

Chinese Bulletin of Botany ›› 2024, Vol. 59 ›› Issue (5): 800-809.DOI: 10.11983/CBB23152 cstr: 32102.14.CBB23152
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Yuze Liu1, Yifei Wang1, Weizhen Ren1, Hao Li2, Bin Lu1, Bingshe Lu1, Xiaoyue Yu1,*( )
)
Received:2023-11-13
Accepted:2024-07-14
Online:2024-09-10
Published:2024-08-19
Contact:
Xiaoyue Yu
Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’[J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809.
| No. | 75% ethanol (s) | 0.1% HgCl2 (min) | 10% H2O2 (min) |
|---|---|---|---|
| A1 | 30 | 8 | 0 |
| A2 | 30 | 10 | 0 |
| A3 | 30 | 12 | 0 |
| A4 | 30 | 14 | 0 |
| A5 | 30 | 16 | 0 |
| A6 | 30 | 18 | 0 |
| A7 | 30 | 10 | 10 |
| A8 | 30 | 12 | 10 |
| A9 | 30 | 14 | 10 |
| A10 | 30 | 16 | 10 |
Table 1 Different sterilization methods
| No. | 75% ethanol (s) | 0.1% HgCl2 (min) | 10% H2O2 (min) |
|---|---|---|---|
| A1 | 30 | 8 | 0 |
| A2 | 30 | 10 | 0 |
| A3 | 30 | 12 | 0 |
| A4 | 30 | 14 | 0 |
| A5 | 30 | 16 | 0 |
| A6 | 30 | 18 | 0 |
| A7 | 30 | 10 | 10 |
| A8 | 30 | 12 | 10 |
| A9 | 30 | 14 | 10 |
| A10 | 30 | 16 | 10 |
| No. | Auxin species | Auxin concentration (mg·L-1) | 6-BA (mg·L-1) |
|---|---|---|---|
| B1 | NAA | 0.2 | 1.0 |
| B2 | NAA | 0.5 | 2.0 |
| B3 | NAA | 1.0 | 3.0 |
| B4 | IBA | 0.2 | 2.0 |
| B5 | IBA | 0.5 | 3.0 |
| B6 | IBA | 1.0 | 1.0 |
| B7 | 2,4-D | 0.2 | 3.0 |
| B8 | 2,4-D | 0.5 | 1.0 |
| B9 | 2,4-D | 1.0 | 2.0 |
Table 2 Experimental scheme of primary culture
| No. | Auxin species | Auxin concentration (mg·L-1) | 6-BA (mg·L-1) |
|---|---|---|---|
| B1 | NAA | 0.2 | 1.0 |
| B2 | NAA | 0.5 | 2.0 |
| B3 | NAA | 1.0 | 3.0 |
| B4 | IBA | 0.2 | 2.0 |
| B5 | IBA | 0.5 | 3.0 |
| B6 | IBA | 1.0 | 1.0 |
| B7 | 2,4-D | 0.2 | 3.0 |
| B8 | 2,4-D | 0.5 | 1.0 |
| B9 | 2,4-D | 1.0 | 2.0 |
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) |
|---|---|---|
| C1 | 1.0 | 0.05 |
| C2 | 1.0 | 0.10 |
| C3 | 1.0 | 0.20 |
| C4 | 1.5 | 0.05 |
| C5 | 1.5 | 0.10 |
| C6 | 1.5 | 0.20 |
| C7 | 2.0 | 0.05 |
| C8 | 2.0 | 0.10 |
| C9 | 2.0 | 0.20 |
