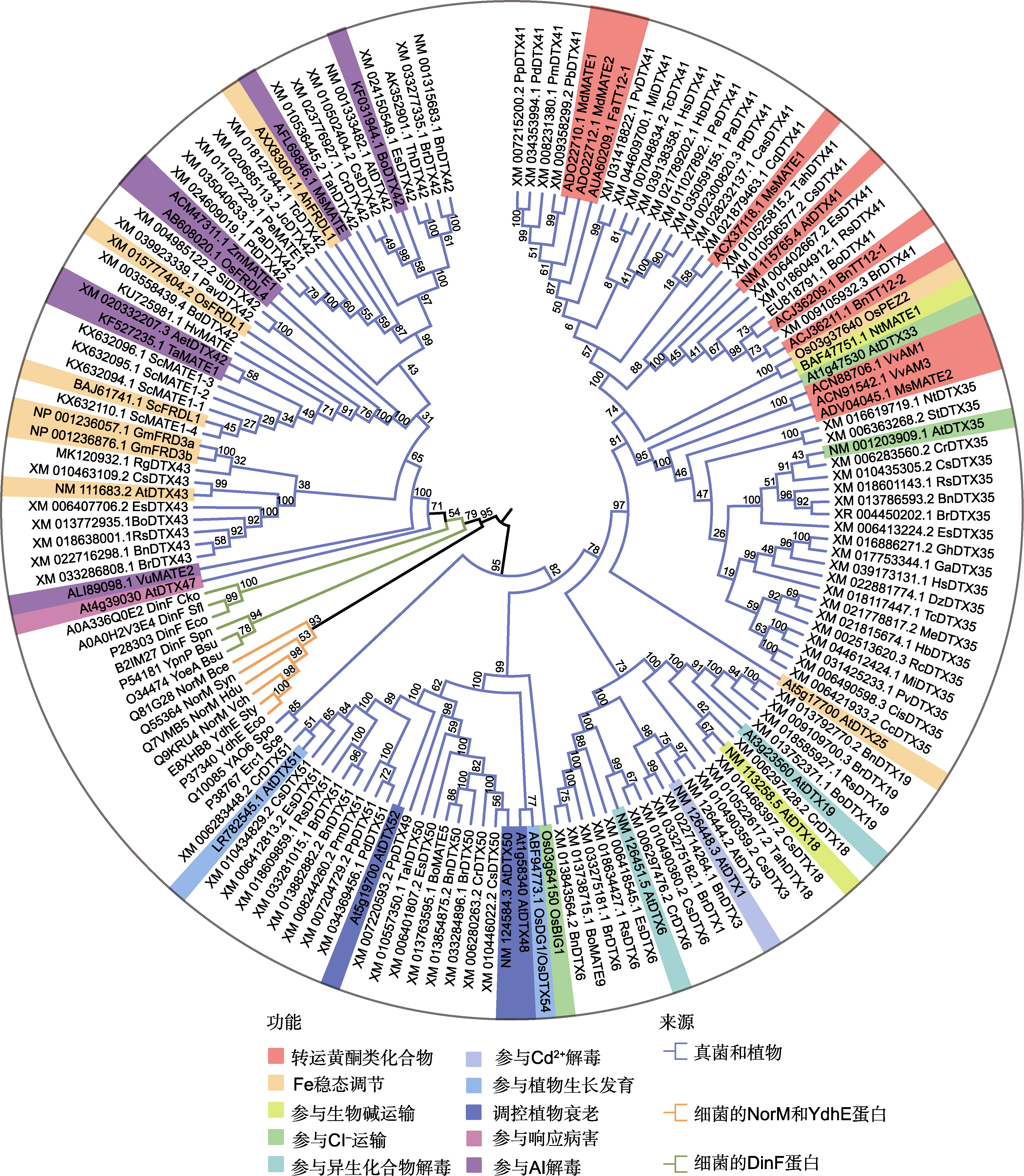
Figure 1 Phylogenetic tree of MATE/DTXs proteins The sources of MATE/DTXs are as follows: Arabidopsis thaliana (At), Camelina sativa (Cs), Eutrema salsugineum (Es), Thellungiella halophila (Th), Brassica rapa (Br), B. napus (Bn), Raphanus sativus (Rs), Rehmannia glutinosa (Rg), Tarenaya hassleriana (Tah), Secale cereale (Sc), Prunus mume (Pm), Jatropha curcas (Jc), Populus euphratica (Pe), P. trichocarpa (Pt), Prunus dulcis (Pd), P. persica (Pp), Theobroma cacao (Tc), P. alba (Pa), Brachypodium distachyon (Bd), Pistacia vera (Pv), Chenopodium quinoa (Cq), Hibiscus syriacus (Hs), Triticum aestivum (Ta), Hordeum vulgare (Hv), Aegilops tauschii (Aet), Panicum virgatum (Pav), Setaria italica (Si), Hevea brasiliensis (Hb), Mangifera indica (Mi), Pyrus bretschneideri (Pb), Camellia sinensis (Cas), Manihot esculenta (Me), Ricinus communis (Rc), Gossypium hirsutum (Gh), G. arboreum (Ga), Durio zibethinus (Dz), Citrus sinensis (Cis), C. clementina (Cc), Nicotiana tabacum (Nt), Solanum tuberosum (St), Oryza sativa (Os), Glycine max (Gm), Arachis hypogaea (Ah), Zea mays (Zm), Vitis vinifera (Vv), Medicago truncatula (Mt), Malus domestica (Md), M. sativa (Ms), Vigna umbellata (Vu), Fragaria x ananassa (Fa), Synechocystis sp. (Syn), Haemophilus ducreyi (Hdu), Bacillus cereus (Bce), Vibrio cholerae (Vch), Escherichia coli (Eco), Salmonella typhimurium (Sty), Saccharomyces cerevisiae (Sce), Schizosaccharomyces pombe (Spo), Shigella flexneri (Sfl), Citrobacter koseri (Cko), Streptococcus pneumoniae (Spn), and Bacillus subtilis (Bsu). Multiple alignment was performed by the Clustal W. A phylogenetic tree of MATE/DTXs was constructed by the neighbor-joining algorithm using MEGA 6.0 software.
