

Chinese Bulletin of Botany ›› 2020, Vol. 55 ›› Issue (2): 126-136.DOI: 10.11983/CBB19242 cstr: 32102.14.CBB19242
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang( )
)
Received:2019-12-16
Accepted:2020-02-17
Online:2020-03-01
Published:2020-02-17
Contact:
Chao Wang
Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang. A Role of Arabidopsis Phosphoinositide Kinase, FAB1, in Root Hair Growth[J]. Chinese Bulletin of Botany, 2020, 55(2): 126-136.
| Primer name | Primer sequence (5'-3') | Purpose |
|---|---|---|
| F1 | GGCGAGGGATATTGA GTTCAG | Genotyping of fab1b-2 and RT-PCR |
| R1 | GTCATACATGTGGGA TCACCG | Genotyping of fab1b-2 and RT-PCR |
| F2 | TGGGAGAAAACAGCAA TGAAC | Genotyping of fab1c-2 and RT-PCR |
| R2 | CACGACAACTTCCCCG AAGCACAA | Genotyping of fab1c-2 and RT-PCR |
| F3 | AGGTTGGGATGAATGG TTTTG | Genotyping of fab1d-2 and RT-PCR |
| R3 | AGGTCGTGCCGTATC TCTTTC | Genotyping of fab1d-2 and RT-PCR |
| sgtDs3'-1 | GGTTCCCGTCCGATT TCGACT | Genotyping of fab1c-2 |
| LBb1.3 | ATTTTGCCGATTTCG GAAC | Genotyping of fab1b-2 and fab1d-2 |
| AtACTIN-F | GTCGTACAACCGGTA TTGTG | Internal control for RT-PCR |
| AtACTIN-R | GAGCTGGTCTTTGAG GTTTC | Internal control for RT-PCR |
Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') | Purpose |
|---|---|---|
| F1 | GGCGAGGGATATTGA GTTCAG | Genotyping of fab1b-2 and RT-PCR |
| R1 | GTCATACATGTGGGA TCACCG | Genotyping of fab1b-2 and RT-PCR |
| F2 | TGGGAGAAAACAGCAA TGAAC | Genotyping of fab1c-2 and RT-PCR |
| R2 | CACGACAACTTCCCCG AAGCACAA | Genotyping of fab1c-2 and RT-PCR |
| F3 | AGGTTGGGATGAATGG TTTTG | Genotyping of fab1d-2 and RT-PCR |
| R3 | AGGTCGTGCCGTATC TCTTTC | Genotyping of fab1d-2 and RT-PCR |
| sgtDs3'-1 | GGTTCCCGTCCGATT TCGACT | Genotyping of fab1c-2 |
| LBb1.3 | ATTTTGCCGATTTCG GAAC | Genotyping of fab1b-2 and fab1d-2 |
| AtACTIN-F | GTCGTACAACCGGTA TTGTG | Internal control for RT-PCR |
| AtACTIN-R | GAGCTGGTCTTTGAG GTTTC | Internal control for RT-PCR |
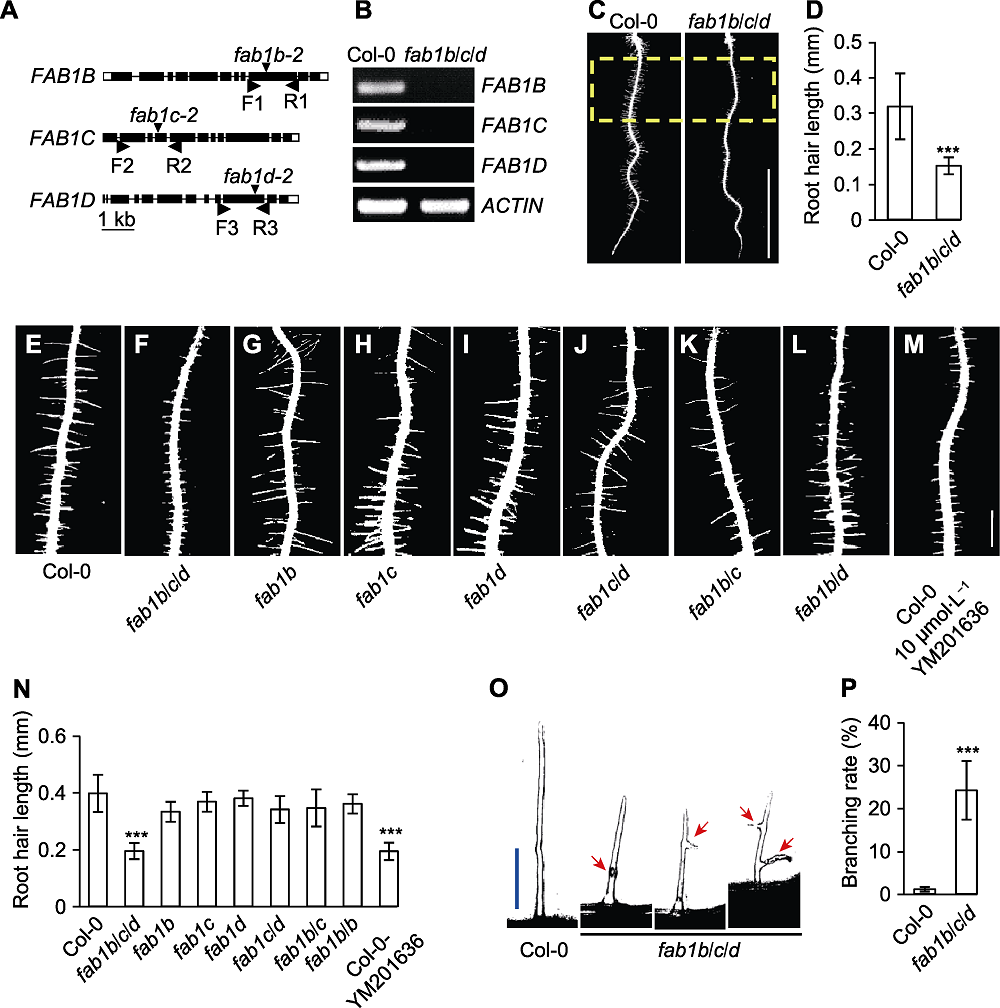