| C10 | 3.0 | 0.05 |
| C11 | 3.0 | 0.10 |
| C12 | 3.0 | 0.20 |
| C13 | 4.0 | 0.05 |
| C14 | 4.0 | 0.10 |
| C15 | 4.0 | 0.20 |
Table 3 Treatments with different plant growth regulation combinations in subculture
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) |
|---|---|---|
| C1 | 1.0 | 0.05 |
| C2 | 1.0 | 0.10 |
| C3 | 1.0 | 0.20 |
| C4 | 1.5 | 0.05 |
| C5 | 1.5 | 0.10 |
| C6 | 1.5 | 0.20 |
| C7 | 2.0 | 0.05 |
| C8 | 2.0 | 0.10 |
| C9 | 2.0 | 0.20 |
| C10 | 3.0 | 0.05 |
| C11 | 3.0 | 0.10 |
| C12 | 3.0 | 0.20 |
| C13 | 4.0 | 0.05 |
| C14 | 4.0 | 0.10 |
| C15 | 4.0 | 0.20 |
| No. | Basic media | NAA (mg·L-1) | Sugar (mg·L-1) | AC (g·L-1) | IBA (mg·L-1) |
|---|---|---|---|---|---|
| D1 | 1/2MS | 0.05 | 20 | 0 | 1.0 |
| D2 | 1/2MS | 0.05 | 20 | 1.0 | 1.5 |
| D3 | 1/2MS | 0.05 | 20 | 1.5 | 2.0 |
| D4 | 1/2MS | 0.05 | 25 | 0 | 1.5 |
| D5 | 1/2MS | 0.05 | 25 | 1.0 | 1.0 |
| D6 | 1/2MS | 0.05 | 25 | 1.5 | 2.0 |
| D7 | 1/2MS | 0.05 | 30 | 0 | 2.0 |
| D8 | 1/2MS | 0.05 | 30 | 1.0 | 1.0 |
| D9 | 1/2MS | 0.05 | 30 | 1.5 | 1.5 |
Table 4 Experimental scheme for rooting culture
| No. | Basic media | NAA (mg·L-1) | Sugar (mg·L-1) | AC (g·L-1) | IBA (mg·L-1) |
|---|---|---|---|---|---|
| D1 | 1/2MS | 0.05 | 20 | 0 | 1.0 |
| D2 | 1/2MS | 0.05 | 20 | 1.0 | 1.5 |
| D3 | 1/2MS | 0.05 | 20 | 1.5 | 2.0 |
| D4 | 1/2MS | 0.05 | 25 | 0 | 1.5 |
| D5 | 1/2MS | 0.05 | 25 | 1.0 | 1.0 |
| D6 | 1/2MS | 0.05 | 25 | 1.5 | 2.0 |
| D7 | 1/2MS | 0.05 | 30 | 0 | 2.0 |
| D8 | 1/2MS | 0.05 | 30 | 1.0 | 1.0 |
| D9 | 1/2MS | 0.05 | 30 | 1.5 | 1.5 |

Figure 1 Effect of different treatments on sterilization and germination rate of seed embryo (A) Effect of different treatments on sterilization; (B) Effect of different low temperature storage time on germination rate. Different lowercase letters indicate significant differences among different treatmets at P<0.05 level. A1-A10 are the same as shown in Table 1.
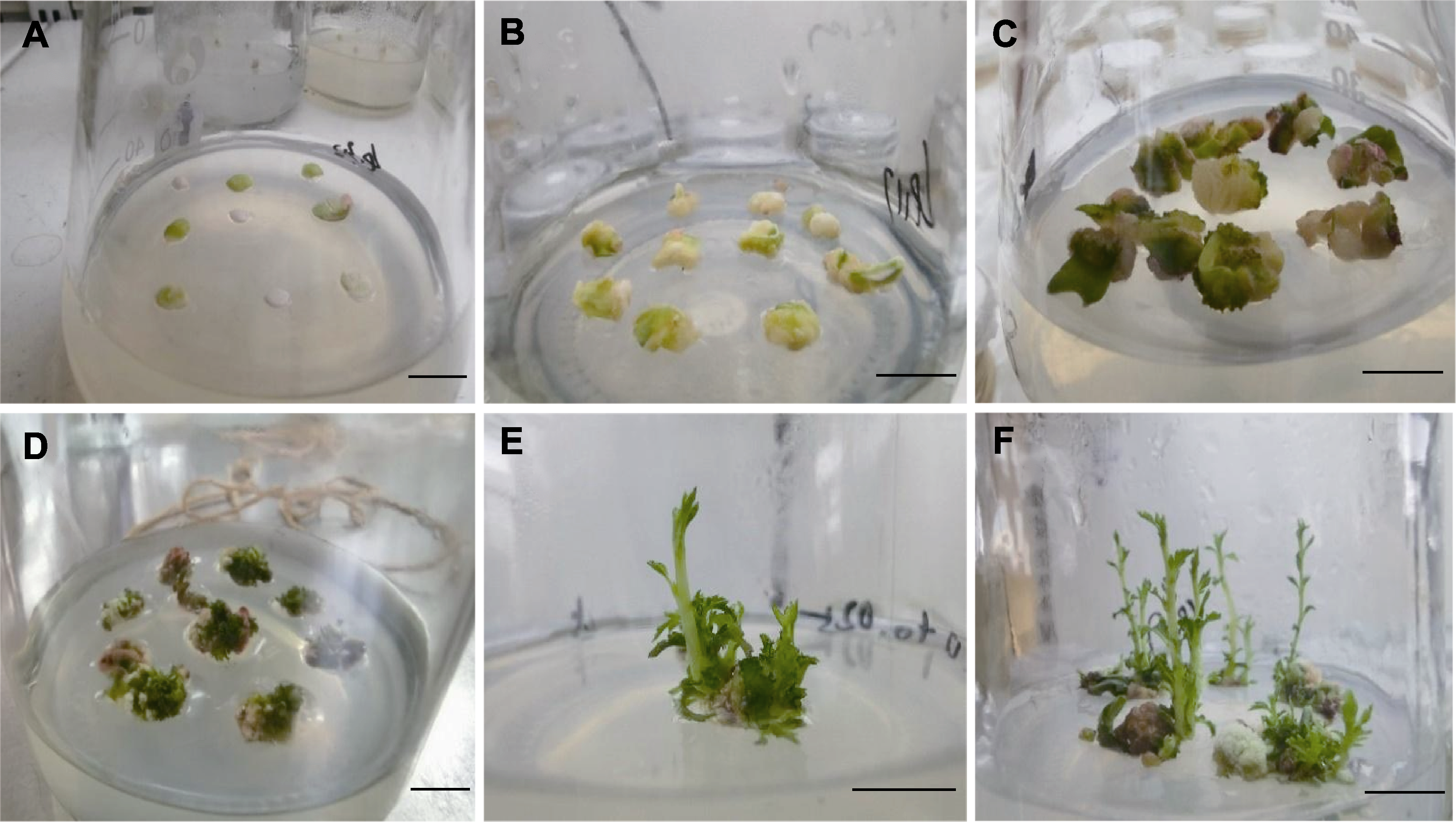
