

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (5): 596-604.DOI: 10.11983/CBB20016 cstr: 32102.14.CBB20016
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Long Ma, Guilin Li, Shipeng Li, Su Jiang*( )
)
Received:2020-02-05
Accepted:2020-06-05
Online:2020-09-01
Published:2020-09-03
Contact:
Su Jiang
Long Ma, Guilin Li, Shipeng Li, Su Jiang. An Improved Protocol for Whole Mount Clearing of Plant Root Tip[J]. Chinese Bulletin of Botany, 2020, 55(5): 596-604.
| No. | HCG-1 | ||
|---|---|---|---|
| Composition | pH | ||
| 1 | H2O | 9 mL | 1.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 2 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 3 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| HCG-2 | |||
| Composition | pH | ||
| 4 | H2O | 9 mL | 1.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 5 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 6 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
Table 1 Solution HCG-1 and HCG-2 in original pH, the pH used for Arabidopsis thaliana culture and neutral pH
| No. | HCG-1 | ||
|---|---|---|---|
| Composition | pH | ||
| 1 | H2O | 9 mL | 1.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 2 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| 3 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 24 g | ||
| Glycerol | 3 mL | ||
| HCG-2 | |||
| Composition | pH | ||
| 4 | H2O | 9 mL | 1.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 5 | H2O | 9 mL | 5.8 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
| 6 | H2O | 9 mL | 7.2 |
| Chloral hydrate | 12 g | ||
| Glycerol | 3 mL | ||
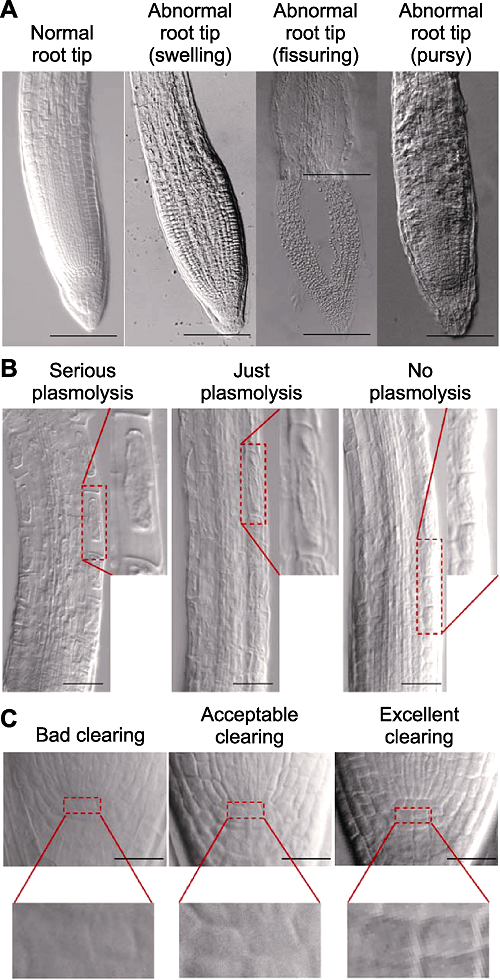
Figure 1 Criteria of Arabidopsis thaliana root tip clearing Root tips morphology (A), elongation zone epidermal cells plasmolysis (B) and quiescent center (QC) cells clarity (C) of cleared Arabidopsis thaliana Col-0 seedlings ((A) Bars=100 μm; (B) Bars=50 μm; (C) Bars=20 μm).
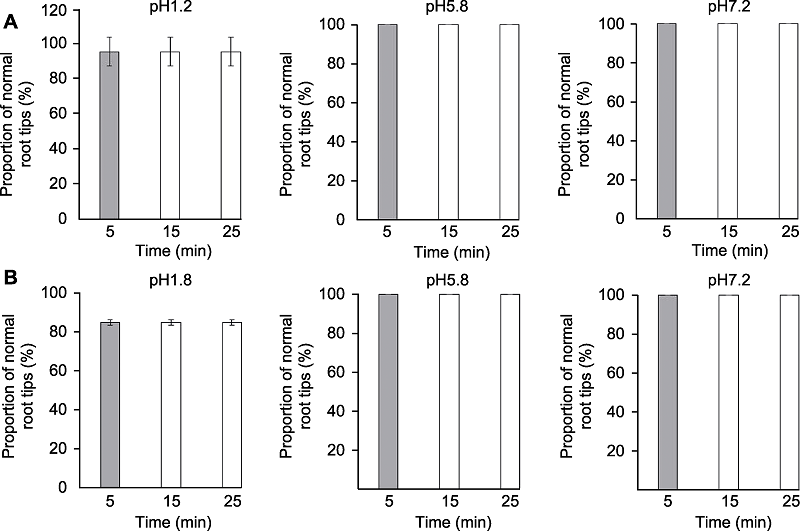
Figure 2 The effects of clearing times on Arabidopsis thaliana root tip morphology Proportion of normal root tips of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively.
