

Chinese Bulletin of Botany ›› 2023, Vol. 58 ›› Issue (2): 308-315.DOI: 10.11983/CBB22184 cstr: 32102.14.CBB22184
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Lulu Ren1, Youze Zhang1, Kelin Huang1, Xiaochun Wan1, Zhaoliang Zhang1,*( ), Mulan Zhu2,3,*(
), Mulan Zhu2,3,*( ), Chaoling Wei1,*(
), Chaoling Wei1,*( )
)
Received:2022-08-03
Accepted:2022-11-15
Online:2023-03-01
Published:2023-03-15
Contact:
*E-mail: Lulu Ren, Youze Zhang, Kelin Huang, Xiaochun Wan, Zhaoliang Zhang, Mulan Zhu, Chaoling Wei. An Efficient System for Regenerating Adventitious Buds in Stem Segments of Tea Plants[J]. Chinese Bulletin of Botany, 2023, 58(2): 308-315.

Figure 1 Adventitious budding system of stem segment (A) Explant (bar=1 cm); (B) Normal bud induction (bar=1 cm); (C) Adventitious bud induction (bar=1 cm); (D) Adventitious bud elongation (bar=1 cm); (E) Adventitious root induction (bar=1.5 cm); (F) Transplanted plants (bar=2 cm)
| Basic medium | State of growth |
|---|---|
| WPM | The bottom callus starts quickly and grows well, the petiole turns red, a small number of normal buds |
| MS | The bottom callus is thickened, the fixed buds are robust, a small number of normal buds, the foliage is emerald green, and the whole culture is viable |
| N6 | The bottom callus starts quickly, the petiole is reddish, and the leaves are thin |
| DCR | There is less callus at the bottom and the leaves are thin and yellowish |
Table 1 Effects of different basic media on stem induction
| Basic medium | State of growth |
|---|---|
| WPM | The bottom callus starts quickly and grows well, the petiole turns red, a small number of normal buds |
| MS | The bottom callus is thickened, the fixed buds are robust, a small number of normal buds, the foliage is emerald green, and the whole culture is viable |
| N6 | The bottom callus starts quickly, the petiole is reddish, and the leaves are thin |