Figure 1 FAB1B, FAB1C and FAB1D regulate root hair growth in Arabidopsis (A) FAB1 gene structure and T-DNA insertion sites; (B) Analysis of gene expression of FAB1B, FAB1C and FAB1D by RT-PCR; (C) Assay of root hair length in 5-day-old seedlings (the yellow dotted box represents quantitative area) (Bar=5 mm); (D) Quantification of root hair length; (E)-(M) The root hair phenotype of the FAB1 single mutants, double mutants and YM201636 (FAB1-specific inhibitor) treatment of Col-0 seedling (Bar=0.5 mm); (N) Quantification of (E)-(M) mutant root hair length; (O) Images of the typical root hair morphologies, the root hair morphologies were categorized as swollen or branched (red arrow indicates) (Bar=75 μm); (P) Quantification of frequency of branched root hairs (%). *** P<0.001 (Student’s t-test)
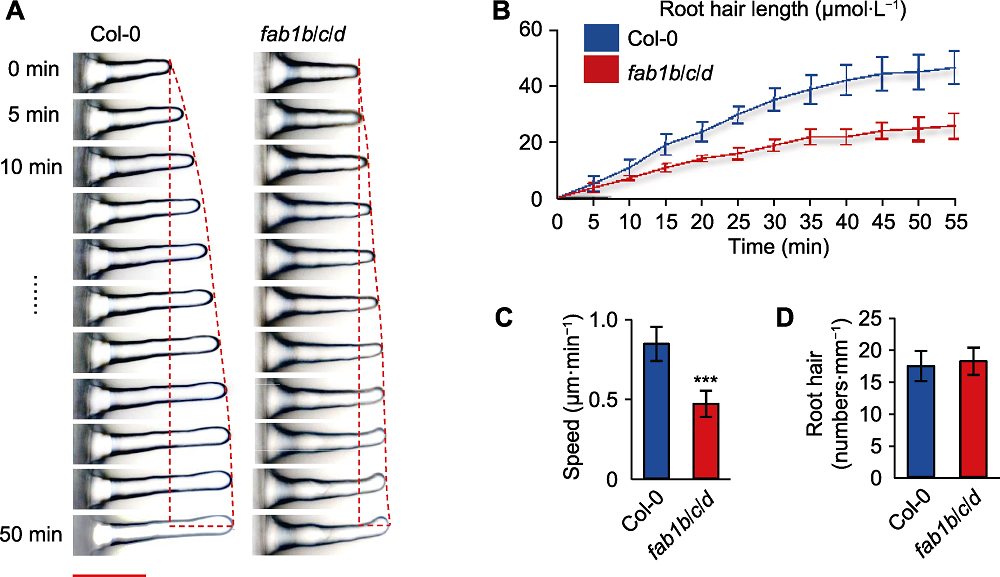
Figure 2 Slow growth of Arabidopsis fab1b/c/d mutant root hairs (A) Growth dynamics of individual Col-0 and fab1b/c/d root hairs (fluorescence microscopy was used to assess root hair elongation, showing consecutive frames of growing root hairs for a period of 50 minutes, pictures were taken every 5 minutes) (Bar=50 µm); (B) Root hair length per unit time; (C) Root hairs growth speed (*** P<0.001, Student’s t-test); (D) Average root hairs number.
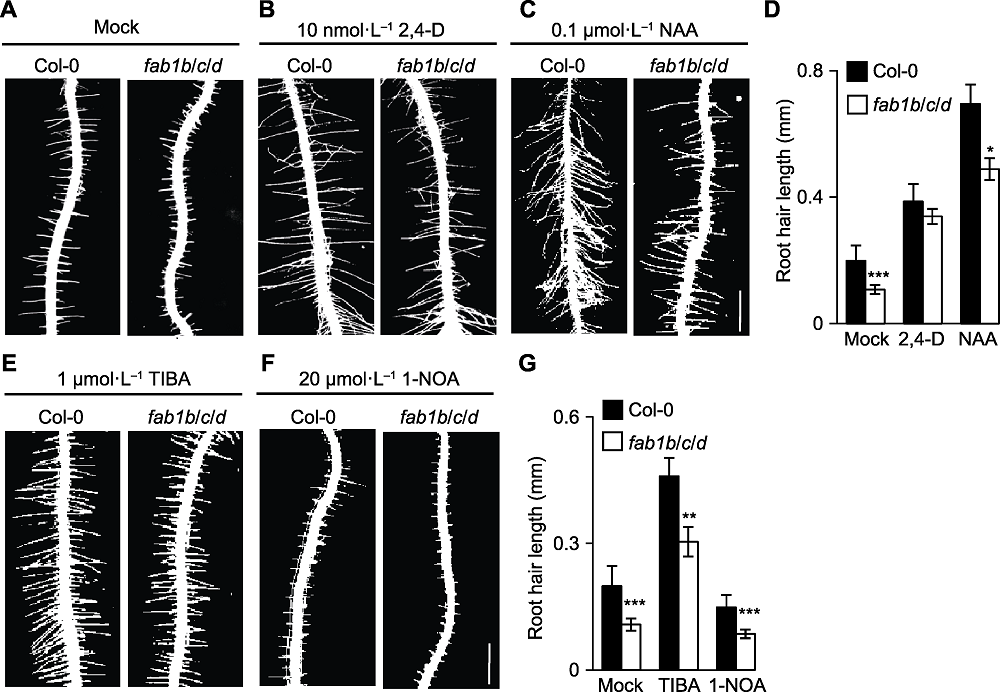
Figure 3 Exogenous auxin application partially rescued the root hair defect of Arabidopsis fab1b/c/d mutants (A)-(C) Root hairs of fab1b/c/d treated with DMSO (Mock), 10 nmol·L-1 2,4-D and 0.1 μmol·L-1 NAA, respectively, the phenotype of root hairs was rescued partially (Bar=0.5 mm); (D) Quantification of root hair length; (E), (F) Col-0 and fab1b/c/d seedlings were transferred to plates containing TIBA (auxin efflux inhibitors) and 1-NOA (auxin influx inhibitor) (Bar=0.5 mm); (G) Quantitative analysis of root hair length. * P<0.05; ** P<0.01; *** P<0.001 (Student’s t-test)
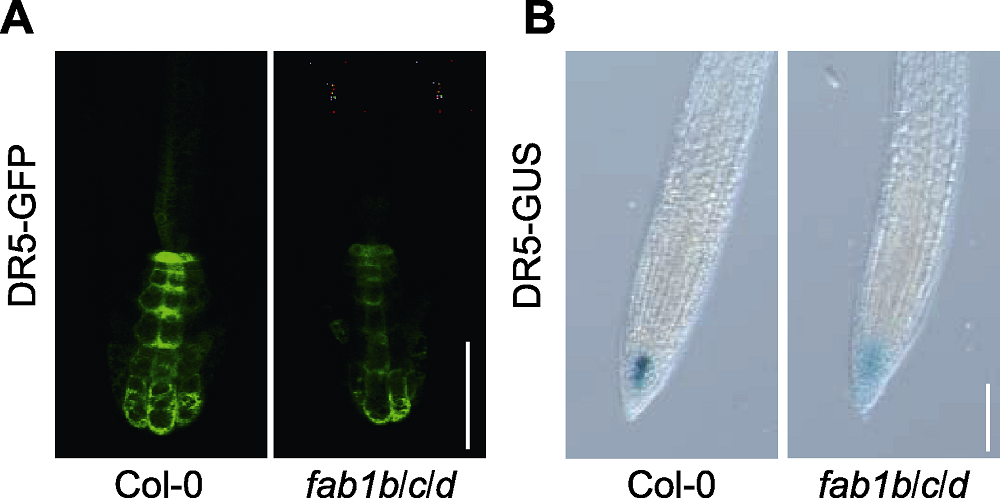
Figure 4 FAB1 affects auxin distribution in Arabidopsis (A) The expression analysis of DR5-GFP for auxin distribution in the root (Bar=75 µm); (B) DR5-GUS expression in Col-0 and fab1b/c/d (Bar=100 µm)
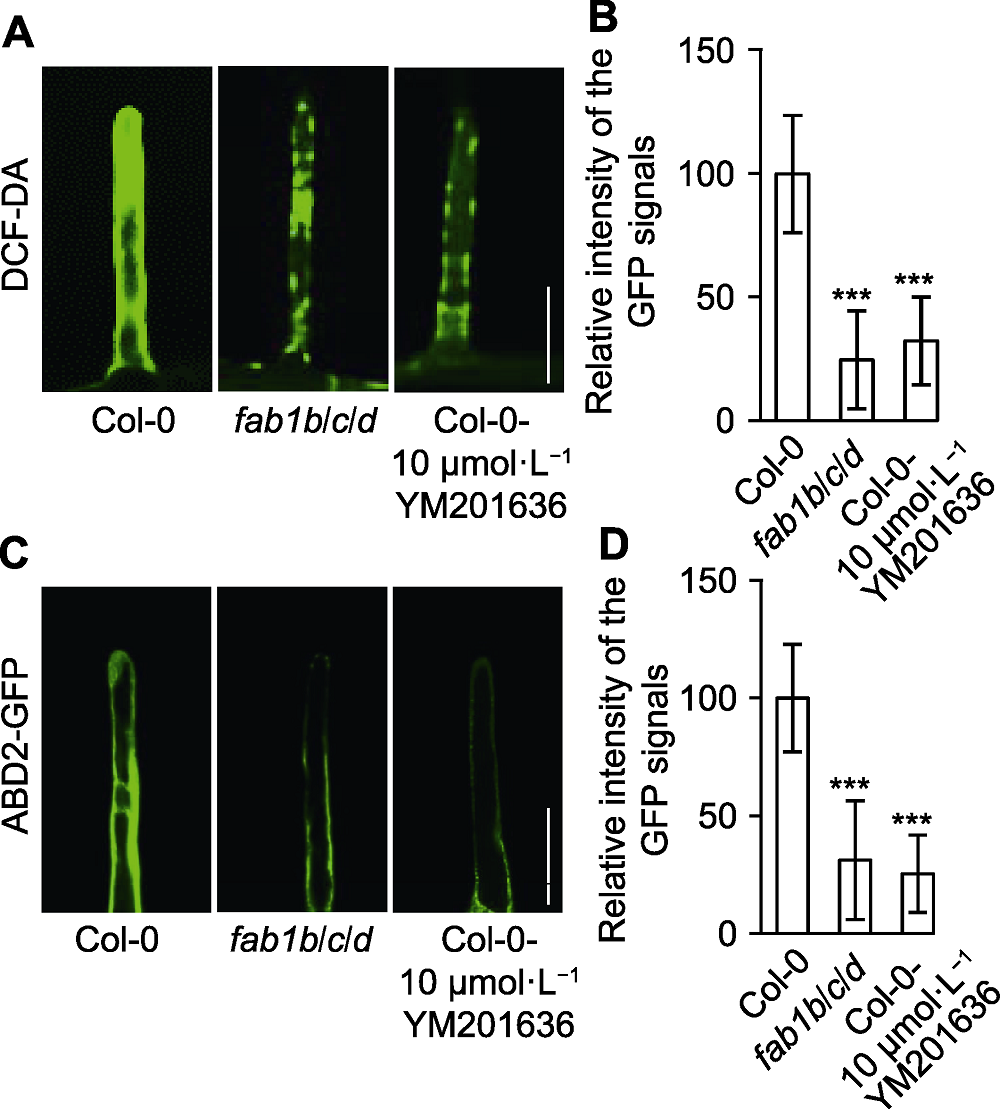
