

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (5): 634-641.DOI: 10.11983/CBB18193 cstr: 32102.14.CBB18193
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Ying Feng1,*( ),Lianwen Qian1,Qingliang Lin2
),Lianwen Qian1,Qingliang Lin2
Received:2018-09-11
Accepted:2019-02-19
Online:2019-09-01
Published:2020-03-10
Contact:
Ying Feng
Ying Feng,Lianwen Qian,Qingliang Lin. The Effect of Different Hormones on Explant Browning and Callus Browning in Cyclocarya paliurus[J]. Chinese Bulletin of Botany, 2019, 54(5): 634-641.
| Treatment | 6-BA content (mg·L-1) | GA3 content (mg·L-1) | NAA content (mg·L-1) |
|---|---|---|---|
| 6-BA1 | 1.0 | 1.0 | 0.3 |
| 6-BA2 | 2.0 | 1.0 | 0.3 |
| 6-BA3 | 3.0 | 1.0 | 0.3 |
| GA31 | 1.0 | 0.0 | 0.3 |
| GA32 | 1.0 | 0.5 | 0.3 |
| GA33 | 1.0 | 1.0 | 0.3 |
| GA34 | 1.0 | 1.5 | 0.3 |
| NAA1 | 1.0 | 1.0 | 0.0 |
| NAA2 | 1.0 | 1.0 | 0.1 |
| NAA3 | 1.0 | 1.0 | 0.3 |
| NAA4 | 1.0 | 1.0 | 0.5 |
Table 1 6-BA+GA3+NAA hormone combination
| Treatment | 6-BA content (mg·L-1) | GA3 content (mg·L-1) | NAA content (mg·L-1) |
|---|---|---|---|
| 6-BA1 | 1.0 | 1.0 | 0.3 |
| 6-BA2 | 2.0 | 1.0 | 0.3 |
| 6-BA3 | 3.0 | 1.0 | 0.3 |
| GA31 | 1.0 | 0.0 | 0.3 |
| GA32 | 1.0 | 0.5 | 0.3 |
| GA33 | 1.0 | 1.0 | 0.3 |
| GA34 | 1.0 | 1.5 | 0.3 |
| NAA1 | 1.0 | 1.0 | 0.0 |
| NAA2 | 1.0 | 1.0 | 0.1 |
| NAA3 | 1.0 | 1.0 | 0.3 |
| NAA4 | 1.0 | 1.0 | 0.5 |
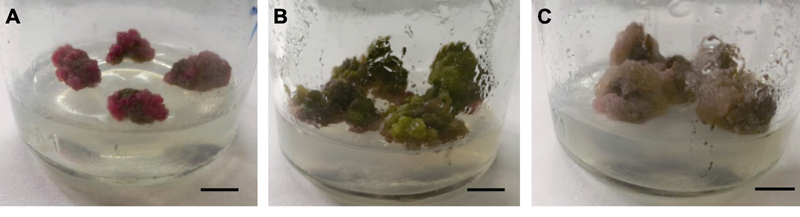
Figure 1 The type of callus induced from leaves of Cyclocarya paliurus (A) Callus of the type I; (B) Callus of the type II; (C) Callus of the type III. Bars=1 cm
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 100.00±0.00 a | 0.00±0.00 a | I, II | I (++), II (+) |
| 2.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 95.24±8.25 a | 4.76±8.25 b | I, II | I (+++), II (++) |
| 3.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 66.67±18.04 b | 33.33±18.04 b | I, III | I (++), III (+) |
Table 2 The effect of 6-BA on leaf browning in Cyclocarya paliurus (means±SD)