Figure 2 Induced differentiation process of embryo (A) Dark culture for 2-4 days; (B) Dark culture for 6-7 days; (C) Dark culture for 14 days; (D) After dark culture for 21 days, it was converted to light culture; (E) Inoculation for about 30 days; (F) About 45 days after inoculation. Bars=1 cm
| No. | Callus rate (%) | Differentiation rate (%) | Number of adventitious buds regenerated | Callus form |
|---|---|---|---|---|
| B1 | 60.56±2.55 e | 77.22±2.55 c | 3.27±0.04 b | Yellowish green, white, sticky lump |
| B2 | 66.11±2.55 d | 72.78±2.55 d | 2.79±0.04 c | Yellowish green, white, loose |
| B3 | 74.44±2.55 c | 70.56±1.92 d | 2.86±0.03 c | Light green, white, ropy |
| B4 | 72.22±2.55 c | 89.44±1.92 a | 3.79±0.06 a | Light green, loose |
| B5 | 85.56±1.92 b | 82.78±1.92 b | 3.86±0.03 a | Chartreuse, white, ropy |
| B6 | 93.33±1.67 a | 63.89±1.92 e | 2.67±0.03 d | Chartreuse, white, ropy |
| B7 | 82.78±2.55 b | 81.67±2.89 b | 3.59±0.04 b | Yellow, white, clumpy and compact |
| B8 | 86.67±3.33 b | 60.56±1.92 e | 2.69±0.05 d | Light yellow, granular sphericity, compact and fragile |
| B9 | 91.67±2.89 a | 61.67±1.67 e | 2.38±0.04 e | Light yellow, white, waterlogged callus |
Table 5 Effect of different plant growth regulator combinations on the primary culture of seed embryos
| No. | Callus rate (%) | Differentiation rate (%) | Number of adventitious buds regenerated | Callus form |
|---|---|---|---|---|
| B1 | 60.56±2.55 e | 77.22±2.55 c | 3.27±0.04 b | Yellowish green, white, sticky lump |
| B2 | 66.11±2.55 d | 72.78±2.55 d | 2.79±0.04 c | Yellowish green, white, loose |
| B3 | 74.44±2.55 c | 70.56±1.92 d | 2.86±0.03 c | Light green, white, ropy |
| B4 | 72.22±2.55 c | 89.44±1.92 a | 3.79±0.06 a | Light green, loose |
| B5 | 85.56±1.92 b | 82.78±1.92 b | 3.86±0.03 a | Chartreuse, white, ropy |
| B6 | 93.33±1.67 a | 63.89±1.92 e | 2.67±0.03 d | Chartreuse, white, ropy |
| B7 | 82.78±2.55 b | 81.67±2.89 b | 3.59±0.04 b | Yellow, white, clumpy and compact |
| B8 | 86.67±3.33 b | 60.56±1.92 e | 2.69±0.05 d | Light yellow, granular sphericity, compact and fragile |