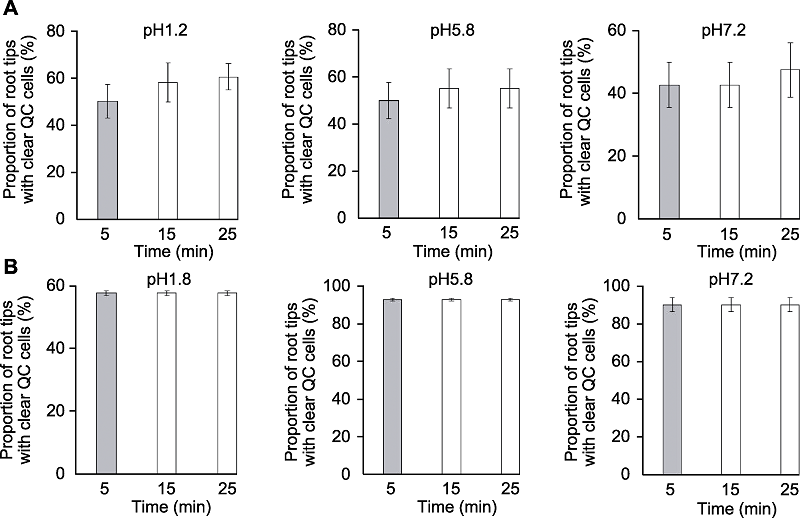
Figure 3 The effects of clearing times on clarity of quiescent center (QC) cells of Arabidopsis thaliana root tips Proportion of root tips with clear QC cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively.
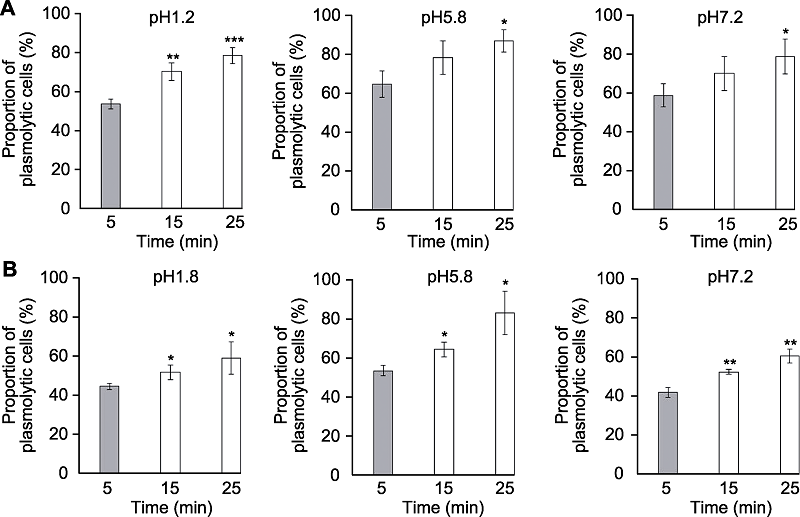
Figure 4 The effects of clearing times on plasmolysis of Arabidopsis thaliana root tip cells Proportion of plasmolytic cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5, 15, 25 min, respectively (* P<0.05, ** P<0.01, *** P<0.001).