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 100.00±0.00 a | 0.00±0.00 a | I, II | I (++), II (+) |
| 2.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 95.24±8.25 a | 4.76±8.25 b | I, II | I (+++), II (++) |
| 3.0 mg·L-1 6-BA+1.0 mg·L-1 GA3+0.3 mg·L-1 NAA | 66.67±18.04 b | 33.33±18.04 b | I, III | I (++), III (+) |
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 48.96±4.77 c | 51.04±4.77 a | I, III | I (++), III (+) |
| 0.5 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 86.67±10.41 b | 13.33±10.41 b | I, II, III | I (++), II (+), III (-) |
| 1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 100.00±0.00 a | 0.00±0.00 c | I, II | I (++), II (+) |
| 1.5 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 86.11±2.78 b | 13.89±2.78 b | I | I (++) |
Table 3 The effect of GA3 on leaf browning in Cyclocarya paliurus (means±SD)
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 48.96±4.77 c | 51.04±4.77 a | I, III | I (++), III (+) |
| 0.5 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 86.67±10.41 b | 13.33±10.41 b | I, II, III | I (++), II (+), III (-) |
| 1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 100.00±0.00 a | 0.00±0.00 c | I, II | I (++), II (+) |
| 1.5 mg·L-1 GA3+1.0 mg·L-1 6-BA+0.3 mg·L-1 NAA | 86.11±2.78 b | 13.89±2.78 b | I | I (++) |
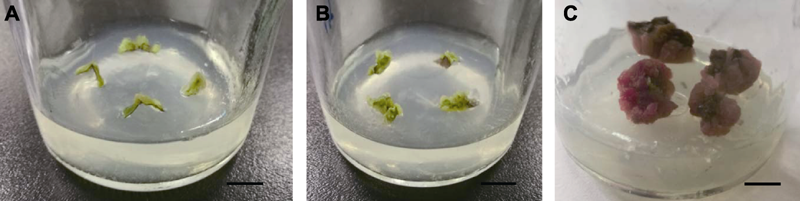
Figure 2 The effect of GA3 on callus induction of leaves in Cyclocarya paliurus (A) Callus in the induction medium without GA3; (B) Callus cultured for 15 days in the induction medium with GA3; (C) Callus cultured for 30 days in the induction medium with GA3. Bars=1 cm
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 23.15±11.25 c | 76.85±11.25 a | I | I (-) |
| 0.1 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 71.39±7.01 b | 18.61±7.01 b | I, II | I (++), II (+) |
| 0.3 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 100.00±0.00 a | 0.00±0.00 c | I, II | I (++), II (+) |
| 0.5 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 96.99±0.20 a | 3.11±0.20 c | I, II | I (+), II (+) |