| 基因名称 | 物种 | 功能 | 参考文献 | |
|---|---|---|---|---|
| AtDTX43 | 拟南芥 | 调节铁稳态 | Durrett et al., | |
| OsFRDL1 | 水稻 | 调节铁稳态 | Yokosho et al., | |
| ScFRDL1 | 黑麦 | 调节铁稳态 | Yokosho et al., | |
| AtDTX25 | 拟南芥 | 调节铁稳态 | Hoang et al., | |
| AhFRDL1 | 花生 | 调节铁稳态 | Qiu et al., | |
| MsMATE1 | 苜蓿 | 调节铁稳态 | 高铭等, | |
| OsPEZ2 | 水稻 | 调节铁稳态 | Bashir et al., | |
| HvAACT1 | 大麦 | 铝解毒 | Furukawa et al., | |
| SbMATE | 高粱 | 铝解毒 | Magalhaes et al., | |
| AtDTX42 | 拟南芥 | 铝解毒 | Liu et al., | |
| PvMATEa/b | 豇豆 | 铝解毒 | Rangel et al., | |
| VuMATE2 | 赤小豆 | 铝解毒 | Liu, | |
| ZmMATE1 | 玉米 | 铝解毒 | Maron et al., | |
| ScFRDL2 | 黑麦 | 铝解毒 | Yokosho et al., | |
| OsFRDL4 | 水稻 | 铝解毒 | Yokosho et al., | |
| BoMATE | 甘蓝 | 铝解毒 | 吴新新, | |
| MsMATE | 苜蓿 | 铝解毒 | 张宝云, | |
| GmMATE2 | 丹波黑大豆 | 铝解毒 | 胡俊等, | |
| CsMATE9 | 茶树 | 铝解毒 | 陈益, | |
| GmMATE13 | 大豆 | 铝解毒 | 龚莉, | |
| AtDTX1 | 拟南芥 | 响应镉胁迫 | Li et al., | |
| AtDTX19 | 拟南芥 | 外排四甲基铵 | Diener et al., | |
| AtDTX6 | 拟南芥 | 外排百草枯 | Xia et al., | |
| AtDTX41 | 拟南芥 | 转运花青素前体 | Marinova, | |
| MtMATE1 | 蒺藜苜蓿 | 转运E3′G | Zhao and Dixon, | |
| MtMATE2 | 蒺藜苜蓿 | 转运类黄酮 | Zhao et al., | |
| FaTT12-1 | 草莓 | 转运原花青素 | Chen et al., | |
| NtMATE3 | 烟草 | 转运尼古丁 | Shitan et al., | |
| NtMATE1 | 烟草 | 转运生物碱 | Shoji et al., | |
| AtDTX18 | 拟南芥 | 参与抗病 | Dobritzsch et al., | |
| OsMATE1 | 水稻 | 参与抗病 | Tiwari et al., | |
| OsMATE2 | 水稻 | 参与抗病 | Tiwari et al., | |
| AtDTX51 | 拟南芥 | 参与生长素合成 | Li et al., | |
| AtDTX50 | 拟南芥 | 转运脱落酸 | Zhang et al., | |
| OsDTX54 | 水稻 | 转运脱落酸 | Qin et al., | |
| AtDTX47 | 拟南芥 | 转运水杨酸 | Nawrath et al., | |
| AtDTX33 | 拟南芥 | 转运Cl- | Zhang et al., | |
| AtDTX35 | 拟南芥 | 转运Cl- | Zhang et al., | |
| OsBIG1 | 水稻 | 转运Cl- | Ren et al., | |
Table 1 MATE/DTXs genes in higher plants
| 基因名称 | 物种 | 功能 | 参考文献 | |
|---|---|---|---|---|
| AtDTX43 | 拟南芥 | 调节铁稳态 | Durrett et al., | |
| OsFRDL1 | 水稻 | 调节铁稳态 | Yokosho et al., | |
| ScFRDL1 | 黑麦 | 调节铁稳态 | Yokosho et al., | |
