

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (4): 407-420.DOI: 10.11983/CBB20009 cstr: 32102.14.CBB20009
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Fangfang He,Huize Chen,Jinlin Feng,Lin Gao,Jiao Niu,Rong Han( )
)
Received:2020-01-15
Accepted:2020-05-15
Online:2020-07-01
Published:2020-05-21
Contact:
Rong Han
Fangfang He,Huize Chen,Jinlin Feng,Lin Gao,Jiao Niu,Rong Han. Response of Arabidopsis Cohesin RAD21 to Cell Division after Enhanced UV-B Radiation[J]. Chinese Bulletin of Botany, 2020, 55(4): 407-420.
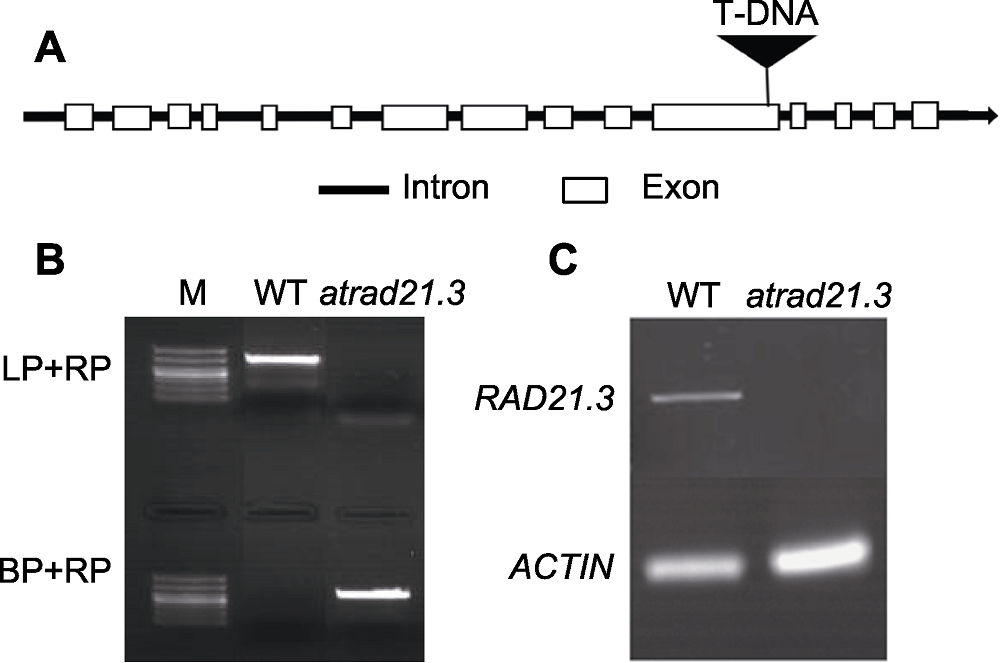
Figure 1 T-DNA insertions and identification of atrad21.3 (A) Gene structure and T-DNA insertions of atrad21.3; (B) Identification of DNA level in atrad21.3(M: Marker); (C) Transcriptional level of RAD21.3 gene in atrad21.3. WT: Wild type