| DCR | There is less callus at the bottom and the leaves are thin and yellowish |
| 6-BA (mg?L-1) | Bud induction rate (%) | Bottom expansion rate (%) | State of growth |
|---|---|---|---|
| 0 | 5.56±1.92 d | 1.11±1.92 d | The growth is weak and the base becomes brown |
| 1 | 51.11±5.09 c | 61.11±3.84 c | The growth is better, with fewer buds and calli form at the base |
| 2 | 77.78±5.09 a | 80.00±3.33 b | The growth is strong, with fixed buds at the axillary buds and callus at the base |
| 3 | 84.44±1.92 a | 78.89±6.93 b | The growth is better, with fixed buds at the axillary buds and more callus at the base |
| 4 | 58.89±3.84 b | 88.89±5.09 a | The growth is weak, the axillary buds are yellow, and the chassis is expanded and partially lobed |
Table 2 Effect of different concentrations of 6-BA on the normal bud induction of stem segments
| 6-BA (mg?L-1) | Bud induction rate (%) | Bottom expansion rate (%) | State of growth |
|---|---|---|---|
| 0 | 5.56±1.92 d | 1.11±1.92 d | The growth is weak and the base becomes brown |
| 1 | 51.11±5.09 c | 61.11±3.84 c | The growth is better, with fewer buds and calli form at the base |
| 2 | 77.78±5.09 a | 80.00±3.33 b | The growth is strong, with fixed buds at the axillary buds and callus at the base |
| 3 | 84.44±1.92 a | 78.89±6.93 b | The growth is better, with fixed buds at the axillary buds and more callus at the base |
| 4 | 58.89±3.84 b | 88.89±5.09 a | The growth is weak, the axillary buds are yellow, and the chassis is expanded and partially lobed |
| Number | 6-BA (mg?L-1) | NAA (mg?L-1) | Adventitious bud elongation rate (%) |
|---|---|---|---|
| 1 | 0.2 | 0.02 | 24.44±6.94 c |
| 2 | 0.4 | 0.04 | 30.00±3.33 c |
| 3 | 0.6 | 0.06 | 58.89±1.92 b |
| 4 | 0.8 | 0.08 | 70.00±8.82 a |
| 5 | 1 | 0.1 | 51.11±5.09 b |