| AtDTX25 | 拟南芥 | 调节铁稳态 | Hoang et al., | |
| AhFRDL1 | 花生 | 调节铁稳态 | Qiu et al., | |
| MsMATE1 | 苜蓿 | 调节铁稳态 | 高铭等, | |
| OsPEZ2 | 水稻 | 调节铁稳态 | Bashir et al., | |
| HvAACT1 | 大麦 | 铝解毒 | Furukawa et al., | |
| SbMATE | 高粱 | 铝解毒 | Magalhaes et al., | |
| AtDTX42 | 拟南芥 | 铝解毒 | Liu et al., | |
| PvMATEa/b | 豇豆 | 铝解毒 | Rangel et al., | |
| VuMATE2 | 赤小豆 | 铝解毒 | Liu, | |
| ZmMATE1 | 玉米 | 铝解毒 | Maron et al., | |
| ScFRDL2 | 黑麦 | 铝解毒 | Yokosho et al., | |
| OsFRDL4 | 水稻 | 铝解毒 | Yokosho et al., | |
| BoMATE | 甘蓝 | 铝解毒 | 吴新新, | |
| MsMATE | 苜蓿 | 铝解毒 | 张宝云, | |
| GmMATE2 | 丹波黑大豆 | 铝解毒 | 胡俊等, | |
| CsMATE9 | 茶树 | 铝解毒 | 陈益, | |
| GmMATE13 | 大豆 | 铝解毒 | 龚莉, | |
| AtDTX1 | 拟南芥 | 响应镉胁迫 | Li et al., | |
| AtDTX19 | 拟南芥 | 外排四甲基铵 | Diener et al., | |
| AtDTX6 | 拟南芥 | 外排百草枯 | Xia et al., | |
| AtDTX41 | 拟南芥 | 转运花青素前体 | Marinova, | |
| MtMATE1 | 蒺藜苜蓿 | 转运E3′G | Zhao and Dixon, | |
| MtMATE2 | 蒺藜苜蓿 | 转运类黄酮 | Zhao et al., | |
| FaTT12-1 | 草莓 | 转运原花青素 | Chen et al., | |
| NtMATE3 | 烟草 | 转运尼古丁 | Shitan et al., | |
| NtMATE1 | 烟草 | 转运生物碱 | Shoji et al., | |
| AtDTX18 | 拟南芥 | 参与抗病 | Dobritzsch et al., | |
| OsMATE1 | 水稻 | 参与抗病 | Tiwari et al., | |
| OsMATE2 | 水稻 | 参与抗病 | Tiwari et al., | |
| AtDTX51 | 拟南芥 | 参与生长素合成 | Li et al., | |
| AtDTX50 | 拟南芥 | 转运脱落酸 | Zhang et al., | |
| OsDTX54 | 水稻 | 转运脱落酸 | Qin et al., | |
| AtDTX47 | 拟南芥 | 转运水杨酸 | Nawrath et al., | |
| AtDTX33 | 拟南芥 | 转运Cl- | Zhang et al., | |
| AtDTX35 | 拟南芥 | 转运Cl- | Zhang et al., | |
| OsBIG1 | 水稻 | 转运Cl- | Ren et al., | |
| [1] | 安婷婷, 黄帝, 王浩, 张一, 陈应龙 (2021). 植物响应镉胁迫的生理生化机制研究进展. 植物学报 56, 347-362. |
| [2] | 陈益 (2019). 铝处理对茶树体内铝钾镁分布及CsMATE9表达的影响. 硕士论文. 南京: 南京农业大学. pp. 25-41. |
| [3] | 丁海燕, 温丹妮, 王苗全, 钱海丰 (2013). 水稻抗铝机制的研究进展. 生命科学 25, 532-537. |
| [4] | 董碧莹 (2019). 木豆MATE基因抗金属胁迫作用解析. 硕士论文. 北京: 北京林业大学. pp. 11-21. |
| [5] |
高铭, 周思莹, 李钧儒, 石卓, 郭长虹, 郭东林 (2021). 紫花苜蓿MsMATE1基因克隆及其抵御铁胁迫功能的鉴定. 植物遗传资源学报 22, 512-520.