Figure 2 Phenotypic analysis of wild type (WT) and atrad21.3 (A) Seed of WT; (B) Rosette leaf (day 12) of WT; (C) Bolting of WT; (D) Seed of atrad21.3; (E) Rosette leaf (day 12) of atrad21.3; (F) Bolting of atrad21.3; (G) Plant height of WT and atrad21.3. (A), (D) Bars=10 μm; (B), (C), (E)-(G) Bars=1.0 cm
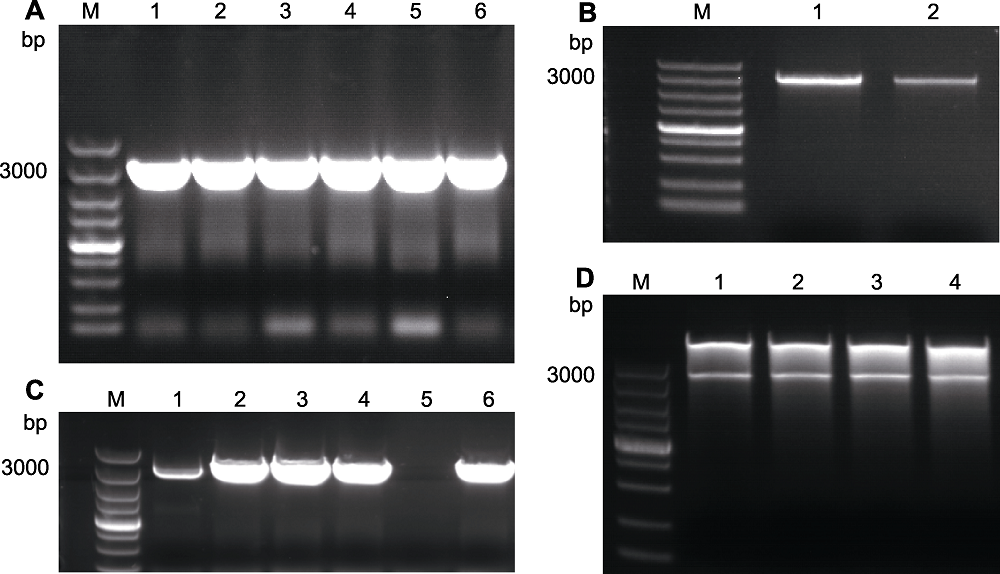
Figure 3 Cloning of AtRAD21.3 (A) PCR products of AtRAD21.3 (1-6: Products); (B) Gel cutting recovery result of AtRAD21.3 (1, 2: Gel cutting recovery products); (C) Bacterial PCR result (1-4, 6: Positive single colony; 5: Negative control products); (D) Dual-restriction digestion of plasmid (1-4: Dual-restriction result). M: DNA marker
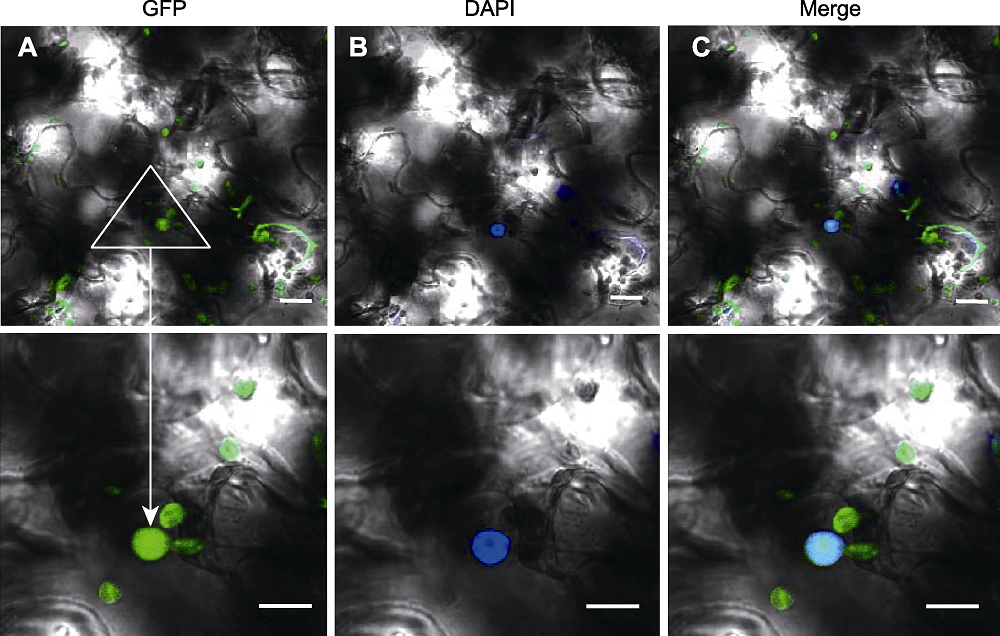