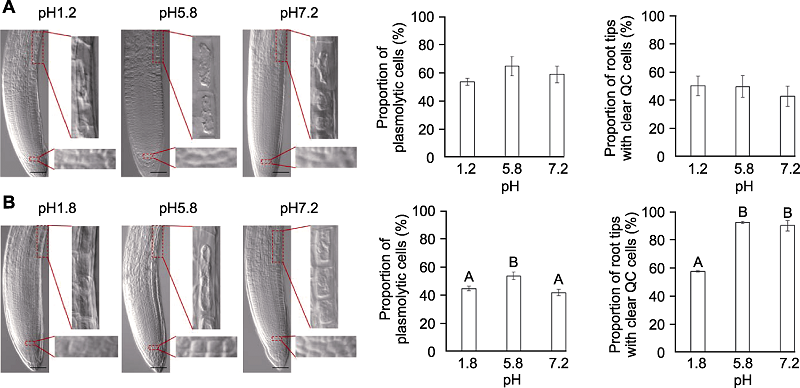
Figure 5 The effects of pH values on Arabidopsis thaliana root tip clearing Proportion of plasmolytic cells and proportion of root tips with clear quiescent center (QC) cells of Arabidopsis thaliana seedlings cleared by HCG-1 (A) and HCG-2 (B) for 5 min under pH1.2/1.8, pH5.8, pH7.2, respectively. Different uppercase letters indicate extremely significant differences (P<0.01). Bars=50 μm
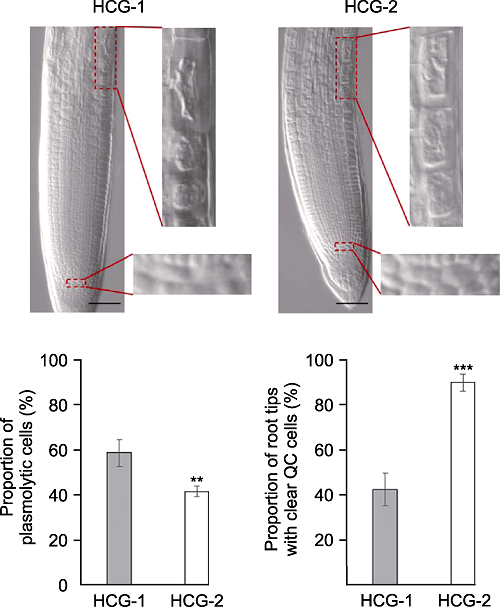
Figure 6 The comparison of solution HCG-1 with HCG-2 on Arabidopsis thaliana root tip clearing Proportion of plasmolytic cells and proportion of root tips with clear QC cells of Arabidopsis thaliana seedlings cleared by HCG-1 and HCG-2 for 5 min under pH7.2 (** P<0.01, *** P< 0.001). Bars=50 μm
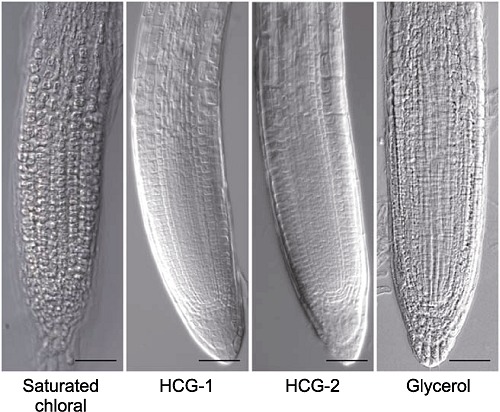
Figure 7 The comparison of root tips cleared by gradient concentrations of chloral solutions Morphology of root tips of Arabidopsis thaliana seedlings cleared by saturated chloral, HCG-1, HCG-2 and 25% glycerol solutions for 5 min, respectively. Bars=50 μm
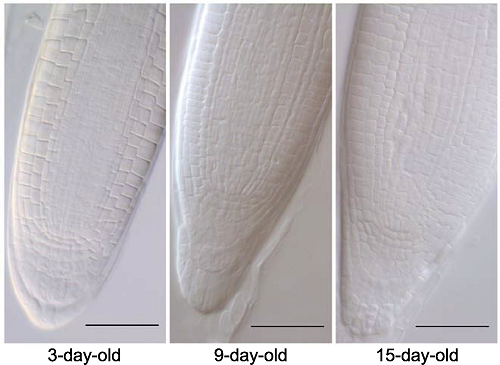
Figure 8 Arabidopsis thaliana seedlings in different growth stages were used to identify the optimized protocol 3-day-old, 9-day-old and 15-day-old seedlings of Arabidopsis thaliana were cleared by HCG-2 (pH7.2) for 5 min. Bars=50 μm
| [1] | 程蔚玲, 丁兰, 李金平, 刘国安, 杨宁 ( 2017). Leukamenin E调节拟南芥幼苗生长发育的模式及其机制. 生态学杂志 36, 676-686. |
| [2] | 郝建华, 强胜 ( 2007). 整体透明技术在植物生物学中的应用实例及其剖析. 植物学通报 24, 490-497. |
| [3] | 李芳芳, 杨娜, 钱猛, 甘立军 ( 2018). 生长素参与三十烷醇诱导的拟南芥侧根发育. 南京农业大学学报 41, 473-480. |
| [4] | 李彦坤, 臧巩固, 赵立宁, 李育君, 唐蜻, 程超华 ( 2011). 整体透明技术观察悬铃叶苎麻胚胎发育的方法研究. 中国麻业科学 33, 142-146. |
| [5] | 任媛媛, 朱炎 ( 2017). INO80参与拟南芥气孔数量调控的分子机制. 复旦学报(自然科学版) 56, 653-661. |
| [6] | 王培新, 张丹, 尚爱加, 侯冰 ( 2016). 组织透明技术. 神经解剖学杂志 32, 124-128. |
| [7] | 王文婧, 刘婷, 郭磊, 刘春明 ( 2011). SLC/AGO1基因控制拟南芥细胞分裂与定向伸长. 植物学报 46, 370-378. |
| [8] | 杨弘远 ( 1986). 用整体染色与透明技术观察胚囊、胚、胚乳和胚状体. 植物学报 28, 575-581. |
| [9] | 杨弘远 ( 1988). 植物胚胎学中的整体透明技术. 植物学通报 5(2), 114-116. |