Table 4 Effects of plant growth regulators on adventitious bud elongation
| Number | 6-BA (mg?L-1) | NAA (mg?L-1) | Adventitious bud elongation rate (%) |
|---|---|---|---|
| 1 | 0.2 | 0.02 | 24.44±6.94 c |
| 2 | 0.4 | 0.04 | 30.00±3.33 c |
| 3 | 0.6 | 0.06 | 58.89±1.92 b |
| 4 | 0.8 | 0.08 | 70.00±8.82 a |
| 5 | 1 | 0.1 | 51.11±5.09 b |
| Number | Medium | IBA (mg?L-1) | Rooting rate (%) |
|---|---|---|---|
| 1 | 1/2MS | 1 | 38.89±5.88 b |
| 2 | 2 | 42.22±4.01 b | |
| 3 | 3 | 85.56±2.94 a | |
| 4 | 4 | 46.67±5.77 b | |
| 5 | MS | 1 | 5.56±2.22 c |
| 6 | 2 | 18.89±2.94 c | |
| 7 | 3 | 8.89±4.01 c | |
| 8 | 4 | 11.11±4.01 c |
Table 5 Effects of different concentration of IBA and basal medium on the induction of adventitious root
| Number | Medium | IBA (mg?L-1) | Rooting rate (%) |
|---|---|---|---|
| 1 | 1/2MS | 1 | 38.89±5.88 b |
| 2 | 2 | 42.22±4.01 b | |
| 3 | 3 | 85.56±2.94 a | |
| 4 | 4 | 46.67±5.77 b | |
| 5 | MS | 1 | 5.56±2.22 c |
| 6 | 2 | 18.89±2.94 c | |
| 7 | 3 | 8.89±4.01 c | |
| 8 | 4 | 11.11±4.01 c |
| Primer | Nucleotide sequence (5°-3°) | Number of amplification band | Size range of band (bp) |
|---|---|---|---|
| S29 | GGGTAACGCC | 8 | 750-3000 |
| S30 | CTGCTGGGAC | 12 | 300-5000 |
| S31 | TGTCATCCCC | 9 | 400-3000 |
| S49 | CTCACCGTCC | 7 | 500-3000 |
| S132 | CAGCTCACGA | 8 | 250-2000 |
| S133 | CTCTCCGCCA | 9 | 300-2000 |
| S144 | GGAAGTCGCC | 6 | 500-2000 |
| S169 | TGGAGAGCAG | 4 | 750-3000 |
| S172 | AGAGGGCACA | 5 | 500-2000 |
| S175 | TCATCCGAGG | 6 | 500-3000 |
| S181 | CTACTGCGCT | 9 | 250-3000 |
| S208 | AACGGCGACA | 8 | 300-3000 |
| S221 | TGACGCATGG | 5 | 600-2000 |
| S226 | ACGCCCAGGT | 10 | 500-3000 |
| S230 | GGACCTGCTG | 7 | 500-2000 |
| Total | 113 | ||
Table 6 The random amplification polymorphic DNA (RAPD) primers used in the study and their amplification profile
| Primer | Nucleotide sequence (5°-3°) | Number of amplification band | Size range of band (bp) |