Table 4 The effect of NAA on leaf browning in Cyclocarya paliurus (means±SD)
| Medium | The rate of callus induction (%) | The rate of leaf browning (%) | Callus | |
|---|---|---|---|---|
| Type | Growth | |||
| 1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 23.15±11.25 c | 76.85±11.25 a | I | I (-) |
| 0.1 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 71.39±7.01 b | 18.61±7.01 b | I, II | I (++), II (+) |
| 0.3 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 100.00±0.00 a | 0.00±0.00 c | I, II | I (++), II (+) |
| 0.5 mg·L-1 NAA+1.0 mg·L-1 GA3+1.0 mg·L-1 6-BA | 96.99±0.20 a | 3.11±0.20 c | I, II | I (+), II (+) |
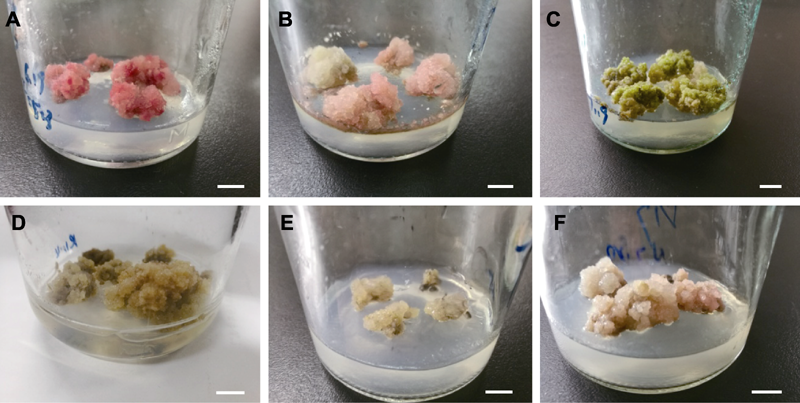
Figure 3 The type of callus propagation in Cyclocarya paliurus (A), (B) The callus of type I; (C), (D) The callus of type II; (E), (F) The callus of type III. Bars=1 cm
| Plant growth regulator (mg·L-1) | The time of callus propagation (time) | The rate of callus browning (%) | Callus | ||
|---|---|---|---|---|---|
| 6-BA | NAA | Type | Growth | ||
| 0.2 | 0.05 | 1.25 de | 0 e | II | II (+++) |
| 0.5 | 0.05 | 3.87 b | 0 e | I, II | I (+++), II (++) |
| 0.8 | 0.05 | 3.51 b | 0 e | I, II | I (++), II (+++) |
| 1.0 | 0.05 | 0.69 e | 0 e | I, III | I (++), III (+) |
| 0.2 | 0.2 | 0.75 e | 0 e | I, II, III | I (+), II (+), III (+) |
| 0.5 | 0.2 | 4.80 a | 0 e | I, II | I (++), II (+++) |
| 0.8 | 0.2 | 3.55 b | 0 e | I, II, III | I (++), II (+++), III (++++) |
| 1.0 | 0.2 | 1.30 de | 40.00 a | I, II | I (++), II (+) |
| 0.2 | 0.4 | 0.97 de | 33.33 ab | I, III | I (+), III (+) |
| 0.5 | 0.4 | 1.65 d | 28.33 bc | II, III | II (+), III (+) |
| 0.8 | 0.4 | 2.57 c | 20.00 d | II, III | II (++), III (+) |
| 1.0 | 0.4 | 1.75 d | 21.67 cd | II, III | II (+), III (+) |
Table 5 The effect of 6-BA+NAA on callus browning in Cyclocarya paliurus
| Plant growth regulator (mg·L-1) | The time of callus propagation (time) | The rate of callus browning (%) | Callus | ||
|---|---|---|---|---|---|