Figure 4 Transient expression of pSuper1300-RAD21.3-GFP in leaves of Nicotiana benthamiana (A) GFP signal of pSuper1300-RAD21.3-GFP; (B) DAPI staining of pSuper1300-RAD21.3-GFP in leaves of Nicotiana benthamiana; (C) Merged image of A and B. Bars=10 μm
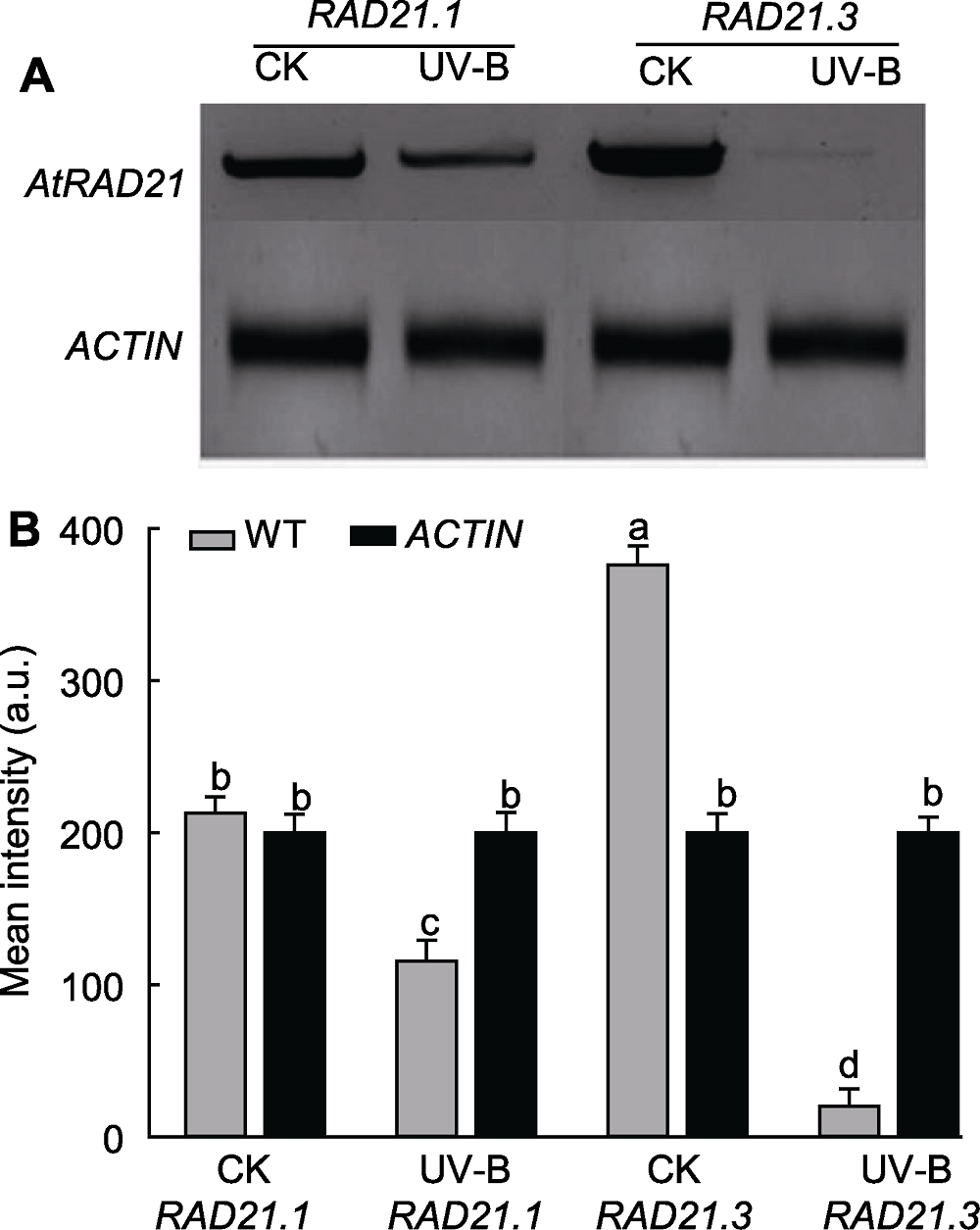
Figure 6 Detection of UV-B radiation on AtRAD21 expres-sion quantity (A) Result of RT-PCR; (B) Gray value analysis. WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
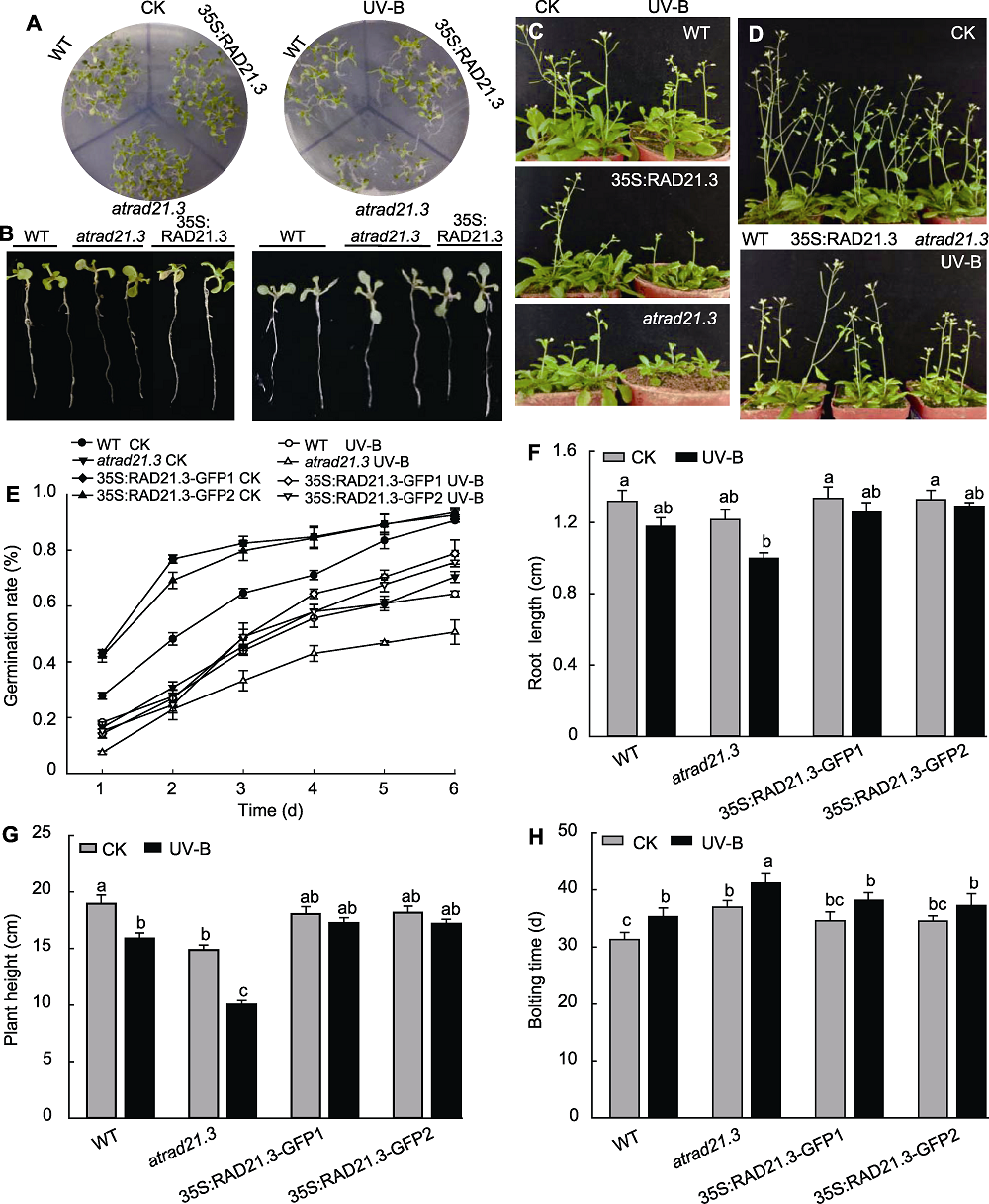
Figure 7 The seed germination rate, root length, plant height and bolting time of Arabidopsis thaliana with UV-B treatment (A), (E) The seed germination rate of Arabidopsis thaliana with UV-B treatment; (B), (F) The root length of Arabidopsis thaliana seedling with UV-B treatment; (C), (H) The bolting time of Arabidopsis thaliana with UV-B treatment; (D), (G) The plant height of Arabidopsis thaliana with UV-B treatment. WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