|---|---|---|---|
| S29 | GGGTAACGCC | 8 | 750-3000 |
| S30 | CTGCTGGGAC | 12 | 300-5000 |
| S31 | TGTCATCCCC | 9 | 400-3000 |
| S49 | CTCACCGTCC | 7 | 500-3000 |
| S132 | CAGCTCACGA | 8 | 250-2000 |
| S133 | CTCTCCGCCA | 9 | 300-2000 |
| S144 | GGAAGTCGCC | 6 | 500-2000 |
| S169 | TGGAGAGCAG | 4 | 750-3000 |
| S172 | AGAGGGCACA | 5 | 500-2000 |
| S175 | TCATCCGAGG | 6 | 500-3000 |
| S181 | CTACTGCGCT | 9 | 250-3000 |
| S208 | AACGGCGACA | 8 | 300-3000 |
| S221 | TGACGCATGG | 5 | 600-2000 |
| S226 | ACGCCCAGGT | 10 | 500-3000 |
| S230 | GGACCTGCTG | 7 | 500-2000 |
| Total | 113 | ||
| Primer | Nucleotide sequence (5°-3°) | Number of amplification band | Size range of band (bp) |
|---|---|---|---|
| UBC824 | TCTCTCTCTCTCTCTCG | 4 | 500-2000 |
| UBC835 | CTCTCTCTCTCTCTCTYC | 6 | 300-1500 |
| UBC840 | GAGAGAGAGAGAGAGAYT | 2 | 500-1500 |
| UBC844 | CTCTCTCTCTCTCTCTRC | 5 | 500-1500 |
| UBC853 | TCTCTCTCTCTCTCTCRT | 5 | 600-1500 |
| UBC854 | TCTCTCTCTCTCTCTCRG | 6 | 500-2000 |
| UBC856 | ACACACACACACACACYA | 5 | 500-1500 |
| UBC859 | TGTGTGTGTGTGTGTGRC | 4 | 500-3000 |
| UBC873 | GACAGACAGACAGACA | 5 | 750-3000 |
| UBC880 | GGAGAGGAGAGGAGA | 6 | 500-2000 |
| Total | 48 | ||
Table 7 The inter-simple sequence repeat (ISSR) primers used in the study and their amplification profile
| Primer | Nucleotide sequence (5°-3°) | Number of amplification band | Size range of band (bp) |
|---|---|---|---|
| UBC824 | TCTCTCTCTCTCTCTCG | 4 | 500-2000 |
| UBC835 | CTCTCTCTCTCTCTCTYC | 6 | 300-1500 |
| UBC840 | GAGAGAGAGAGAGAGAYT | 2 | 500-1500 |
| UBC844 | CTCTCTCTCTCTCTCTRC | 5 | 500-1500 |
| UBC853 | TCTCTCTCTCTCTCTCRT | 5 | 600-1500 |
| UBC854 | TCTCTCTCTCTCTCTCRG | 6 | 500-2000 |
| UBC856 | ACACACACACACACACYA | 5 | 500-1500 |
| UBC859 | TGTGTGTGTGTGTGTGRC | 4 | 500-3000 |
| UBC873 | GACAGACAGACAGACA | 5 | 750-3000 |