| 6-BA | NAA | Type | Growth | ||
| 0.2 | 0.05 | 1.25 de | 0 e | II | II (+++) |
| 0.5 | 0.05 | 3.87 b | 0 e | I, II | I (+++), II (++) |
| 0.8 | 0.05 | 3.51 b | 0 e | I, II | I (++), II (+++) |
| 1.0 | 0.05 | 0.69 e | 0 e | I, III | I (++), III (+) |
| 0.2 | 0.2 | 0.75 e | 0 e | I, II, III | I (+), II (+), III (+) |
| 0.5 | 0.2 | 4.80 a | 0 e | I, II | I (++), II (+++) |
| 0.8 | 0.2 | 3.55 b | 0 e | I, II, III | I (++), II (+++), III (++++) |
| 1.0 | 0.2 | 1.30 de | 40.00 a | I, II | I (++), II (+) |
| 0.2 | 0.4 | 0.97 de | 33.33 ab | I, III | I (+), III (+) |
| 0.5 | 0.4 | 1.65 d | 28.33 bc | II, III | II (+), III (+) |
| 0.8 | 0.4 | 2.57 c | 20.00 d | II, III | II (++), III (+) |
| 1.0 | 0.4 | 1.75 d | 21.67 cd | II, III | II (+), III (+) |
| Plant growth regulator | The time of callus propagation (time) | The rate of callus browning (%) |
|---|---|---|
| 0.2 mg·L-1 6-BA | 0.98±0.33 b | 11.11±17.64 b |
| 0.5 mg·L-1 6-BA | 3.44±1.47 a | 9.44±15.09 b |
| 0.8 mg·L-1 6-BA | 3.21±0.67 a | 6.67±10.00 b |
| 1.0 mg·L-1 6-BA | 1.25±0.55 b | 20.56±17.40 a |
| 0.05 mg·L-1 NAA | 2.33±1.49 a | 0.00±0.00 c |
| 0.2 mg·L-1 NAA | 2.59±1.75 a | 10.00±18.09 b |
| 0.4 mg·L-1 NAA | 1.73±0.74 b | 25.83±8.75 a |
Table 6 The effect of 6-BA and NAA on callus browning in Cyclocarya paliurus, respectively (means±SD)
| Plant growth regulator | The time of callus propagation (time) | The rate of callus browning (%) |
|---|---|---|
| 0.2 mg·L-1 6-BA | 0.98±0.33 b | 11.11±17.64 b |
| 0.5 mg·L-1 6-BA | 3.44±1.47 a | 9.44±15.09 b |
| 0.8 mg·L-1 6-BA | 3.21±0.67 a | 6.67±10.00 b |
| 1.0 mg·L-1 6-BA | 1.25±0.55 b | 20.56±17.40 a |
| 0.05 mg·L-1 NAA | 2.33±1.49 a | 0.00±0.00 c |
| 0.2 mg·L-1 NAA | 2.59±1.75 a | 10.00±18.09 b |
| 0.4 mg·L-1 NAA | 1.73±0.74 b | 25.83±8.75 a |
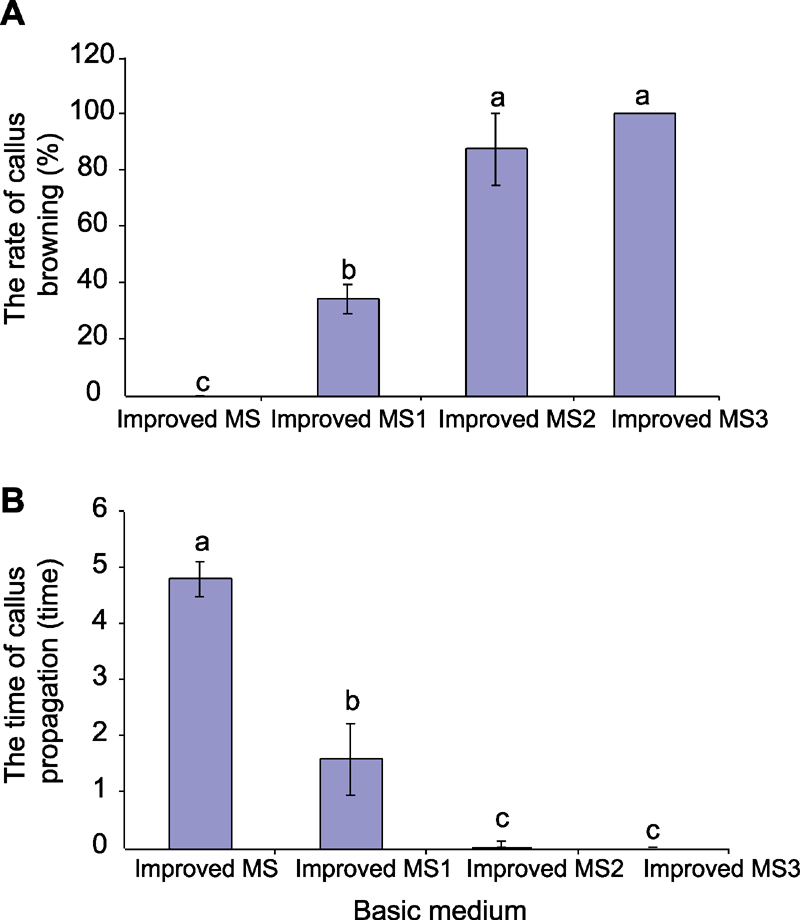
Figure 4 The effect of basic medium on callus browning (A) and callus propagation (B) in Cyclocarya paliurus (means±SD) Different lowercase letters indicate significant differences at P<0.05.