| [10] | 于明明, 李兴国, 张宪省 ( 2009). APETALA1启动子驱动AtIPT4在转基因拟南芥中表达导致花和花器官发育异常. 植物学报 44, 59-68. |
| [11] | 赵林姝, 刘录祥, 古佳玉, 郭会君, 李军辉, 谢永盾, 赵世荣 ( 2014). 一种小麦叶片气孔保卫细胞观察样品的制备方法. 植物学报 49, 120-126. |
| [12] |
Beemster GTS, Baskin TI ( 1998). Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116, 1515-1526.
DOI URL PMID |
| [13] |
Bougourd S, Marrison J, Haseloff J ( 2000). An aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24, 543-550.
URL PMID |
| [14] |
Bruzzese E, Hasan S ( 1983). A whole leaf clearing and staining technique for host specificity studies of rust fungi. Plant Pathol 32, 335-338.
DOI URL |
| [15] | Crane CF ( 1978). Apomixis and Crossing Incompatibilities in Some Zephyrantheae. Ph.D. thesis. Austin: University of Texas. |
| [16] |
Derbyshire P, Findlay K, McCann MC, Roberts K ( 2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J Exp Bot 58, 2079-2089.
DOI URL PMID |
| [17] |
Hager A ( 2003). Role of the plasma membrane H+-ATPase in auxin-induced elongation growth: historical and new aspects. J Plant Res 116, 483-505.
DOI URL PMID |
| [18] |
Haseloff J ( 2003). Old botanical techniques for new microscopes. BioTechniques 34, 1174-1182.
DOI URL PMID |
| [19] |
Herr JMJ ( 1971). A new clearing-squash technique for the study of ovule development in angiosperms. Am J Bot 58, 785-790.
DOI URL |
| [20] | Herr JMJ (1993). Clearing techniques for the study of vascular plant tissues in whole structures and thick sections. In: Tested Studies for Laboratory Teaching. Proceedings of the Fifth Workshop/Conference of the Association for Biology Laboratory Education (ABLE). Toronto: ABLE. pp. 63-84. |
| [21] | Hoyer H ( 1882). Beiträge zur histologischen Technik. 3. Einschlussflüssigkeiten. Biologisches Zentralblatt 2, 23-24. |
| [22] | Itoh K, Nakamura Y, Kawata H, Yamada T, Ohta E, Sakata M ( 1987). Effect of osmotic stress on turgor pressure in mung bean root cells. Plant Cell Physiol 28, 987-994. |
| [23] |
Ivanov VB, Dubrovsky JG ( 2013). Longitudinal zonation pattern in plant roots: conflicts and solutions. Trends Plant Sci 18, 237-243.