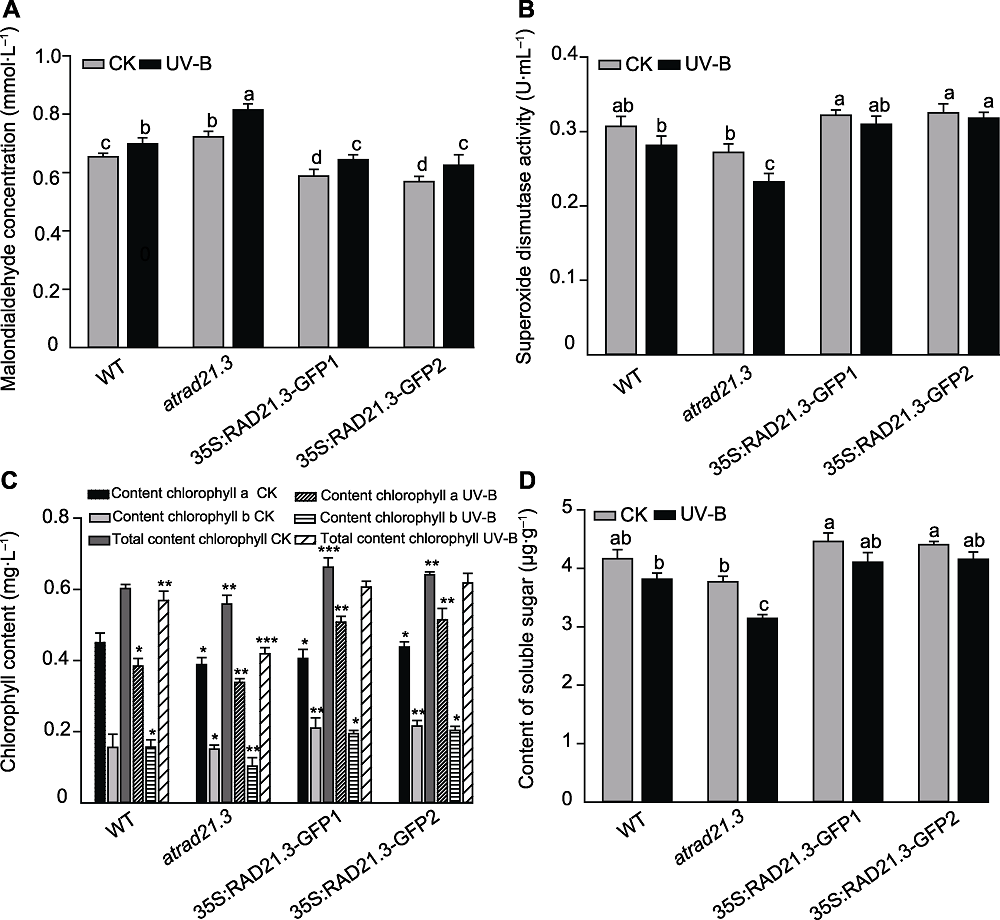
Figure 8 The influence of physiological and biochemical in Arabidopsis thaliana with UV-B treatment (A) Effect of UV-B radiation on Arabidopsis thaliana malondialdehyde (MDA) concentration; (B) Effect of UV-B radiation on Arabidopsis thaliana superoxide dismutase (SOD) activity; (C) Effect of UV-B radiation on chlorophyll content in Arabidopsis thaliana; (D) Effect of UV-B radiation on content of soluble sugar of Arabidopsis thaliana. WT: Wild type. Different lowercase letters (or * ) show significant differences (Duncan method, P<0.05).
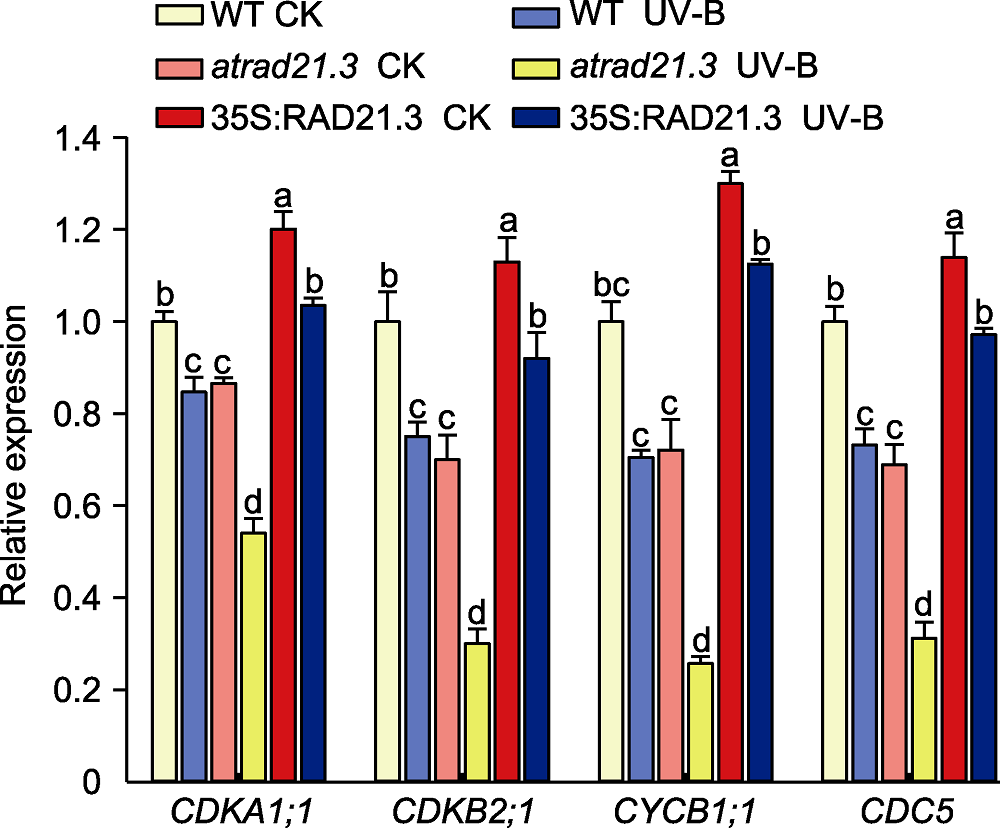
Figure 9 Relative expression of cell cycle related genes in Arabidopsis thaliana WT: Wild type. Different lowercase letters show significant differences (Duncan method, P<0.05).