| B9 | 91.67±2.89 a | 61.67±1.67 e | 2.38±0.04 e | Light yellow, white, waterlogged callus |
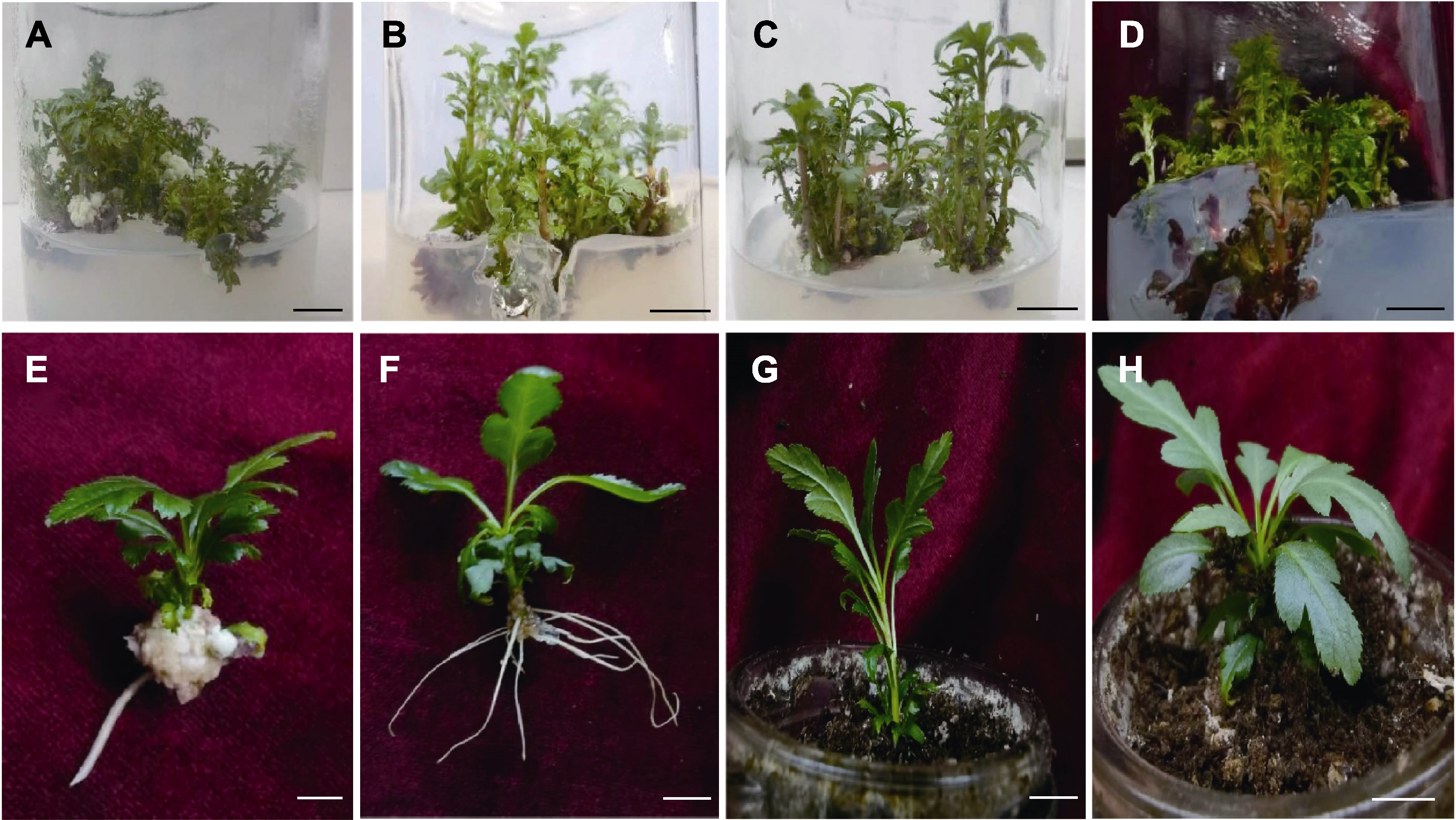
Figure 3 Adventitious bud proliferation and tissue culture seedling transplanting (A)-(D) Adventitious bud proliferation when 6-BA concentration was 1.0,1.5, 2.0 and 4.0 mg·L-1; (E) Rooting condition of the treatment group without adding active carbon; (F) Rooting condition of the treatment group added active carbon; (G), (H) Transplant. Bars=1 cm
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) | Multiplication rates (%) | Multiplication coefficient | Growth condition |
|---|---|---|---|---|---|
| C1 | 1.0 | 0.05 | 91.11±0.96 d | 3.17±0.17 efg | Average, short |
| C2 | 1.0 | 0.1 | 87.22±0.96 e | 3.00±0.44 fg | Average, short |
| C3 | 1.0 | 0.2 | 95.00±1.67 c | 2.89±0.10 g | Average |
| C4 | 1.5 | 0.05 | 100.00±0.00 a | 4.17±0.44 bc | Stronger |
| C5 | 1.5 | 0.1 | 100.00±0.00 a | 5.22±0.35 a | Stronger, large leaf blade, dark green |
| C6 | 1.5 | 0.2 | 100.00±0.00 a | 4.11±0.10 bcd | Stronger, dark green |
| C7 | 2.0 | 0.05 | 100.00±0.00 a | 3.67±0.17 cde | Stronger, dark green |
| C8 | 2.0 | 0.1 | 100.00±0.00 a | 4.50±0.33 b | Stronger |
| C9 | 2.0 | 0.2 | 100.00±0.00 a | 3.61±0.19 cdef | Stronger |
| C10 | 3.0 | 0.05 | 95.56±0.96 c | 3.61±0.69 cdef | Average growth, small leaf blade |
| C11 | 3.0 | 0.1 | 97.22±0.96 b | 3.50±0.44 defg | Average growth, small leaf blade |
| C12 | 3.0 | 0.2 | 100.00±0.00 a | 3.28±0.19 efg | Average growth, dwarf tufted bud, deformed seedling |
| C13 | 4.0 | 0.05 | 96.11±0.96 bc | 3.94±0.35 bcd | Weak growth, fewer leaves, red stems and leaves, glass seedlings |
| C14 | 4.0 | 0.1 | 92.22±0.96 d | 3.67±0.29 cde | Small leaf blade, dwarf tufted bud, glass seedlings, deformed seedling |
| C15 | 4.0 | 0.2 | 100.00±0.00 a | 3.56±0.19 cdef | Malformed leaf, glass seedlings |
Table 6 Effect of different plant growth regulator combinations on adventitious bud proliferation