DOI URL PMID |
| [24] | Janicka-Russak M (2011). Plant plasma membrane H+- ATPase in adaptation of plants to abiotic stresses. In: Shanker A, ed. Abiotic Stress Response in Plants—Physiological, Biochemical and Genetic Perspectives. Rijeka: Intech Open Press. pp. 197-218. |
| [25] |
Kim YX, Stumpf B, Sung J, Lee SJ ( 2018). The relationship between turgor pressure change and cell hydraulics of midrib parenchyma cells in the leaves of Zea mays. Cells 7, 180.
DOI URL |
| [26] |
Kurihara D, Mizuta Y, Sato Y, Higashiyama T ( 2015). ClearSee: a rapid optical clearing reagent for whole-plant fluorescence imaging. Development 142, 4168-4179.
DOI URL PMID |
| [27] |
Lang I, Sassmann S, Schmidt B, Komis G ( 2014). Plasmolysis: loss of turgor and beyond. Plants (Basel) 3, 583-593.
DOI URL PMID |
| [28] |
Lersten NR ( 1986). Modified clearing method to show sieve tubes in minor veins of leaves. Stain Technol 61, 231-234.
DOI URL PMID |
| [29] |
Li SP, Chen M, Yu DL, Ren SC, Sun SF, Liu LD, Ketelaar T, Emons AMC, Liu CM ( 2013). EXO70A1-mediated vesicle trafficking is critical for tracheary element development in Arabidopsis. Plant Cell 25, 1774-1786.
URL PMID |
| [30] |
Li SP, van Os GMA, Ren SC, Yu DL, Ketelaar T, Emons AMC, Liu CM ( 2010). Expression and functional analyses of EXO70 genes in Arabidopsis implicate their roles in regulating cell type-specific exocytosis. Plant Physiol 154, 1819-1830.
DOI URL PMID |
| [31] |
Liberato JR, Barreto RW, Shivas RG ( 2005). Leaf-clearing and staining techniques for the observation of conidiophores in the Phyllactinioideae (Erysiphaceae). Australas Plant Pathol 34, 401-404.
DOI URL |
| [32] |
Pavelescu I, Vilarrasa-Blasi J, Planas-Riverola A, González-García MP, Caño-Delgado AI, Ibañes M ( 2018). A Sizer model for cell differentiation in Arabidopsis thaliana root growth. Mol Syst Biol 14, e7687.
DOI URL PMID |
| [33] |
Richmond PA, Métraux JP, Taiz L ( 1980). Cell expansion patterns and directionality of wall mechanical properties in Nitella. Plant Physiol 65, 211-217.
DOI URL PMID |
| [34] |
Shabala S, Babourina O, Newman I ( 2000). Ion-specific mechanisms of osmoregulation in bean mesophyll cells. J Exp Bot 51, 1243-1253.
URL PMID |
| [35] |
Shabala SN, Lew RR ( 2002). Turgor regulation in osmotically stressed Arabidopsis epidermal root cells. Direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129, 290-299.
DOI URL PMID |
| [36] |
Takatsuka H, Umeda M ( 2014). Hormonal control of cell division and elongation along differentiation trajectories in roots. J Exp Bot 65, 2633-2643.
DOI URL PMID |
| [37] |
Ursache R, Andersen TG, Marhavý P, Geldner N ( 2018). A protocol for combining fluorescent proteins with histological stains for diverse cell wall components. Plant J 93, 399-412.
URL PMID |
| [38] |
Verbelen JP, de Cnodder T, Le J, Vissenberg K, Baluška F ( 2006). The root apex of Arabidopsis thaliana consists of four distinct zones of growth activities. Plant Signal Behav 1, 296-304.
DOI URL PMID |
| [39] |
Villani TS, Koroch AR, Simon JE ( 2013). An improved clearing and mounting solution to replace chloral hydrate in microscopic applications. Appl Plant Sci 1, apps. 1300016.