DOI |
| [6] | 龚莉 (2020). GmMATE13在铝诱导大豆根系柠檬酸分泌中的作用分析. 硕士论文. 长春: 吉林大学. pp. 9-37. |
| [7] | 胡俊, 刘卢生, 韩蓉蓉, 魏运民, 汪莹, 蒋曹德, 玉永雄 (2019). 丹波黑大豆GmMATE2基因的克隆及功能鉴定. 农业生物技术学报 27, 1161-1170. |
| [8] | 李洪有, 钟长春, 蔡芳, 霍冬敖, 张晓娜, 陈庆富 (2018). 甜荞柠檬酸转运蛋白基因FeFRD3的克隆及表达分析. 西北植物学报 38, 409-415. |
| [9] | 梁俊超, 孙建, 颜廷献, 乐美旺, 饶月亮, 颜小文, 周红英 (2021). 芝麻MATE基因家族的全基因组鉴定与表达分析. 基因组学与应用生物学 40, 735-746. |
| [10] | 刘青松 (2012). 香橙CjMATE基因的克隆与功能初步分析. 硕士论文. 重庆: 西南大学. pp. 25. |
| [11] | 蒲时 (2020). 雷公藤跨膜转运蛋白TwMATE2、TwMATE3克隆与表达分析. 硕士论文. 杨凌: 西北农林科技大学. pp. 2-43. |
| [12] | 王碧莹 (2018). 长春花转运基因CrMATE1的基因克隆及功能鉴定. 硕士论文. 大连: 大连工业大学. pp. 33-53. |
| [13] | 王甲水 (2010). MaMATE1基因功能的初步研究. 硕士论文. 海口: 海南大学. pp. 55. |
| [14] | 王璐, 戴思兰, 金雪花, 黄河, 洪艳 (2014). 植物花青素苷转运机制的研究进展. 生物工程学报 30, 848-863. |
| [15] | 王玉琪 (2021). 桑树多药与毒素排出蛋白MulMATE的功能及作用机制研究. 硕士论文. 泰安: 山东农业大学. pp. 35-44. |
| [16] | 文开新, 王成章, 严学兵, 吴鹏举, 李振田 (2010). 黄酮类化合物生物学活性研究进展. 草业科学 27(6), 115-122. |
| [17] | 吴楠 (2014). 棕色棉纤维原花青素转运蛋白基因GhTT12的克隆与表达分析. 硕士论文. 合肥: 安徽农业大学. pp. 23-36. |
| [18] | 吴平治, 栾升, 李东屏 (2006). 拟南芥中MATE基因家族的研究进展. 遗传 28, 906-910. |
| [19] | 吴新新 (2014). 甘蓝耐铝基因MATE和ALMT克隆与功能验证. 博士论文. 北京: 中国农业大学. pp. 8-30. |
| [20] |
谢玲玲, 王金龙, 伍国强 (2021). 植物CBL-CIPK信号系统响应非生物胁迫的调控机制. 植物学报 56, 614-626.
DOI |
| [21] | 张宝云 (2017). 紫花苜蓿铝胁迫响应基因MsMATE与MsSTOP1的功能与表达调控研究. 博士论文. 重庆: 重庆大学. pp. 93-96. |
| [22] | 张凡 (2012). 柿单宁及花青苷跨膜相关基因的克隆与表达分析. 硕士论文. 武汉: 华中农业大学. pp. 28-48. |
| [23] | 张郎织, 李季肤, 黄杰, 王志勇, 陈志坚 (2021). 地毯草AcMATE1基因的克隆与表达分析. 热带作物学报 42, 1860-1867. |
| [24] |
张艳艳, 王倩, 李晓旭, 孙亭亭, 龚达平, 杨明磊, 刘贯山 (2015). 普通烟草MATE基因家族分析及功能预测. 植物遗传资源学报 16, 1307-1314.
DOI |
| [25] | 张玉倩 (2018). 文多灵在大鼠体内外代谢及对细胞色素P450酶活性影响的研究. 硕士论文. 石家庄: 河北医科大学. pp. 82-83. |
| [26] |
Baetz U, Martinoia E (2014). Root exudates: the hidden part of plant defense. Trends Plant Sci 19, 90-98.
DOI PMID |
| [27] |
Bashir K, Ishimaru Y, Shimo H, Kakei Y, Senoura T, Takahashi R, Sato Y, Sato Y, Uozumi N, Nakanishi H, Nishizawa NK (2011). Rice phenolics efflux transporter 2 (PEZ2) plays an important role in solubilizing apoplasmic iron. Soil Sci Plant Nutr 57, 803-812.
DOI URL |
| [28] |
Blanco P, Hernando-Amado S, Reales-Calderon JA, Corona F, Lira F, Alcalde-Rico M, Bernardini A, Sanchez MB, Martinez JL (2016). Bacterial multidrug efflux pumps: much more than antibiotic resistance determinants. Microorganisms 4, 14.
DOI URL |
| [29] | Briat JF, Lebrun M (1999). Plant responses to metal toxicity. C R Acad Sci III 322, 43-54. |
| [30] |
Brown MH, Paulsen IT, Skurray RA (1999). The multidrug efflux protein NorM is a prototype of a new family of transporters. Mol Microbiol 31, 394-395.
DOI PMID |
| [31] |
Chai YR, Lei B, Huang HL, Li JN, Yin JM, Tang ZL, Wang R, Chen L (2009). TRANSPARENT TESTA 12 genes from Brassica napus and parental species: cloning, evolution, and differential involvement in yellow seed trait. Mol Genet Genom 281, 109-123.