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) | Multiplication rates (%) | Multiplication coefficient | Growth condition |
|---|---|---|---|---|---|
| C1 | 1.0 | 0.05 | 91.11±0.96 d | 3.17±0.17 efg | Average, short |
| C2 | 1.0 | 0.1 | 87.22±0.96 e | 3.00±0.44 fg | Average, short |
| C3 | 1.0 | 0.2 | 95.00±1.67 c | 2.89±0.10 g | Average |
| C4 | 1.5 | 0.05 | 100.00±0.00 a | 4.17±0.44 bc | Stronger |
| C5 | 1.5 | 0.1 | 100.00±0.00 a | 5.22±0.35 a | Stronger, large leaf blade, dark green |
| C6 | 1.5 | 0.2 | 100.00±0.00 a | 4.11±0.10 bcd | Stronger, dark green |
| C7 | 2.0 | 0.05 | 100.00±0.00 a | 3.67±0.17 cde | Stronger, dark green |
| C8 | 2.0 | 0.1 | 100.00±0.00 a | 4.50±0.33 b | Stronger |
| C9 | 2.0 | 0.2 | 100.00±0.00 a | 3.61±0.19 cdef | Stronger |
| C10 | 3.0 | 0.05 | 95.56±0.96 c | 3.61±0.69 cdef | Average growth, small leaf blade |
| C11 | 3.0 | 0.1 | 97.22±0.96 b | 3.50±0.44 defg | Average growth, small leaf blade |
| C12 | 3.0 | 0.2 | 100.00±0.00 a | 3.28±0.19 efg | Average growth, dwarf tufted bud, deformed seedling |
| C13 | 4.0 | 0.05 | 96.11±0.96 bc | 3.94±0.35 bcd | Weak growth, fewer leaves, red stems and leaves, glass seedlings |
| C14 | 4.0 | 0.1 | 92.22±0.96 d | 3.67±0.29 cde | Small leaf blade, dwarf tufted bud, glass seedlings, deformed seedling |
| C15 | 4.0 | 0.2 | 100.00±0.00 a | 3.56±0.19 cdef | Malformed leaf, glass seedlings |
| No. | Rooting rate (%) | Average number of roots | Maximum root length (cm) | Rooting situation | The base case |
|---|---|---|---|---|---|
| D1 | 15.74±3.21 f | 0.43±0.04 g | 3.10 | Rooting is rare, taproot is short and thick | More callus, white |
| D2 | 82.63±4.24 ab | 3.63±0.08 a | 10.50 | Taproot is long, more fibrous roots | Callus is rare or absent |
| D3 | 73.15±4.24 b | 2.19±0.06 c | 14.90 | Taproot is long, more fibrous roots | Minor callus |
| D4 | 28.70±3.21 e | 1.19±0.06 e | 2.60 | Rarely rooting | More callus, white |
| D5 | 85.19±4.24 a | 2.37±0.07 b | 12.90 | Taproot is long, fibrous roots are well developed | Callus is rare or absent |