Figure 5 Reactive oxygen species (ROS) intensity and actin stability were altered in root hairs of Arabidopsis fab1b/c/d seedlings (A) Total ROS generated by oxidation of DCF-DA in wild type, fab1b/c/d and Col-0-YM201636 root (Bar=25 µm); (B) Relative intensity of the GFP signals; (C) Distribution of actin cytoskeleton marker ABD2 in root hair of Col-0, fab1b/c/d and Col-0-YM201636 treatment (Bar=25 µm); (D) Average relative intensity of the ABD2-GFP signals. *** P<0.001 (Student’s t-test)
| [1] | 李林, 谭康, 唐秀光, 晁晓婷, 汶晨曦, 白壮东, 丰华玲, 刘文哲, 苏慧 ( 2016). 拟南芥根毛衰老死亡过程的PCD检测. 植物学报 51, 194-201. |
| [2] | 王立德, 廖红, 王秀荣, 严小龙 ( 2004). 植物根毛的发生、发育及养分吸收. 植物学通报 21, 649-659. |
| [3] | Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU, Lee Y ( 2013). Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 25, 2202-2216. |
| [4] | Balla T ( 2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93, 1019-1173. |
| [5] | Carol RJ, Dolan L ( 2006). The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot 57, 1829-1834. |
| [6] | Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang HM, Casero P, Sandberg G, Bennett MJ ( 2003). Dissecting Arabidopsis lateral root development. Trends Plant Sci 8, 165-171. |
| [7] | Cui SK, Suzaki T, Tominaga-Wada R, Yoshida S ( 2018). Regulation and functional diversification of root hairs. Semin Cell Dev Biol 83, 115-122. |
| [8] | De Craene JO, Bertazzi D, Bär S, Friant S ( 2017). Phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. Int J Mol Sci 18, 634. |
| [9] | Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M ( 2005). Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9, 109-119. |
| [10] | Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH ( 1997). Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390, 187-192. |
| [11] | Dove SK, Dong KZ, Kobayashi T, Williams FK, Michell RH ( 2009). Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve under PPIn endo-lysosome function. Biochem J 419, 1-13. |
| [12] | Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT ( 2010). Differential auxin-transporting activities of PIN- FORMED proteins in Arabidopsis root hair cells. Plant Physiol 153, 1046-1061. |