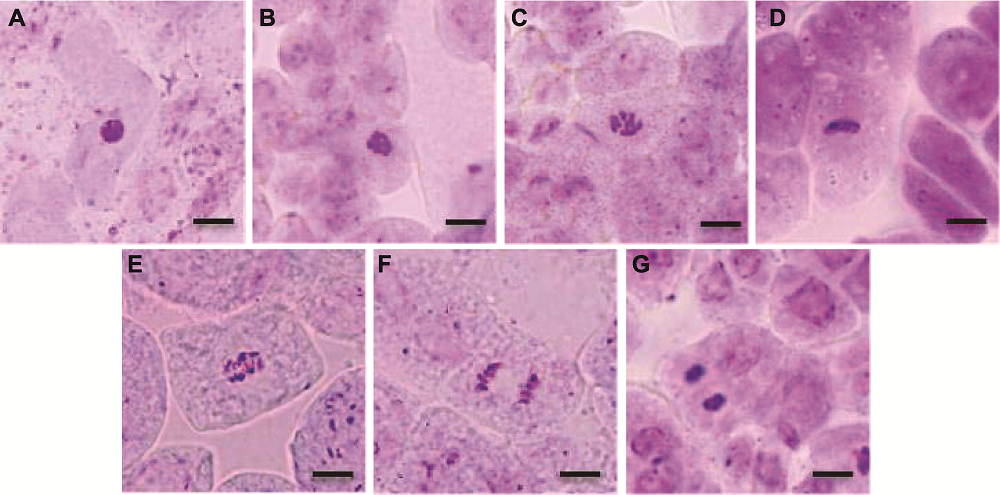
Figure 10 Normal phase types of mitosis in root tip of Arabidopsis thaliana (A) Interphase; (B), (C) Prophase; (D) Metaphase; (E) Meta-anaphase; (F) Anaphase; (G) Telephase. Bars=20 μm
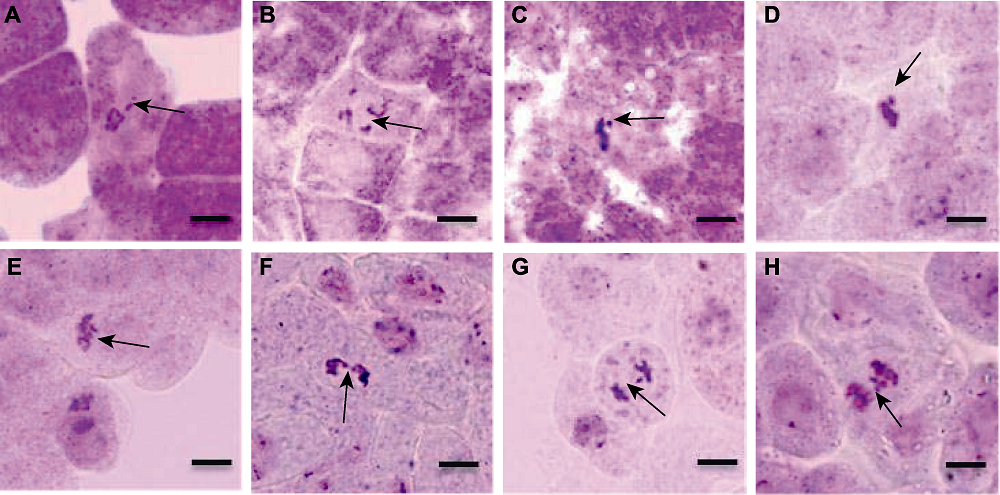
Figure 11 Different types of chromosome aberration in root tip of Arabidopsis thaliana (A) Unorientation at prophase (arrow); (B) Cofusion at prophase (arrow); (C) Fragments chromosomes (arrow); (D) Fragments chromosomes (arrow); (E) Asymmetric at anaphase (arrow); (F) Chromosome bridge (arrow); (G) Lagging chromosome (arrow); (H) Lagging chromosome (arrow). Bars=20 μm
| Treatment | Total of observing cells | Total of dividing cells | Total of aberration cells | Percentage of dividing cells (%) | Percentage of chromosomal aberration (%) |
|---|---|---|---|---|---|
| WT CK | 5390 | 195 | 8 | 3.6±0.202 a | 0.148±0.102 c |
| WT UV-B | 5376 | 166 | 25 | 3.08±0.258 c | 0.465±0.056 b |
| atrad21.3 CK | 6998 | 215 | 32 | 3.07±0.195 c | 0.457±0.085 b |
| atrad21.3 UV-B | 7044 | 194 | 45 | 2.75±0.182 d | 0.638±0.105 a |
| 35S:RAD21.3 CK | 5603 | 186 | 7 | 3.3±0.132 ab | 0.125±0.136 c |
| 35S:RAD21.3 UV-B | 5536 | 193 | 21 | 3.48±0.129 ab | 0.379±0.125 bc |
Table1 Effects of the UV-B radiation on mitosis of Arabidopsis thaliana
| Treatment | Total of observing cells | Total of dividing cells | Total of aberration cells | Percentage of dividing cells (%) | Percentage of chromosomal aberration (%) |
|---|---|---|---|---|---|
| WT CK | 5390 | 195 | 8 | 3.6±0.202 a | 0.148±0.102 c |
| WT UV-B | 5376 | 166 | 25 | 3.08±0.258 c | 0.465±0.056 b |
| atrad21.3 CK | 6998 | 215 | 32 | 3.07±0.195 c | 0.457±0.085 b |
| atrad21.3 UV-B | 7044 | 194 | 45 | 2.75±0.182 d | 0.638±0.105 a |
| 35S:RAD21.3 CK | 5603 | 186 | 7 | 3.3±0.132 ab | 0.125±0.136 c |
| 35S:RAD21.3 UV-B | 5536 | 193 | 21 | 3.48±0.129 ab | 0.379±0.125 bc |
| [1] | 陈慧泽, 韩榕 (2015). 植物响应UV-B辐射的研究进展. 植物学报 50, 790-801. |
| [2] |
陈建权, 程晨, 张梦恬, 张向前, 张尧, 王爱英, 祝建波 (2018). 天山雪莲SiSAD基因与拟南芥AtFAB2基因转化烟草的抗寒性分析. 植物学报 53, 603-611.