| UBC880 | GGAGAGGAGAGGAGA | 6 | 500-2000 |
| Total | 48 | ||
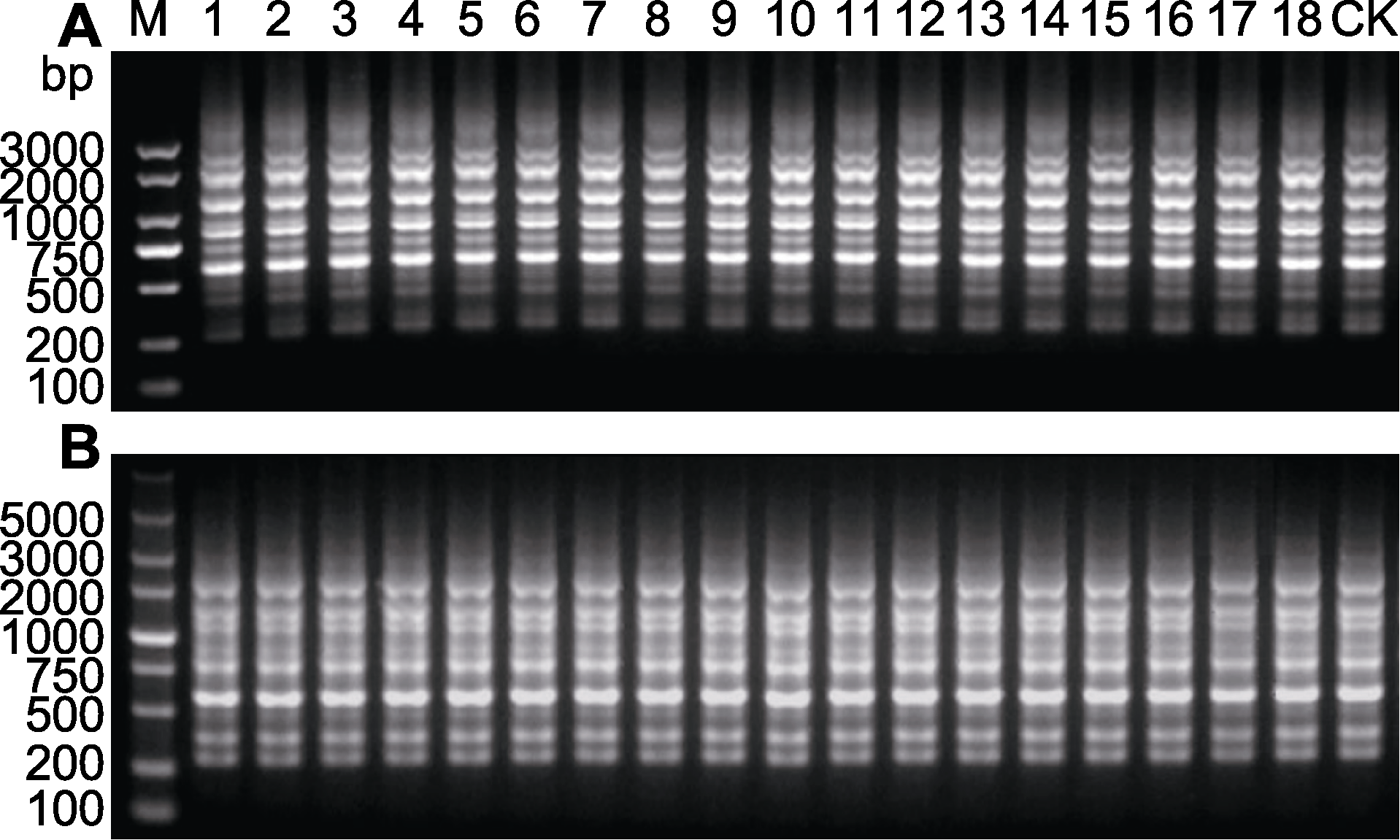
Figure 2 Random amplification polymorphic DNA (RAPD) banding profiles of regenerated plants of Shuchazao (A) Primer S132; (B) Primer S133. 1-18: In vitro regenerated plant population of the same tea plant stem segment; 1-6: First generation regenerated plants of Shuchazao; 7-12: Second generation regenerated plants of Shuchazao; 13-18: Third generation regenerated plants of Shuchazao; CK: Mother plant
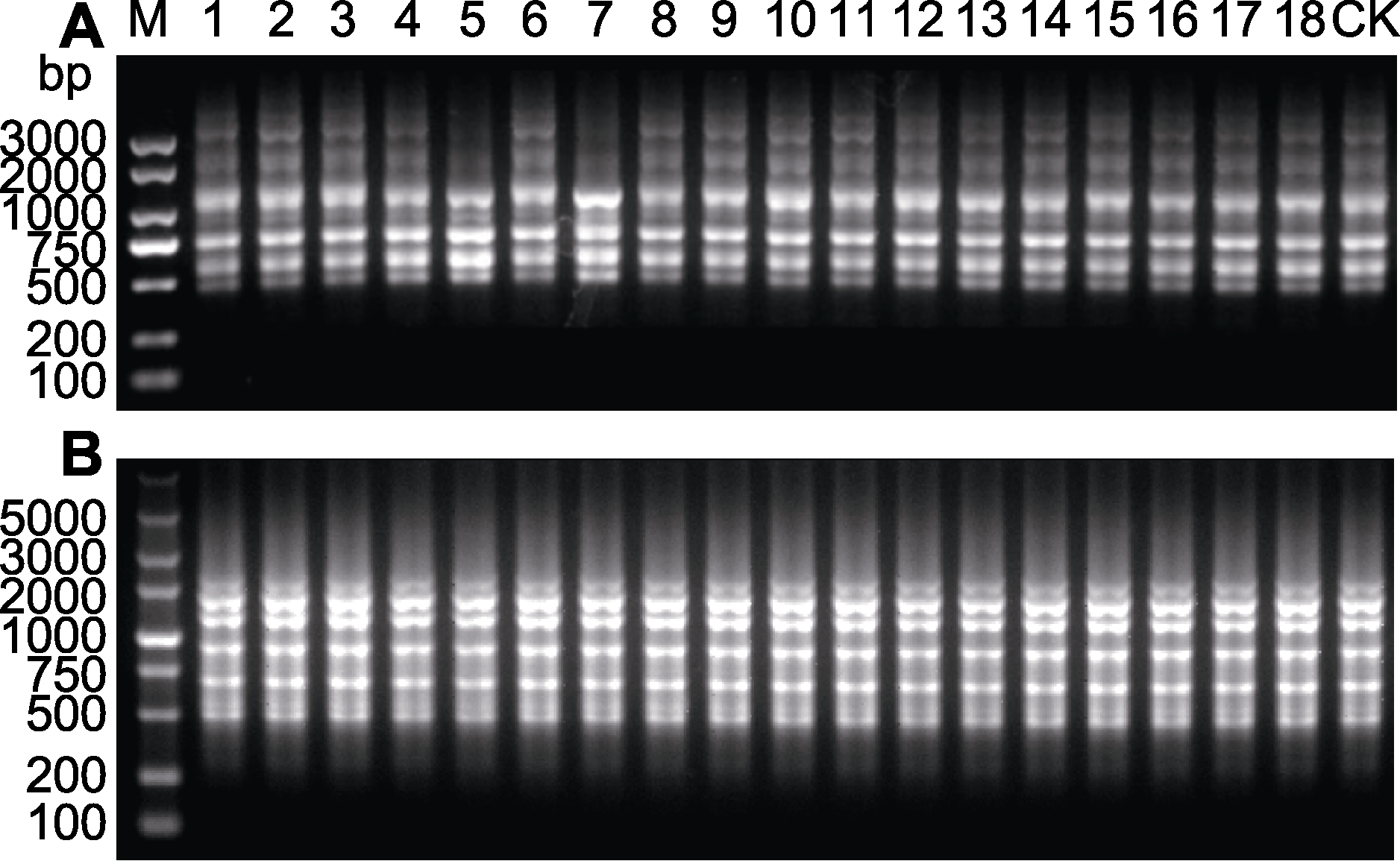
Figure 3 Inter-simple sequence repeat (ISSR) banding pro- files of regenerated plants of Shuchazao (A) Primer UBC854; (B) Primer UBC880. 1-18: In vitro regenerated plant population of the same tea plant stem segment; 1-6: First generation regenerated plants of Shuchazao; 7-12: Second generation regenerated plants of Shuchazao; 13-18: Third generation regenerated plants of Shuchazao; CK: Mother plant
| Number | 6-BA (mg?L-1) | NAA (mg?L-1) | Adventitious bud induction rate (%) | Average number of shoots |
|---|---|---|---|---|
| 1 | 0 | 0 | 16.67±5.78 d | 0.33±0.15 d |
| 2 | 1 | 0.1 | 54.44±5.09 c | 1.33±0.08 c |
| 3 | 2 | 0.2 | 88.89±6.93 a | 7.88±0.23 a |
| 4 | 3 | 0.3 | 71.11±6.93 b | 3.48±0.25 b |
Table 3 Effects of plant growth regulators on the induction of adventitious bud proliferation
| Number | 6-BA (mg?L-1) | NAA (mg?L-1) | Adventitious bud induction rate (%) | Average number of shoots |
|---|---|---|---|---|
| 1 | 0 | 0 | 16.67±5.78 d | 0.33±0.15 d |
| 2 | 1 | 0.1 | 54.44±5.09 c | 1.33±0.08 c |
| 3 | 2 | 0.2 | 88.89±6.93 a | 7.88±0.23 a |
| 4 | 3 | 0.3 | 71.11±6.93 b | 3.48±0.25 b |
| [1] | 陈立杰, 杨霞, 高倩, 李悦, 陈红 (2019). 花溪古茶树离体繁殖技术研究初报. 种业导刊 (8), 10-13. |
| [2] | 陈泽雄, 刘奕清, 黄登艳 (2009). ‘渝茶1号’离体培养及植株再生研究. 西南大学学报(自然科学版) 31(12),67-70. |
| [3] | 陈宗懋 (1994). 中国茶经. 上海: 上海文化出版社. pp. 5. |