| [13] | Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD ( 1998). Fab1p is essential for PtdIns(3)P5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143, 65-79. |
| [14] | Griersona C, Nielsen E, Ketelaarc T, Schiefelbein J ( 2014). Root hairs. Arabidopsis Book 12, e0172. |
| [15] | Hasegawa J, Strunk BS, Weisman LS ( 2017). PI5P and PI(3,5)P2: minor, but essential phosphoinositides. Cell Struct Funct 42, 49-60. |
| [16] | Hirano T, Konno H, Takeda S, Dolan L, Kato M, Aoyama T, Higaki T, Takigawa-Imamura H, Sato MH ( 2018). PtdIns(3,5)P2 mediates root hair shank hardening in Arabidopsis. Nat Plants 4, 888-897. |
| [17] | Hirano T, Matsuzawa T, Takegawa K, Sato MH ( 2011). Loss-of-function and gain-of-function mutations in FAB1A/B impair endomembrane homeostasis, conferring pleiotropic developmental abnormalities in Arabidopsis. Plant Physiol 155, 797-807. |
| [18] | Hirano T, Munnik T, Sato MH ( 2015). Phosphatidylinositol 3-phosphate 5-kinase, FAB1/PIKfyve kinase mediates endosome maturation to establish endosome-cortical microtubule interaction in Arabidopsis. Plant Physiol 169, 1961-1974. |
| [19] | Hirano T, Stecker K, Munnik T, Xu HX, Sato MH ( 2017). Visualization of phosphatidylinositol 3,5-bisphosphate dynamics by a tandem ML1N-based fluorescent protein probe in Arabidopsis. Plant Cell Physiol 58, 1185-1195. |
| [20] | Ishida T, Kurata T, Okada K, Wada T ( 2008). A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59, 365-386. |
| [21] | Jin N, Lang MJ, Weisman LS ( 2016). Phosphatidylinositol 3,5-bisphosphate: regulation of cellular events in space and time. Biochem Soc Trans 44, 177-184. |
| [22] | Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HMO, Grierson CS ( 2009). Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol 11, 78-84. |
| [23] | Jones MA, Raymond MJ, Yang ZB, Smirnoff N ( 2007). NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot 58, 1261-1270. |
| [24] | Ketelaar T, de Ruijter NCA, Emons AMC ( 2003). Unstable F-Actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15, 285-292. |