DOI URL |
| [32] | Chen L, Liu YS, Liu HD, Kang LM, Geng JM, Gai YZ, Ding YL, Sun HY, Li YD (2015). Identification and expression analysis of MATE genes involved in flavonoid transport in blueberry plants. PLoS One 10, e0118578. |
| [33] |
Chen SY, Tang YM, Hu YY, Wang Y, Sun B, Wang XR, Tang HR, Chen Q (2018). FaTT12-1, a multidrug and toxin extrusion (MATE) member involved in proanthocyanidin transport in strawberry fruits. Sci Hortic 231, 158-165.
DOI URL |
| [34] |
Coleman J, Blake-Kalff M, Davies E (1997). Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci 2, 144-151.
DOI URL |
| [35] |
Curie C, Alonso JM, Le Jean M, Ecker JR, Briat JF (2000). Involvement of NRAMP1 from Arabidopsis thaliana in iron transport. Biochem J 347, 749-755.
DOI URL |
| [36] |
De Angeli A, Zhang JB, Meyer S, Martinoia E (2013). AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat Commun 4, 1804.
DOI |
| [37] |
Delhaize E, Craig S, Beaton CD, Bennet RH, Jagadish VC, Randall PJ (1993). Aluminum tolerance in wheat (Triticum aestivum L.) (I. Uptake and distribution of aluminum in root apices). Plant Physiol 103, 685-693.
PMID |
| [38] |
Delhaize E, Ma JF, Ryan PR (2012). Transcriptional regulation of aluminium tolerance genes. Trends Plant Sci 17, 341-348.
DOI PMID |
| [39] |
Diédhiou CJ, Golldack D (2006). Salt-dependent regulation of chloride channel transcripts in rice. Plant Sci 170, 793-800.
DOI URL |
| [40] |
Diener AC, Gaxiola RA, Fink GR (2001). Arabidopsis ALF5, a multidrug efflux transporter gene family member, confers resistance to toxins. Plant Cell 13, 1625-1638.
DOI PMID |
| [41] |
Dobritzsch M, Lübken T, Eschen-Lippold L, Gorzolka K, Blum E, Matern A, Marillonnet S, Böttcher C, Dräger B, Rosahl S (2016). MATE transporter-dependent export of hydroxycinnamic acid amides. Plant Cell 28, 583-596.
DOI URL |
| [42] |
Durrett TP, Gassmann W, Rogers EE (2007). The FRD3- mediated efflux of citrate into the root vasculature is necessary for efficient iron translocation. Plant Physiol 144, 197-205.
DOI URL |
| [43] |
Frank S, Keck M, Sagasser M, Niehaus K, Weisshaar B, Stracke R (2011). Two differentially expressed MATE factor genes from apple complement the Arabidopsis transparent testa 12 mutant. Plant Biol 13, 42-50.
DOI URL |
| [44] |
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007). An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48, 1081-1091.
DOI PMID |
| [45] |
Gomez C, Terrier N, Torregrosa L, Vialet S, Fournier- Level A, Verriès C, Souquet JM, Mazauric JP, Klein M, Cheynier V, Ageorges A (2009). Grapevine MATE-type proteins act as vacuolar H+-dependent acylated anthocyanin transporters. Plant Physiol 150, 402-415.
DOI PMID |
| [46] |
Green LS, Rogers EE (2004). FRD3 controls iron localization in Arabidopsis. Plant Physiol 136, 2523-2531.
DOI URL |
| [47] |
Harada H, Kuromori T, Hirayama T, Shinozaki K, Leigh RA (2004). Quantitative trait loci analysis of nitrate storage in Arabidopsis leading to an investigation of the contribution of the anion channel gene, AtCLC-c, to variation in nitrate levels. J Exp Bot 55, 2005-2014.
DOI URL |
| [48] |
He X, Szewczyk P, Karyakin A, Evin M, Hong WX, Zhang QH, Chang G (2010). Structure of a cation-bound multidrug and toxic compound extrusion transporter. Nature 467, 991-994.
DOI |
| [49] |
Hoang MTT, Almeida D, Chay S, Alcon C, Corratge-Fail- alie C, Curie C, Mari S (2021). AtDTX25 a member of the multidrug and toxic compound extrusion family, is a vacuolar ascorbate transporter that controls intracellular iron cycling in Arabidopsis. New Phytol 231, 1956-1967.
DOI URL |
| [1] |
Ishimaru Y, Suzuki M, Tsukamoto T, Suzuki K, Nakazono M, Kobayashi T, Wada Y, Watanabe S, Matsuhashi S, Takahashi M, Nakanishi H, Mori S, Nishizawa NK (2006). Rice plants take up iron as an Fe3+-phytosiderophore and as Fe2+. Plant J 45, 335-346.
DOI PMID |
| [2] |
Kuroda T, Tsuchiya T (2009). Multidrug efflux transporters in the MATE family. Biochim Biophys Acta 1794, 763-768.