| [4] | 陈祖枝 (2018). 福安茶树良种繁育现状及存在问题分析. 福建茶叶 40(2), 1-2. |
| [5] | 成浩, 曾建明, 周健, 王丽鸳, 常杰, 葛滢, 袁海波, 谷保静, 张小飞 (2007). 茶树种苗工厂化快速繁育技术. 茶叶科学 27, 231-235. |
| [6] | 李华锋, 滕杰, 莫岚, 曾雯, 晏嫦妤, 黄亚辉 (2016). 连南县茶树种质资源遗传多样性的RAPD分析. 茶叶学报 57, 166-171. |
| [7] | 李晓东, 向勤锃, 高吉刚, 张丽霞 (2010). 不同成熟度茶籽外植体诱导发生体胚的研究. 中国农学通报 26(18), 54-58. |
| [8] | 李旭云 (2020). 安溪县茶树种苗繁育及种质资源保护利用. 福建茶叶 42(11), 11-12. |
| [9] | 刘静 (2020). 茶树带腋芽茎段组织培养研究. 陕西农业科学 66(10), 43-45. |
| [10] | 刘青, 赵德刚, 赵懿琛 (2021). 古茶树种质资源遗传多样性ISSR分析. 种子 40(5), 7-14. |
| [11] | 罗军武, 施兆鹏, 沈程文, 刘春林, 龚志华, 黄意欢 (2004). 茶树种质资源遗传多样性的RAPD分析. 作物学报 30, 266-269. |
| [12] | 欧少云, 陈珊, 陈春兰, 刘细群 (2012). 不同外源激素对清远笔架茶愈伤组织诱导的影响. 湖北农业科学 51, 3626-3627. |
| [13] | 亓峥, 庞志强, 蓝增全 (2020). 云南小叶种茶树叶片和茎段愈伤组织诱导及继代培养. 科学技术与工程 20, 7206-7212. |
| [14] | 谭和平, 徐利远, 余桂蓉, 杜文平, 王宇星, 钟昌松 (2006). 茶树种质资源ISSR分子标记初步研究. 核农学报 20, 113-115. |
| [15] | 谢恩俊, 田维丽, 陈正武, 李岩, 赵德刚 (2020). ‘中黄1号’茶树带腋芽茎段组培快繁体系建立. 分子植物育种 18, 5071-5080. |
| [16] | 许益娟 (2012). 茶树组织培养再生体系优化与遗传转化的研究. 硕士论文. 南京: 南京农业大学. pp. 1-64. |
| [17] |
鄢东海, 刘声传, 罗显扬, 魏杰, 陆建良, 范方媛 (2015). 贵州地方茶树品种资源遗传多样性RAPD分析. 中国农学通报 31(19), 30-34.
DOI |
| [18] | 姚明哲, 黄海涛, 余继忠, 陈亮 (2005). ISSR在茶树品种分子鉴别和亲缘关系研究中的适用性分析. 茶叶科学 25, 153-157. |
| [19] | 岳翠男, 王治会, 江新凤, 杨普香 (2018). 茶树组培技术研究进展. 蚕桑茶叶通讯 2(3), 27-31. |
| [20] | Doyle JJ, Doyle JL (1987). A rapid DNA isolation procedure for small quantities of fresh leaf tissue. Phytochem Bull 19, 11-15. |
| [21] |
Sun J, Lei PD, Zhang ZZ, Shi GH, Tang ZJ, Zhu SY, Jiang CJ, Wan XC (2012). Shoot basal ends as novel explants for in vitro plantlet regeneration in an elite clone of tea. J Hortic Sci Biotechnol 87, 71-76.
DOI URL |
| [1] | Lü Xiuli, Zhang Qun, Chen Xiangbo, Li Pujin, Wu Wei, Guan Yuan. Mass Propagation and Genetic Stability of Bergenia Species [J]. Chinese Bulletin of Botany, 2018, 53(5): 643-652. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||