| [25] | Kirsch SA, Kugemann A, Carpaneto A, Böckmann RA, Dietrich P ( 2018). Phosphatidylinositol-3,5-bisphosphate lipid-binding-induced activation of the human two-pore channel 2. Cell Mol Life Sci 75, 3803-3815. |
| [26] | Kusano H, Testerink C, Vermeer JEM, Tsuge T, Shimada H, Oka A, Munnik T, Aoyama T ( 2008). The Arabidopsis phosphatidylinositol phosphate 5-Kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20, 367-380. |
| [27] | Lee RDW, Cho HT ( 2013). Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front Plant Sci 4, 448. |
| [28] | Lee SH, Cho HT ( 2006). PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18, 1604-1616. |
| [29] | Lee Y, Bak G, Choi Y, Chuang WI, Cho HT, Lee Y ( 2008). Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol 147, 624-635. |
| [30] | Mangano S, Denita-Juarez SP, Choi HS, Marzol E, Hwang Y, Ranocha P, Velasquez SM, Borassi C, Barberini ML, Aptekmann AA, Muschietti JP, Nadra AD, Dunand C, Cho HT, Estevez JM ( 2017). Molecular link between auxin and ROS-mediated polar growth. Proc Natl Acad Sci USA 114, 5289-5294. |
| [31] | McCartney AJ, Zhang YL, Weisman LS ( 2014). Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. BioEssays 36, 52-64. |
| [32] | Mendrinna A, Persson S ( 2015). Root hair growth: it's a one way street. F1000Prime Rep 7, 23. |
| [33] | Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S ( 2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104, 20996-21001. |
| [34] | Mueller-Roeber B, Pical C ( 2002). Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130, 22-46. |
| [35] | Nakamura M, Claes AR, Grebe T, Hermkes R, Viotti C, Ikeda Y, Grebe M ( 2018). Auxin and ROP GTPase signaling of polar nuclear migration in root epidermal hair cells. Plant Physiol 176, 378-391. |
| [36] | Nishimura T, Hayashi K, Suzuki H, Gyohda A, Takaoka C, Sakaguchi Y, Matsumoto S, Kasahara H, Sakai T, Kato J, Kamiya Y, Koshiba T ( 2014). Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J 77, 352-366. |
| [37] | Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS ( 2011). Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana(L.) Heynh. under elevated CO2. Plant Cell Environ 34, 1304-1317. |