DOI PMID |
| [3] |
Kusakizako T, Claxton DP, Tanaka Y, Maturana AD, Kuroda T, Ishitani R, Mchaourab HS, Nureki O (2019). Structural basis of H+-dependent conformational change in a bacterial MATE transporter. Structure 27, 293-301.
DOI PMID |
| [4] |
Leung J, Giraudat J (1998). Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49, 199-222.
DOI URL |
| [5] |
Li LG, He ZY, Pandey GK, Tsuchiya T, Luan S (2002). Functional cloning and characterization of a plant efflux carrier for multidrug and heavy metal detoxification. J Biol Chem 277, 5360-5368.
DOI PMID |
| [6] | Li RX, Li JR, Li SB, Qin GJ, Novák O, Pěnčík A, Ljung K, Aoyama T, Liu JJ, Murphy A, Gu HY, Tsuge T, Qu LJ (2014). ADP1 affects plant architecture by regulating local auxin biosynthesis. PLoS Genet 10, e1003954. |
| [7] |
Li WYF, Wong FL, Tsai SN, Phang TH, Shao GH, Lam HM (2006). Tonoplast-located GmCLC1 and GmNHX1 from soybean enhance NaCl tolerance in transgenic bright yellow (BY)-2 cells. Plant Cell Environ 29, 1122-1137.
DOI URL |
| [8] |
Liu JP, Magalhaes JV, Shaff J, Kochian LV (2009). Aluminum-activated citrate and malate transporters from the MATE and ALMT families function independently to confer Arabidopsis aluminum tolerance. Plant J 57, 389-399.
DOI URL |
| [9] |
Liu MY, Lou HQ, Chen WW, Piñeros MA, Xu JM, Fan W, Kochian LV, Zheng SJ, Yang JL (2018). Two citrate transporters coordinately regulate citrate secretion from rice bean root tip under aluminum stress. Plant Cell Environ 41, 809-822.
DOI URL |
| [10] |
Lu M, Radchenko M, Symersky J, Nie RX, Guo Y (2013a). Structural insights into H+-coupled multidrug extrusion by a MATE transporter. Nat Struct Mol Biol 20, 1310-1317.
DOI |
| [11] |
Lu M, Symersky J, Radchenko M, Koide A, Guo Y, Nie RX, Koide S (2013b). Structures of a Na+-coupled, substrate-bound MATE multidrug transporter. Proc Natl Acad Sci USA 110, 2099-2104.
DOI URL |
| [12] |
Lv ZY, Zhao MM, Wang WJ, Wang Q, Huang MQ, Li CQ, Lian QC, Xia JQ, Qi J, Xiang CB, Tang HR, Ge XC (2021). Changing Gly311 to an acidic amino acid in the MATE family protein DTX6 enhances Arabidopsis resistance to the dihydropyridine herbicides. Mol Plant 14, 2115-2125.
DOI URL |
| [13] |
Magalhaes JV, Liu JP, Guimarães CT, Lana UGP, Alves VMC, Wang YH, Schaffert RE, Hoekenga OA, Piñeros MA, Shaff JE, Klein PE, Carneiro NP, Coelho CM, Trick HN, Kochian LV (2007). A gene in the multidrug and toxic compound extrusion (MATE) family confers aluminum tolerance in sorghum. Nat Genet 39, 1156-1161.
DOI PMID |
| [14] |
Marinova K, Pourcel L, Weder B, Schwarz M, Barron D, Routaboul JM, Debeaujon I, Klein M (2007). The Arabidopsis MATE transporter TT12 acts as a vacuolar flavonoid/H+-antiporter active in proanthocyanidin-accumulating cells of the seed coat. Plant Cell 19, 2023-2038.
DOI URL |
| [15] |
Maron LG, Piñeros MA, Guimarães CT, Magalhaes JV, Pleiman JK, Mao CZ, Shaff J, Belicuas SNJ, Kochian LV (2010). Two functionally distinct members of the MATE (multi-drug and toxic compound extrusion) family of transporters potentially underlie two major aluminum tolerance QTLs in maize. Plant J 61, 728-740.
DOI URL |
| [16] |
Meyer S, Scholz-Starke J, De Angeli A, Kovermann P, Burla B, Gambale F, Martinoia E (2011). Malate transport by the vacuolar AtALMT6 channel in guard cells is subject to multiple regulation. Plant J 67, 247-257.
DOI URL |
| [17] |
Miyauchi H, Moriyama S, Kusakizako T, Kumazaki K, Nakane T, Yamashita K, Hirata K, Dohmae N, Nishizawa T, Ito K, Miyaji T, Moriyama Y, Ishitani R, Nureki O (2017). Structural basis for xenobiotic extrusion by eukaryotic MATE transporter. Nat Commun 8, 1633.
DOI PMID |
| [18] |
Morita Y, Kodama K, Shiota S, Mine T, Kataoka A, Mizushima T, Tsuchiya T (1998). NorM, a putative multidrug efflux protein, of Vibrio parahaemolyticus and its homolog in Escherichia coli. Antimicrob Agents Chemother 42, 1778-1782.