| D6 | 60.19±4.24 c | 1.26±0.04 de | 2.50 | Taproot is thin and long, minuscule fibrous roots | Minor callus |
| D7 | 25.93±4.24 e | 0.84±0.04 f | 11.90 | Rarely rooting, a few taproots are thick and short | Large callus, white |
| D8 | 51.85±4.24 d | 1.31±0.06 d | 12.50 | Taproot is slender with a few fibrous roots | Moderate callus |
| D9 | 65.74±4.24 c | 3.59±0.08 a | 9.40 | Taproot is slender with a few fibrous roots | Moderate callus |
Table 7 Effects of different concentrations of sucrose, active carbon (AC) and IBA on the rooting of adventitious buds
| No. | Rooting rate (%) | Average number of roots | Maximum root length (cm) | Rooting situation | The base case |
|---|---|---|---|---|---|
| D1 | 15.74±3.21 f | 0.43±0.04 g | 3.10 | Rooting is rare, taproot is short and thick | More callus, white |
| D2 | 82.63±4.24 ab | 3.63±0.08 a | 10.50 | Taproot is long, more fibrous roots | Callus is rare or absent |
| D3 | 73.15±4.24 b | 2.19±0.06 c | 14.90 | Taproot is long, more fibrous roots | Minor callus |
| D4 | 28.70±3.21 e | 1.19±0.06 e | 2.60 | Rarely rooting | More callus, white |
| D5 | 85.19±4.24 a | 2.37±0.07 b | 12.90 | Taproot is long, fibrous roots are well developed | Callus is rare or absent |
| D6 | 60.19±4.24 c | 1.26±0.04 de | 2.50 | Taproot is thin and long, minuscule fibrous roots | Minor callus |
| D7 | 25.93±4.24 e | 0.84±0.04 f | 11.90 | Rarely rooting, a few taproots are thick and short | Large callus, white |
| D8 | 51.85±4.24 d | 1.31±0.06 d | 12.50 | Taproot is slender with a few fibrous roots | Moderate callus |
| D9 | 65.74±4.24 c | 3.59±0.08 a | 9.40 | Taproot is slender with a few fibrous roots | Moderate callus |
| [1] | 邓彬 (2019). 玛瑙红樱桃幼胚培养及其遗传转化. 硕士论文. 贵阳: 贵州大学. pp. 1-71. |
| [2] | 邓小敏 (2020). 君子兰未成熟胚离体培养的研究. 农业技术与装备 (08), 55, 57. |
| [3] | 李克壮, 梅梅, 洪晓松, 葛根塔娜, 陆秀君 (2014). 美国红枫组织培养初步研究. 见:第十六届中国科协年会——分11森林培育技术创新与特色资源产业发展学术研讨会论文集. 昆明: 中国科学技术协会, 云南省人民政府. pp. 7. |
| [4] | 李林山, 张黎 (2020). 植物激素对如意皇后粗肋草离体培养的影响. 农业科学研究 41(3), 89-92. |
| [5] | 李孟悦, 刘柳, 刘艳, 张晓曼 (2021). 毛报春(Primula×pubescens)腋芽再生组织培养体系的建立. 植物学报 56, 732- 739. |