| [38] | Payrastre B, Missy K, Giuriato S, Bosin S, Plantavid M, Gratacap MP ( 2001). Phosphoinositides: key players in cell signaling, in time and space. Cell Signal 13, 377-387. |
| [39] | Pei WK, Du F, Zhang Y, He T, Ren HY ( 2012). Control of the actin cytoskeleton in root hair development. Plant Sci 187, 10-18. |
| [40] | Qin H, Huang RF ( 2018). Auxin controlled by ethylene steers root development. Int J Mol Sci 19, 3656. |
| [41] | Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S ( 2002). Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130, 1908-1917. |
| [42] | Schoenaers S, Balcerowicz D, Breen G, Hill K, Zdanio M, Mouille G, Holman TJ, Oh J, Wilson MH, Nikonorova N, Vu LD, De Smet I, Swarup R, De Vos WH, Pintelon I, Adriaensen D, Grierson C, Bennett MJ, Vissenberg K ( 2018). The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr Biol 28, 722-732. |
| [43] | Serrazina S, Dias FV, Malhó R ( 2014). Characterization of FAB1 phosphatidylinositol kinases in Arabidopsis pollen tube growth and fertilization. New Phytol 203, 784-793. |
| [44] | Shibata M, Sugimoto K ( 2019). A gene regulatory network for root hair development. J Plant Res 132, 301-309. |
| [45] | Stenzel I, Ischebeck T, König S, Holubowska A, Sporysz M, Hause B, Heilmann I ( 2008). The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 20, 124-141. |
| [46] | Takasuga S, Horie Y, Sasaki J, Sun-Wada GH, Kawamura N, Iizuka R, Mizuno K, Equchi S, Kofuji S, Kimura H, Yamazaki M, Horie C, Odanaga E, Sato Y, Chida S, Kontani K, Harada A, Katada T, Suzuki A, Wada Y, Ohnishi H, Sasaki T ( 2013). Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proc Natl Acad Sci USA 110, 1726-1731. |
| [47] | Velasquez SM, Barbez E, Kleine-Vehn J, Estevez JM ( 2016). Auxin and cellular elongation. Plant Physiol 170, 1206-1215. |
| [48] | Vijayakumar P, Datta S, Dolan L ( 2016). ROOT HAIR DEFECTIVE SIX-LIKE 4 (RSL4) promotes root hair elongation by transcriptionally regulating the expression of genes required for cell growth. New Physiol 212, 944-953. |