DOI URL |
| [1] | Yuhan Liu, Qijiang Cao, Shihan Zhang, Yihui Li, Jing Wang, Xiaomeng Tan, Xiaoru Liu, Xianling Wang. Mechanism of AtFTCD-L in Root Response to Soil Compaction [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Jiahui Huang, Huimin Yang, Xinyu Chen, Chaoyu Zhu, Yanan Jiang, Chengxiang Hu, Jinjin Lian, Tao Lu, Mei Lu, Weilin Zhang, Yuchun Rao. Response Mechanism of Rice Mutant pe-1 to Low Light Stress [J]. Chinese Bulletin of Botany, 2024, 59(4): 574-584. |
| [3] | Jixuan Yang, Xuefei Wang, Hongya Gu. Genetic Basis of Flowering Time Variations in Tibetan Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 373-382. |
| [4] | Yanxiao Chen, Yaping Li, Jinjun Zhou, Lixia Xie, Yongbin Peng, Wei Sun, Yanan He, onghui Jiang, Zenglan Wang, Chongke Zheng, Xianzhi Xie. Effect of Amino Acid Point Mutations on the Structure and Function of Phytochrome B in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 481-494. |
| [5] | Wenqi Zhou, Yuqian Zhou, Yongsheng Li, Haijun He, Yanzhong Yang, Xiaojuan Wang, Xiaorong Lian, Zhongxiang Liu, Zhubing Hu. ZmICE2 Regulates Stomatal Development in Maize [J]. Chinese Bulletin of Botany, 2023, 58(6): 866-881. |
| [6] | Gang Wang, Ertao Wang. The Broad-spectrum Innate Resistance Against Clubroot Disease Conferred by WeiTsing is Mechanistically Revealed [J]. Chinese Bulletin of Botany, 2023, 58(3): 356-358. |
| [7] | Yang Yongqing, Guo Yan. Analysis of the pH Sensing Mechanism of Plant Apoplasts [J]. Chinese Bulletin of Botany, 2022, 57(4): 409-411. |
| [8] | Tiantian Zhi, Zhou Zhou, Chengyun Han, Chunmei Ren. PAD4 Mutation Accelerating Programmed Cell Death in Arabidopsis thaliana Tyrosine Degradation Deficient Mutant sscd1 [J]. Chinese Bulletin of Botany, 2022, 57(3): 288-298. |
| [9] | Yanyan Li, Yanhua Qi. Advances in Biological Functions of Aux/IAA Gene Family in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 30-41. |
| [10] | Yongmei Che, Yanjun Sun, Songchong Lu, Lixia Hou, Xinxin Fan, Xin Liu. AtMYB77 Involves in Lateral Root Development via Regulating Nitric Oxide Biosynthesis under Drought Stress in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2021, 56(4): 404-413. |
| [11] | Ting Wang, Huanhuan Yang, Hongwei Zhao, Josef Voglmeir, Li Liu. Changes of Protein N-glycosylation in the Growth of Arabidopsis thaliana and Effects of Enzymatic Deglycosylation on Root Development [J]. Chinese Bulletin of Botany, 2021, 56(3): 262-274. |
| [12] | Yuqing Lin, Yanhua Qi. Advances in Auxin Efflux Carrier PIN Proteins [J]. Chinese Bulletin of Botany, 2021, 56(2): 151-165. |
| [13] | Fangfang He,Huize Chen,Jinlin Feng,Lin Gao,Jiao Niu,Rong Han. Response of Arabidopsis Cohesin RAD21 to Cell Division after Enhanced UV-B Radiation [J]. Chinese Bulletin of Botany, 2020, 55(4): 407-420. |
| [14] | Nan Zhang,Ziguang Liu,Shichen Sun,Shengyi Liu,Jianhui Lin,Yifang Peng,Xiaoxu Zhang,He Yang,Xi Cen,Juan Wu. Response of AtR8 lncRNA to Salt Stress and Its Regulation on Seed Germination in Arabidopsis [J]. Chinese Bulletin of Botany, 2020, 55(4): 421-429. |
| [15] | Zeyuan Zuo,Wanlin Liu,Jie Xu. Evolution and Functional Analysis of Gene Clusters in Anther Tapetum Cells of Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2020, 55(2): 147-162. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||