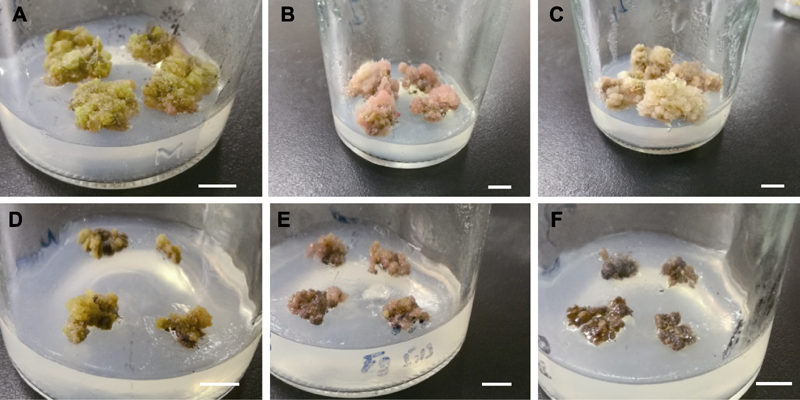
Figure 5 Callus of Cyclocarya paliurus growth in the different basic medium (A) Callus growth in the improved MS basic medium; (B), (C) Callus growth in the improved MS1 basic medium; (D), (E) Callus growth in the improved MS2 basic medium; (F) Callus growth in the improved MS3 basic medium. Bars=1 cm
| 1 | 陈雪, 张金柱, 潘兵兵, 桑成瑾, 马雪, 杨涛, 车代弟 (2011). 月季愈伤组织的诱导及植株再生. 植物学报 46, 569-574. |
| 2 | 方升佐, 杨万霞 (2003). 青钱柳的开发利用与资源培育. 林业科技开发 17, 49-51. |
| 3 | 侯建华, 李正红, 马宏, 刘秀贤, 万友名 (2015). 地涌金莲组织培养中的褐化抑制. 林业科学研究 28, 217-221. |
| 4 | 郎玉涛, 罗晓芳 (2007). 牡丹愈伤组织的诱导及愈伤褐化抑制的研究. 河南林业科技 27, 4-6, 29. |
| 5 | 卢其能, 杨清 (2007). 激素等外源物质对马铃薯愈伤组织花色苷积累的影响. 西北植物学报 27, 2233-2239. |
| 6 | 鲁萌, 阮氏钏, 王纪, 方升佐 (2013). 青钱柳茎段腋芽的离体培养技术. 南京林业大学学报(自然科学版) 37(6), 6-10. |
| 7 | 任丽梅, 张洁, 赵立红, 王冬梅 (2008). 红叶石楠愈伤组织抗褐化研究以及悬浮细胞系的建立. 河北农业大学学报 31(5), 46-51. |
| 8 | 阮氏钏, 方升佐, 尚旭岚, 杨万霞 (2014). 青钱柳愈伤组织不定芽诱导技术. 南京林业大学学报(自然科学版) 38(2), 52-56. |
| 9 | 上官新晨, 郭春兰, 蒋艳, 沈勇根, 吴少福, 胡冬南 (2006). 培养基和植物激素对青钱柳茎段和叶片愈伤组织诱导的研究. 江西农业大学学报 28, 678-682. |
| 10 | 盛长忠, 王淑芳, 王宁宁, 王勇 (2001). 红豆杉愈伤组织培养中褐变现象的初探. 南开大学学报(自然科学版) 34(4), 120-122. |
| 11 | 舒任庚, 舒积成 (2007). 青钱柳中的酚类化学成分. 中草药 38, 507-508. |
| 12 | 唐利球, 唐君海, 陆祖正, 周婧 (2005). 金钱树愈伤组织的诱导及其褐化的防止. 广西热带农业 ( 3), 12-13. |
| 13 | 王纪, 谢寅峰, 方升佐 (2012). 青钱柳愈伤组织诱导与增殖的初步研究. 安徽农业科学 40, 1309-1312. |
| 14 | 吴群英, 徐庆, 李丽亚, 梁荣感, 龚受基 (2008). 青钱柳不同外植体组织培养及褐变防止的研究. 时珍国医国药 19, 1872-1874. |
| 15 | 肖莉杰, 王丽艳, 闵丽, 方淑梅, 韩毅强, 张红梅 (2011). 玉米成熟胚愈伤组织诱导及褐化控制研究. 玉米科学 19(4), 37-42. |
| 16 | 谢寅峰, 张志敏, 尚旭岚, 杨万霞, 王纪, 方升佐 (2011). 青钱柳茎段腋芽萌发和丛生芽增殖. 林业科学 47, 50-55. |
| 17 | 谢寅峰, 张志敏, 张颖颖, 李颖, 尚旭岚, 方升佐 (2015). 3种抗氧化剂对青钱柳愈伤组织褐化的影响. 安徽农业大学学报 42, 493-498. |
| 18 | 谢寅峰, 张志敏, 张颖颖, 孙朦, 尚旭岚, 方升佐 (2012). 青钱柳愈伤组织增殖. 东北林业大学学报 40(6), 16-18. |
| 19 | 杨文婷, 匡倩 (2016). 红豆杉组织培养的防褐变措施研究. 北方园艺 ( 17), 111-114. |
| 20 | 张文泉, 邓洁 (2016). 青钱柳叶片诱导愈伤组织研究. 内蒙古农业大学学报(自然科学版) 37(2), 28-33. |
| 21 | 赵伶俐, 葛红, 范崇辉, 印芳, 李秋香, 周玉杰 (2006). 不同光照强度对蝴蝶兰组培中外植体褐化的影响. 北方园艺 ( 4), 160-161. |
| 22 | 周凤, 于卉, 葛占宇, 周芝辉, 邓燕燕, 付永彩 (2010). 微量元素浓度对3个籼稻品种愈伤组织褐化和分化的影响. 农业生物技术学报 18, 702-706. |
| 23 | Creasy LL (1968). The increase in phenylalanine ammonia- lyase activity in strawberry leaf disks and its correlation with flavonoid synthesis. Phytochemistry 7, 441-446. |
| 24 | Fang SZ, Wang JY, Wei ZY, Zhu ZX (2006). Methods to break seed dormancy in Cyclocarya paliurus(Batal) Iljinskaja. Sci Hortic 110, 305-309. |