DOI URL |
| [3] | 方荧, 刘风珍, 张昆, 张秀荣, 朱素青, 赵炎, 万勇善 (2018). UV-B辐射增强影响作物生长发育的研究进展. 山东农业科学 50, 183-188. |
| [4] | 韩榕 (2002). He-Ne激光对小麦增强UV-B辐射损伤的修复效应及机理. 博士论文. 西安: 西北大学. pp. 52-55. |
| [5] |
李晓阳, 陈慧泽, 韩榕 (2013). UV-B辐射对拟南芥种子萌发和幼苗生长的影响. 植物学报 48, 52-58.
DOI URL |
| [6] | 马兰, 黄华孙, 程汉 (2018). 拟南芥突变体L1.3的表型分析及遗传定位. 分子植物育种 12, 4023-4028. |
| [7] |
王静, 蒋磊, 王艳, 李韶山 (2009). UV-B辐射对拟南芥细胞周期G1/S期转变的影响. 植物学报 44, 426-433.
DOI URL |
| [8] | 徐金龙, 梁爽, 郁飞 (2019). 拟南芥细胞周期基因AtCDC5的功能研究及抗体制备. 江苏农业学报 35, 26-32. |
| [9] | 张亮然 (2006). 水稻RAD21/REC8家族基因的分离与功能分析. 博士论文. 北京: 中国科学院研究生院(植物研究所). pp. 1-3. |
| [10] | 张志良, 瞿伟菁, 李小方 (2009). 植物生理学实验指导(第4版). 北京: 高等教育出版社. pp. 54-229. |
| [11] |
Björn LO, Callaghan TV, Johnsen I, Lee JA, Manetas Y, Paul ND, Sonesson M, Wellburn AR, Coop D, Heide-Jørgensen HS, Gehrke C, Gwynn-Jones D, Johanson U, Kyparissis A, Levizou E, Nikolopoulos D, Petropoulou Y, Stephanou M (1997). The effects of UV-B radiation on European heathland species. Plant Ecol 128, 253-264.
DOI URL |
| [12] |
Björn OL (1996). Effects of ozone depletion and increased UV-B on terrestrial ecosystems. Int J Environ Stud 51, 217-243.
DOI URL |
| [13] | Caldwell MM, Teramura AH, Tevini M, Bornman JF, Björn LO, Kulandaivelu G (1995). Effects of increased solar ultraviolet radiation on terrestrial plants. Ambio 24, 166-173. |
| [14] |
Casadevall R, Rodriguez RE, Debernardi JM, Palatnik JF, Casati P (2013). Repression of growth regulating factors by the microRNA396 inhibits cell proliferation by UV-B radiation in Arabidopsis leaves. Plant Cell 25, 3570-3583.
DOI URL |
| [15] |
Čejka C, Ardan T, Širc J, Michálek J, Beneš J, Brůnová B, Rosina J (2011). Hydration and transparency of the rabbit cornea irradiated with UVB-doses of 0.25 J/cm2 and 0.5 J/cm2 compared with equivalent UVB radiation exposure reaching the human cornea from sunlight. Curr Eye Res 36, 607-613.
DOI URL |
| [16] |
Chen F, Kamradt M, Mulcahy M, Byun Y, Xu HL, McKay MJ, Cryns VL (2002). Caspase proteolysis of the cohesin component RAD21 promotes apoptosis. J Biol Chem 277, 16775-16781.