| [49] | Whitley P, Hinz S, Doughty J ( 2009). Arabidopsis FAB1/PIKfyve proteins are essential for development of viable pollen. Plant Physiol 151, 1812-1822. |
| [50] | Zhang YL, Li E, Feng QN, Zhao XY, Ge FR, Zhang Y, Li S ( 2015). Protein palmitoylation is critical for the polar growth of root hairs in Arabidopsis. BMC Plant Biol 15, 50. |
| [1] | HAN Fei, WANG Ge, WU Shuai-Kai, LIN Mao, DONG Kuan-Hu, $\boxed{\hbox{WANG Chang-Hui}}$ , SU Yuan. Effects of extreme precipitation on soil gross nitrification rate, gross nitrogen mineralization rate and sensitivity of different types of grassland [J]. Chin J Plant Ecol, 2025, 49(5): 697-709. |
| [2] | WANG Bei-Bei, WU Su, WANG Miao-Miao, HU Jin-Tao. Contributions of radiative, structural, and physiological information of solar-induced chlorophyll fluorescence on predicting crop gross primary production across temporal scales [J]. Chin J Plant Ecol, 2025, 49(4): 562-572. |
| [3] | LI Lin, HUANG Jia-Fang, DING Zhong-Hao, GUO Ping-Ping, CAI Yuan-Bin, LI Shi-Hua, LI Yun-Qin, LUO Min. Impact of increased inundation height on the net ecosystem CO2 exchange in a Cyperus malaccensis tidal marsh [J]. Chin J Plant Ecol, 2025, 49(4): 526-539. |
| [4] | HU Xiao-Hui, WANG Xing-Chang, DONG Han-Jun, LIU Yu-Long, YUAN Dan-Yang, LIU Di, WANG Xiao-Chun. Variation and coordination of non-structural carbohydrates among organs in 32 tree species from a temperate conifer-broadleaf mixed forest in Northeast China [J]. Chin J Plant Ecol, 2025, 49(3): 432-445. |
| [5] | Xuemin Cao, Ying Bao, Yuexin Zhang, Ruijie Li, Jianxin Su, Wei Zhang. Tissue Culture, Rapid Propagation and Efficient Transient Expression Systems of Rosa multiflora [J]. Chinese Bulletin of Botany, 2025, 60(2): 235-245. |
| [6] | WANG Yin, TONG Xiao-Juan, ZHANG Jin-Song, LI Jun, MENG Ping, LIU Pei-Rong, ZHANG Jing-Ru. Impact of drought on carbon and water fluxes and their coupling in a Quercus variabilis plantation [J]. Chin J Plant Ecol, 2024, 48(9): 1157-1171. |