| [6] | 李清亚, 路斌, 赵佳伟, 栗浩, 李艳, 苗胜越, 路丙社 (2020). 不同豆梨品种对低温胁迫的生理响应及抗寒性评价. 西北农林科技大学学报(自然科学版) 48, 86-94, 110. |
| [7] | 李学馨 (2020). 梨继代体系优化及再生体系探索的研究. 硕士论文. 南京: 南京农业大学. pp. 1-60. |
| [8] | 廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰 (2023). 平潭野菊混合瓣型株系再生体系的建立. 植物学报 58, 449-460. |
| [9] | 龙达, 刘卫东, 张艳萍, 张雕 (2019). 观赏桃未成熟胚子叶再生植株的研究. 经济林研究 37(3), 173-179. |
| [10] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧 (2022). 大花银莲花愈伤组织诱导及再生体系的建立. 植物学报 57, 217-226. |
| [11] | 栾晓龙, 史昊, 许波, 张倩男, 刘莉 (2023). 活性炭对秋子梨组培幼苗生根的影响. 分子植物育种 (2), 589-593. |
| [12] | 吕德任, 云勇, 潘梅, 王景飞, 戚华沙 (2015). 花叶艳山姜组培苗不定根诱导研究. 中国园艺文摘 (12), 11-12, 52. |
| [13] | 潘晓, 陈瑞丹 (2010). ‘淡丰后’梅胚培养影响因素的研究. 北京林业大学学报 32(S2), 88-92. |
| [14] | 石丽敏, 宋费玲, 许巧贤, 卢华兵, 陶正明, 姜武, 吴志刚 (2021). 蔗糖浓度对白芨种子培养的影响. 浙江农业科学 62, 297-298, 338. |
| [15] | 孙大宽, 马莉, 杨宏艳, 马焕成, 郑晟靖, 唐军荣 (2022). 3种因素对木棉组培苗生根的影响. 云南农业大学学报(自然科学) 37, 137-144. |
| [16] | 孙清荣, 关秋竹, 陶吉寒, 孙洪雁 (2020). 碳源和细胞分裂素对‘库尔勒香梨’离体叶片再生不定芽的影响. 山东农业科学 52(8), 17-20. |
| [17] | 王德芬, 张梅, 李鼎立, 王然, 马春晖, 宋健坤 (2016). 秋子梨叶片高效再生体系的构建. 北方园艺 (4), 97-101. |
| [18] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. |
| [19] | 吴翠云, 阿依买木, 刘丽, 王新建 (2009). 影响野生杜梨种胚离体培养的因素. 西北农业学报 18(2), 209-212, 216. |
| [20] | 徐丽娟, 董梅, 王永香, 王倩 (2016). 激素浓度和配比对蝴蝶兰离体培养的影响. 青岛农业大学学报(自然科学版) 33, 20-23. |
| [21] | 袁振, 廖荣君, 邢刚, 刘卫东, 刘汝成, 冯殿齐 (2020). 欧李无糖组培生根研究. 山东林业科技 50(4), 39-42. |
| [22] | 张仕超 (2022). 南红梨和杜梨多倍体诱导及鉴定. 硕士论文. 杨凌: 西北农林科技大学. pp. 1-63. |
| [23] | 赵亚楠, 张懿, 谭旭, 王胜男, 姜峰, 李天忠, 朱元娣 (2021). 杜梨组培苗两步生根技术体系优化. 中国农业大学学报 26(11), 105-112. |
| [24] | 钟颖, 冯建荣, 樊新民, 任欢喜, 张秀抗, 许竹叶 (2018). 库尔勒香梨离体叶片再生体系的建立. 新疆农业科学 55, 829-836. |
| [25] | 宗宇, 孙萍, 牛庆丰, 滕元文 (2013). 中国北方野生杜梨分布现状及其形态多样性评价. 果树学报 30, 918-923. |
| [26] | Antonelli M, Druart P (1990). The use of a brief 2,4-D treatment to induce leaf regeneration on Prunus canescens Bois. Acta Hortic (280), 45-50. |
| [27] | Caboni E, Tonelli MG, Lauri P, D’Angeli S, Damiano C (1999). In vitro shoot regeneration from leaves of wild pear. Plant Cell Tissue Organ Cult 59, 1-7. |