DOI PMID |
| [19] |
Morrissey J, Guerinot ML (2009). Iron uptake and transport in plants: the good, the bad, and the ionome. Chem Rev 109, 4553-4567.
DOI PMID |
| [20] |
Nakamura A, Fukuda A, Sakai S, Tanaka Y (2006). Molecular cloning, functional expression and subcellular localization of two putative vacuolar voltage-gated chloride channels in rice (Oryza sativa L.). Plant Cell Physiol 47, 32-42.
DOI PMID |
| [21] |
Nawrath C, Heck S, Parinthawong N, Métraux JP (2002). EDS5, an essential component of salicylic acid-dependent signaling for disease resistance in Arabidopsis, is a member of the MATE transporter family. Plant Cell 14, 275-286.
DOI URL |
| [22] |
Pan YL, Liu ZY, Rocheleau H, Fauteux F, Wang YL, McCartney C, Ouellet T (2018). Transcriptome dynamics associated with resistance and susceptibility against fusarium head blight in four wheat genotypes. BMC Genomics 19, 642.
DOI PMID |
| [23] |
Poole K (2000). Efflux-mediated resistance to fluoroquinolones in gram-negative bacteria. Antimicrob Agents Chemother 44, 2233-2241.
DOI PMID |
| [24] | Qin P, Zhang GH, Hu BH, Wu J, Chen WL, Ren ZJ, Liu YL, Xie J, Yuan H, Tu B, Ma BT, Wang YP, Ye LM, Li LG, Xiang CB, Li SG (2021). Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 7, eabc8873. |
| [25] |
Qiu W, Wang NQ, Dai J, Wang TQ, Kochian LV, Liu JP, Zuo YM (2019). AhFRDL1-mediated citrate secretion contributes to adaptation to iron deficiency and aluminum stress in peanuts. J Exp Bot 70, 2873-2886.
DOI PMID |
| [26] |
Rangel AF, Rao IM, Braun HP, Horst WJ (2010). Aluminum resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiol Plant 138, 176-190.
DOI PMID |
| [27] |
Ren ZJ, Bai FL, Xu JW, Wang L, Wang XH, Zhang Q, Feng CX, Niu Q, Zhang LY, Song JL, Bao F, Liu LY, He YK, Ma LG, Tian W, Hou CC, Li LG (2021). A chloride efflux transporter, big rice grain 1, is involved in mediating grain size and salt tolerance in rice. J Integr Plant Biol 63, 2150-2163.
DOI |
| [28] |
Rogers EE, Guerinot ML (2002). FRD3, a member of the multidrug and toxin efflux family, controls iron deficiency responses in Arabidopsis. Plant Cell 14, 1787-1799.
DOI URL |
| [29] |
Rogers EE, Wu XL, Stacey G, Nguyen HT (2009). Two MATE proteins play a role in iron efficiency in soybean. J Plant Physiol 166, 1453-1459.
DOI URL |
| [30] |
Roschzttardtz H, Conéjéro G, Divol F, Alcon C, Verdeil JL, Curie C, Mari S (2013). New insights into Fe localization in plant tissues. Front Plant Sci 4, 350.
DOI PMID |
| [31] |
Sasaki T, Yamamoto Y, Ezaki B, Katsuhara M, Ahn SJ, Ryan PR, Delhaize E, Matsumoto H (2004). A wheat gene encoding an aluminum-activated malate transporter. Plant J 37, 645-653.
DOI PMID |
| [32] |
Serrano M, Wang BJ, Aryal B, Garcion C, Abou-Mansour E, Heck S, Geisler M, Mauch F, Nawrath C, Métraux JP (2013). Export of salicylic acid from the chloroplast requires the multidrug and toxin extrusion-like transporter EDS5. Plant Physiol 162, 1815-1821.
DOI PMID |
| [33] |
Shitan N, Bazin I, Dan K, Obata K, Kigawa K, Ueda K, Sato F, Forestier C, Yazaki K (2003). Involvement of CjMDR1, a plant multidrug-resistance-type ATP-binding cassette protein, in alkaloid transport in Coptis japonica. Proc Natl Acad Sci USA 100, 751-756.
DOI URL |
| [34] |
Shitan N, Dalmas F, Dan K, Kato N, Ueda K, Sato F, Forestier C, Yazaki K (2013). Characterization of Coptis japonica CjABCB2, an ATP-binding cassette protein involved in alkaloid transport. Phytochemistry 91, 109-116.
DOI URL |
| [35] | Shitan N, Minami S, Morita M, Hayashida M, Ito S, Takanashi K, Omote H, Moriyama Y, Sugiyama A, Goossens A, Moriyasu M, Yazaki K (2014). Involvement of the leaf-specific multidrug and toxic compound extrusion (MATE) transporter Nt-JAT2 in vacuolar sequestration of nicotine in Nicotiana tabacum. PLoS One 9, e108789. |
| [36] |
Shoji T, Inai K, Yazaki Y, Sato Y, Takase H, Shitan N, Yazaki K, Goto Y, Toyooka K, Matsuoka K, Hashimoto T (2009). Multidrug and toxic compound extrusion-type transporters implicated in vacuolar sequestration of nicotine in tobacco roots. Plant Physiol 149, 708-718.