| [7] | ZHANG Meng-Di, XIANG Guan-Hai, WEN Yi-Yao, WANG Huan, Hugejile , BAI Yong-Fei, WANG Zhong-Wu, ZHENG Shu-Xia. Response of carbon exchange between shrub and grass patches to increased seasonal precipitation: a comparative analysis based on aboveground net primary productivity and leaf area index standardization [J]. Chin J Plant Ecol, 2024, 48(8): 1035-1049. |
| [8] | Quanquan Jin, Ying Xiang, Hua Wang, Xinqiang Xi. Drosophilidae species diversity and parasitism rate in different types of green spaces in Xianlin university town, Nanjing [J]. Biodiv Sci, 2024, 32(8): 24156-. |
| [9] | Suowei Wu, Xueli An, Xiangyuan Wan. Molecular Mechanisms of Male Sterility and their Applications in Biotechnology-based Male-sterility Hybrid Seed Production in Maize [J]. Chinese Bulletin of Botany, 2024, 59(6): 932-949. |
| [10] | Xiang Song, Luyao Wang, Boxiao Fu, Shuangda Li, Yuanyuan Wei, Yan Hong, Silan Dai. Advances in Identification and Synthesis of Promoter Elements in Higher Plants [J]. Chinese Bulletin of Botany, 2024, 59(5): 691-708. |
| [11] | Siying Qin, Yan Luo, He Zhang, Jun Hu, Jugou Liao. Optimization of Preparation and Detection Methods for Pollen Tube Cell Wall by Atomic Force Microscopy [J]. Chinese Bulletin of Botany, 2024, 59(5): 783-791. |
| [12] | Wenna Chen, Liangtao Li, Lu Zhou, Gang Yao. Recent Uplift of the Taihang Mountains Triggered the Lineage Diversification within the Genus Taihangia (Rosaceae) [J]. Chinese Bulletin of Botany, 2024, 59(5): 763-773. |
| [13] | Yuying Zhou, Hui Chen, Simu Liu. Research Progress on Auxin Responsive Non-canonical Aux/IAA Proteins in Plants [J]. Chinese Bulletin of Botany, 2024, 59(4): 651-658. |
| [14] | Yanxiao Chen, Yaping Li, Jinjun Zhou, Lixia Xie, Yongbin Peng, Wei Sun, Yanan He, onghui Jiang, Zenglan Wang, Chongke Zheng, Xianzhi Xie. Effect of Amino Acid Point Mutations on the Structure and Function of Phytochrome B in Arabidopsis thaliana [J]. Chinese Bulletin of Botany, 2024, 59(3): 481-494. |
| [15] | YANG Yu-Meng, LAI Quan, LIU Xin-Yi. Quantitative analysis of climate change and human activities on vegetation gross primary productivity in Nei Mongol, China [J]. Chin J Plant Ecol, 2024, 48(3): 306-316. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||