| [28] | Long Y, Yang Y, Pan GT, Shen YQ (2022). New insights into tissue culture plant regeneration mechanisms. Front Plant Sci 13, 926752. |
| [29] | Phillips GC, Garda M (2019). Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol Plant 55, 242-257. |
| [30] | Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A, Ćosić T (2021). Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. Int J Mol Sci 22, 8554. |
| [31] | Thomas TD (2008). The role of activated charcoal in plant tissue culture. Biotechnol Adv 26, 618-631. |
| [1] | Jingjing Li, Yanfei Li, Anqi Wang, Jiaying Wang, Chengyan Deng, Min Lu, Jianying Ma, Silan Dai. Establishment of Regeneration and Genetic Transformation System for Chrysanthemum Cultivar ‘Wandai Fengguang’ [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [3] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [4] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [5] | Xiaoyun Wu, Minling Liao, Xueru Li, Zichun Shu, Jiatong Xin, Bohan Zhang, Silan Dai. Establishment of Regeneration System of Chrysanthemum vestitum with Three Floret Forms [J]. Chinese Bulletin of Botany, 2024, 59(2): 245-256. |
| [6] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [7] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [8] | Minling Liao, Ya Pu, Xiaoyun Wu, Chaofeng Ma, Wenkui Wang, Silan Dai. Establishment of Regeneration System of Chrysanthemum indicum in Pingtan with Various Ligulate Floret Form [J]. Chinese Bulletin of Botany, 2023, 58(3): 449-460. |
| [9] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [10] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [11] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [12] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [13] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [14] | Sha Deng, Yanni Wu, Kunlin Wu, Lin Fang, Lin Li, Songjun Zeng. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China [J]. Biodiv Sci, 2020, 28(3): 385-400. |
| [15] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||