| 25 | Kumar S, Mangal M, Dhawan AK, Singh N (2013). Callus induction and plant regeneration from leaf explants of jojoba [Simmondsia chinensis(Link) Schneider]. India J Biotechnol 12, 544-547. |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zheng Guo, Xiangjun Shao, Haiwen Lu, Dan Hou, Simeng Kong, Xiangyu Li, Huaqian Liu, Xinchun Lin. Efficient Induction and Identification of Polyploids in Dendrocalamus asper [J]. Chinese Bulletin of Botany, 2025, 60(2): 246-255. |
| [3] | Xuemin Cao, Ying Bao, Yuexin Zhang, Ruijie Li, Jianxin Su, Wei Zhang. Tissue Culture, Rapid Propagation and Efficient Transient Expression Systems of Rosa multiflora [J]. Chinese Bulletin of Botany, 2025, 60(2): 235-245. |
| [4] | Yuchen Li, Haixia Zhao, Xiping Jiang, Xintian Huang, Yaling Liu, Zhenying Wu, Yan Zhao, Chunxiang Fu. Establishment of Agrobacterium-mediated Transformation System for Agropyron mongolicum [J]. Chinese Bulletin of Botany, 2024, 59(4): 600-612. |
| [5] | Xuping Tian, Kangjie Yue, Jiali Wang, Huixin Liu, Ziyin Shi, Hongwei Kang. Callus Induction and Plant Regeneration of Dracocephalum rupestre [J]. Chinese Bulletin of Botany, 2024, 59(4): 613-625. |
| [6] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [7] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [8] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [9] | ZHANG Jin-Yan, CUN Zhu, SHUANG Sheng-Pu, HONG Jie, MENG Zhen-Gui, CHEN Jun-Wen. Steady-state and dynamic photosynthetic characteristics of shade-tolerant species Panax notoginseng in response to nitrogen levels [J]. Chin J Plant Ecol, 2023, 47(3): 331-347. |
| [10] | Lulu Ren, Youze Zhang, Kelin Huang, Xiaochun Wan, Zhaoliang Zhang, Mulan Zhu, Chaoling Wei. An Efficient System for Regenerating Adventitious Buds in Stem Segments of Tea Plants [J]. Chinese Bulletin of Botany, 2023, 58(2): 308-315. |
| [11] | Lü Xiuli, Yu Zequn, Chen Xiangbo, Fu Renjie, Miao Shanshan, Du An. Rapid Propagation Technology and Field Production of Hemerocallis fulva cv. ‘Fenmeiren’ [J]. Chinese Bulletin of Botany, 2022, 57(3): 350-357. |
| [12] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [13] | Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum [J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235. |
| [14] | Yaqian Xiong, Xianbao Deng, Huihui Zhang, Dong Yang, Heng Sun, Juan Liu, Mei Yang. In Vitro Rapid Propagation of Nelumbo nucifera [J]. Chinese Bulletin of Botany, 2021, 56(5): 605-613. |
| [15] | Yanmin Li, Hui Jiang, Zhenzhu Fu, Jing Zhang, Xin Yuan, Huijuan Wang, Jie Gao, Xiaoyu Dong, Limin Wang, Hechen Zhang. Callus Induction and Somatic Embryogenesis in Anther Culture of Paeonia lactiflora [J]. Chinese Bulletin of Botany, 2021, 56(4): 443-450. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||