DOI URL PMID |
| [17] |
Da Costa-Nunes JA, Bhatt AM, O'Shea S, West CE, Bray CM, Grossniklaus U, Dickinson HG (2006). Characterization of the three Arabidopsis thaliana RAD21 cohesins reveals differential responses to ionizing radiation. J Exp Bot 57, 971-983.
URL PMID |
| [18] |
Da Costa-Nunes JA, Capitão C, Kozak J, Costa-Nunes P, Ducasa GM, Pontes O, Angelis KJ (2014). The At- RAD21.1 and AtRAD21.3 Arabidopsis cohesins play a synergistic role in somatic DNA double strand break damage repair. BMC Plant Biol 14, 353.
DOI URL PMID |
| [19] |
Frohnmeyer H, Staiger D (2003). Ultraviolet-B radiation- mediated responses in plants. Balancing damage and protection. Plant Physiol 133, 1420-1428.
DOI URL PMID |
| [20] |
Hauf S, Waizenegger C, Peters JM (2001). Cohesin cleavage by separase required for anaphase and cytokinesis in human cells. Science 293, 1320-1323.
DOI URL PMID |
| [21] |
Hectors K, Jacques E, Prinsen E, Guisez Y, Verbelen JP, Jansen MK, Vissenberg K (2010). UV radiation reduces epidermal cell expansion in leaves of Arabidopsis thaliana. J Exp Bot 61, 4339-4349.
DOI URL PMID |
| [22] |
Hirano T (2000). Chromosome cohesion, condensation, and separation. Annu Rev Biochem 69, 115-144.
DOI URL PMID |
| [23] |
Hoque MT, Ishikawa F (2002). Cohesin defects lead to premature sister chromatid separation, kinetochore dysfunction, and spindle-assembly checkpoint activation. J Biol Chem 277, 42306-42314.
DOI URL PMID |
| [24] |
Jallepalli PV, Waizenegger IC, Bunz F, Langer S, Speicher MR, Peters JM, Kinzler KW, Vogelstein B, Lengauer C (2001). Securin is required for chromosomal stability in human cells. Cell 105, 445-457.
URL PMID |
| [25] |
Jiang L, Wang Y, Björn LO, Li SS (2011). UV-B-induced DNA damage mediates expression changes of cell cycle regulatory genes in Arabidopsis root tips. Planta 233, 831-841.
DOI URL |
| [26] |
Jiang L, Xia M, Strittmatter LI, Makaroff CA (2007). The Arabidopsis cohesin protein SYN3 localizes to the nucleolus and is essential for gametogenesis. Plant J 50, 1020-1034.
DOI URL PMID |
| [27] | Liu X, Yue M, Ji QR, He JF (2013). Effects of ultraviolet-B radiation on primary photophysical process in photosystem II: a fluorescence spectrum analysis. In: Kuang TY, Lu CM, Zhang LX, eds. Photosynthesis Research for Food, Fuel and the Future. Berlin, Heidelberg: Springer. pp. 642-649. |
| [28] |
Losada A (2007). Cohesin regulation: fashionable ways to wear a ring. Chromosoma 116, 321-329.
DOI URL |
| [29] |
Nasmyth K, Peters JM, Uhlmann F (2000). Splitting the chromosome: cutting the ties that bind sister chromatids. Science 288, 1379-1384.
DOI URL PMID |
| [30] |
Nogués S, Allen DJ, Morison JL, Baker NR (1998). Ultraviolet-B radiation effects on water relations, leaf development, and photosynthesis in droughted pea plants. Plant Physiol 117, 173-181.
URL PMID |
| [31] |
Rao H, Uhlmann F, Nasmyth K, Varshavsky A (2001). Degradation of a cohesin subunit by the N-end rule pathway is essential for chromosome stability. Nature 410, 955-959.
DOI URL PMID |
| [32] |
Robson TM, Klem K, Urban O, Jansen MAK (2015). Re-interpreting plant morphological responses to UV-B radiation. Plant Cell Environ 38, 856-866.
DOI URL PMID |
| [33] |
Sadano H, Sugimoto H, Sakai F, Nomura N, Osumi T (2000). NXP-1, a human protein related to Rad21/Scc1/ Mcd1, is a component of the nuclear matrix. Biochem Biophys Res Commun 267, 418-422.
URL PMID |
| [34] |
Searles PS, Flint SD, Caldwell MM (2001). A meta-analysis of plant field studies simulating stratospheric ozone depletion. Oecologia 127, 1-10.
DOI URL PMID |
| [35] |
Skibbens RV (2009). Establishment of sister chromatid cohesion. Curr Biol 19, R1126-R1132.
DOI URL PMID |
| [36] |
Sugimoto-Shirasu K, Roberts K (2003). ‘Big it up’: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6, 544-553.
DOI URL PMID |
| [37] |
Suzuki G, Nishiuchi C, Tsuru A, Kako E, Li J, Yamamoto M, Mukai Y (2013). Cellular localization of mitotic RAD21 with repetitive amino acid motifs in Allium cepa. Gene 514, 75-81.
DOI URL |
| [38] |
Uhlmann F, Lottspeich F, Nasmyth K (1999). Sister- chromatid separation at anaphase onset is promoted by cleavage of the cohesin subunit Scc1. Nature 400, 37-42.
DOI URL PMID |
| [39] |
Uhlmann F, Wernic D, Poupart MA, Koonin EV, Nasmyth K (2000). Cleavage of cohesin by the CD clan protease separin triggers anaphase in yeast. Cell 103, 375-386.