DOI PMID |
| [37] |
Sun XL, Gilroy EM, Chini A, Nurmberg PL, Hein I, Lacomme C, Birch PRJ, Hussain A, Yun BW, Loake GJ (2011). ADS1 encodes a MATE-transporter that negatively regulates plant disease resistance. New Phytol 192, 471-482.
DOI URL |
| [38] | Takanashi K, Shitan N, Yazaki K (2014). The multidrug and toxic compound extrusion (MATE) family in plants. Plant Biotechnol J 31, 417-430. |
| [39] |
Takahashi M, Terada Y, Nakai I, Nakanishi H, Yoshimura E, Mori S, Nishizawa NK (2003). Role of nicotianamine in the intracellular delivery of metals and plant reproductive development. Plant Cell 15, 1263-1280.
PMID |
| [40] |
Tanaka Y, Iwaki S, Tsukazaki T (2017). Crystal structure of a plant multidrug and toxic compound extrusion family protein. Structure 25, 1455-1460.
DOI PMID |
| [41] |
Tiwari M, Sharma D, Singh M, Tripathi RD, Trivedi PK (2014). Expression of OsMATE1 and OsMATE2 alters development, stress responses and pathogen susceptibility in Arabidopsis. Sci Rep 4, 3964.
DOI |
| [42] |
Upadhyay N, Kar D, Deepak Mahajan B, Nanda S, Rahiman R, Panchakshari N, Bhagavatula L, Datta S (2019). The multitasking abilities of MATE transporters in plants. J Exp Bot 70, 4643-4656.
DOI PMID |
| [43] |
Wang R, Liu XY, Liang S, Ge Q, Li YF, Shao JX, Qi YF, An LJ, Yu F (2015). A subgroup of MATE transporter genes regulates hypocotyl cell elongation in Arabidopsis. J Exp Bot 66, 6327-6343.
DOI PMID |
| [44] |
Xia JQ, Nazish T, Javaid A, Ali M, Liu QQ, Wang L, Zhang ZY, Zhang ZS, Huang YJ, Wu J, Yang ZS, Sun LF, Chen YX, Xiang CB (2021). A gain-of-function mutation of the MATE family transporter DTX6 confers paraquat resistance in Arabidopsis. Mol Plant 14, 2126-2133.
DOI URL |
| [45] |
Yokosho K, Yamaji N, Ma JF (2010). Isolation and characterisation of two MATE genes in rye. Funct Plant Biol 37, 296-303.
DOI URL |
| [46] |
Yokosho K, Yamaji N, Ma JF (2011). An Al-inducible MATE gene is involved in external detoxification of Al in rice. Plant J 68, 1061-1069.
DOI URL |
| [47] |
Yokosho K, Yamaji N, Ueno D, Mitani N, Ma JF (2009). OsFRDL1 is a citrate transporter required for efficient translocation of iron in rice. Plant Physiol 149, 297-305.
DOI PMID |
| [48] |
Yu F, De Luca V (2013). ATP-binding cassette transporter controls leaf surface secretion of anticancer drug components in Catharanthus roseus. Proc Natl Acad Sci USA 110, 15830-15835.
DOI URL |
| [49] |
Yu YQ, Assmann SM (2014). Metabolite transporter regulation of ABA function and guard cell response. Mol Plant 7, 1505-1507.
DOI PMID |
| [50] | Zhang HW, Zhao FG, Tang RJ, Yu YX, Song JL, Wang Y, Li LG, Luan S (2017). Two tonoplast MATE proteins function as turgor-regulating chloride channels in Arabidopsis. Proc Natl Acad Sci USA 114, E2036-E2045. |
| [51] |
Zhang HW, Zhu HF, Pan YJ, Yu YX, Luan S, Li LG (2014). A DTX/MATE-type transporter facilitates abscisic acid efflux and modulates ABA sensitivity and drought tolerance in Arabidopsis. Mol Plant 7, 1522-1532.
DOI URL |
| [52] |
Zhao J, Dixon RA (2009). MATE transporters facilitate vacuolar uptake of epicatechin 3'-O-glucoside for proanthocyanidin biosynthesis in Medicago truncatula and Arabidopsis. Plant Cell 21, 2323-2340.
DOI URL |
| [53] |
Zhao J, Dixon RA (2010). The ‘ins’ and ‘outs’ of flavonoid transport. Trends Plant Sci 15, 72-80.
DOI URL |
| [54] |
Zhao J, Huhman D, Shadle G, He XZ, Sumner LW, Tang YH, Dixon RA (2011). MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in Medicago truncatula. Plant Cell 23, 1536-1555.
DOI URL |
| [55] |
Zifarelli G, Pusch M (2010). CLC transport proteins in plants. FEBS Lett 584, 2122-2127.
DOI PMID |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||