DOI URL PMID |
| [40] |
Vandenbussche F, Yu N, Li WD, Vanhaelewyn L, Hamshou M, Van Der Straeten D, Smagghe G (2018). An ultraviolet B condition that affects growth and defense in Arabidopsis. Plant Sci 268, 54-63.
DOI URL PMID |
| [41] |
Waizenegger IC, Hauf S, Meinke A, Peters JM (2000). Two distinct pathways remove mammalian cohesin from chromosome arms in prophase and from centromeres in ana- phase. Cell 103, 399-410.
DOI URL PMID |
| [42] |
Warren WD, Steffensen S, Lin E, Coelho P, Loupart ML, Cobbe N, Lee JY, McKay M, Orr-Weaver TL, Heck MMS, Sunkel CE (2000). The Drosophila RAD21 cohesin persists at the centromere region in mitosis. Curr Biol 10, 1463-1466.
URL PMID |
| [43] |
Xu HL, Beasley M, Verschoor S, Inselman A, Handel MA, McKay MJ (2004). A new role for the mitotic RAD21/ SCC1 cohesin in meiotic chromosome cohesion and segregation in the mouse. EMBO Rep 5, 378-384.
DOI URL PMID |
| [44] |
Xu HL, Yan YQ, Deb S, Rangasamy D, Germann M, Malaterre J, Eder NC, Ward RL, Hawkins NJ, Tothill RW, Chen L, Mortensen NJ, Fox SB, McKay MJ, Ramsay RG (2014). Cohesin rad21 mediates loss of heterozygosity and is upregulated via Wnt promoting transcriptional dysregulation in gastrointestinal tumors. Cell Rep 9, 1781-1797.
URL PMID |
| [1] | Yanxiao Chen, Yaping Li, Jinjun Zhou, Lixia Xie, Yongbin Peng, Wei Sun, Yanan He, onghui Jiang, Zenglan Wang, Chongke Zheng, Xianzhi Xie. Effect of Amino Acid Point Mutations on the Structure and Function of Phytochrome B in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 481-494. |
| [2] | Jixuan Yang, Xuefei Wang, Hongya Gu. Genetic Basis of Flowering Time Variations in Tibetan Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 373-382. |
| [3] | Gang Wang, Ertao Wang. The Broad-spectrum Innate Resistance Against Clubroot Disease Conferred by WeiTsing is Mechanistically Revealed [J]. Chinese Bulletin of Botany, 2023, 58(3): 356-358. |
| [4] | Yang Yongqing, Guo Yan. Analysis of the pH Sensing Mechanism of Plant Apoplasts [J]. Chinese Bulletin of Botany, 2022, 57(4): 409-411. |
| [5] | Tiantian Zhi, Zhou Zhou, Chengyun Han, Chunmei Ren. PAD4 Mutation Accelerating Programmed Cell Death in Arabidopsis thaliana Tyrosine Degradation Deficient Mutant sscd1 [J]. Chinese Bulletin of Botany, 2022, 57(3): 288-298. |
| [6] | Yanyan Li, Yanhua Qi. Advances in Biological Functions of Aux/IAA Gene Family in Plants [J]. Chinese Bulletin of Botany, 2022, 57(1): 30-41. |
| [7] | Yongmei Che, Yanjun Sun, Songchong Lu, Lixia Hou, Xinxin Fan, Xin Liu. AtMYB77 Involves in Lateral Root Development via Regulating Nitric Oxide Biosynthesis under Drought Stress in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2021, 56(4): 404-413. |
| [8] | Ting Wang, Huanhuan Yang, Hongwei Zhao, Josef Voglmeir, Li Liu. Changes of Protein N-glycosylation in the Growth of Arabidopsis thaliana and Effects of Enzymatic Deglycosylation on Root Development [J]. Chinese Bulletin of Botany, 2021, 56(3): 262-274. |
| [9] | Yuqing Lin, Yanhua Qi. Advances in Auxin Efflux Carrier PIN Proteins [J]. Chinese Bulletin of Botany, 2021, 56(2): 151-165. |
| [10] | Long Ma, Guilin Li, Shipeng Li, Su Jiang. An Improved Protocol for Whole Mount Clearing of Plant Root Tip [J]. Chinese Bulletin of Botany, 2020, 55(5): 596-604. |
| [11] | Nan Zhang,Ziguang Liu,Shichen Sun,Shengyi Liu,Jianhui Lin,Yifang Peng,Xiaoxu Zhang,He Yang,Xi Cen,Juan Wu. Response of AtR8 lncRNA to Salt Stress and Its Regulation on Seed Germination in Arabidopsis [J]. Chinese Bulletin of Botany, 2020, 55(4): 421-429. |
| [12] | Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang. A Role of Arabidopsis Phosphoinositide Kinase, FAB1, in Root Hair Growth [J]. Chinese Bulletin of Botany, 2020, 55(2): 126-136. |
| [13] | Zeyuan Zuo,Wanlin Liu,Jie Xu. Evolution and Functional Analysis of Gene Clusters in Anther Tapetum Cells of Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2020, 55(2): 147-162. |
| [14] | Wanyue Xu,Yingxiang Wang. Chromosome Behaviors of Male Meiocytes by Chromosome Spread in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2019, 54(5): 620-624. |
| [15] | Juqing Kang, Tianshu Sun, Huiting Zhang, Yihao Shi. Quantitative Trait Loci Mapping Platform of Natural Populations of Arabidopsis thaliana along the Yangtze River in China [J]. Chinese Bulletin of Botany, 2016, 51(5): 659-666. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||