

植物学报 ›› 2021, Vol. 56 ›› Issue (6): 687-698.DOI: 10.11983/CBB21025 cstr: 32102.14.CBB21025
王田幸子, 朱峥, 陈悦, 刘玉晴, 燕高伟, 徐珊, 张彤, 马金姣, 窦世娟, 李莉云( ), 刘国振(
), 刘国振( )
)
收稿日期:2021-01-31
接受日期:2021-08-09
出版日期:2021-11-01
发布日期:2021-11-12
通讯作者:
李莉云,刘国振
作者简介:gzhliu@hebau.edu.cn基金资助:
Tianxingzi Wang, Zheng Zhu, Yue Chen, Yuqing Liu, Gaowei Yan, Shan Xu, Tong Zhang, Jinjiao Ma, Shijuan Dou, Liyun Li( ), Guozhen Liu(
), Guozhen Liu( )
)
Received:2021-01-31
Accepted:2021-08-09
Online:2021-11-01
Published:2021-11-12
Contact:
Liyun Li,Guozhen Liu
摘要: Xa21对白叶枯病菌(Xanthomonas oryzae pv. oryzae, Xoo)具有广谱抗性, 是最早被克隆的水稻(Oryza sativa)抗白叶枯病基因。前期研究表明, OsWRKY42可能在Xa21介导的抗病反应中发挥作用。在Xa21基因遗传背景下制备了OsWRKY42的RNA干扰株系, 将经免疫印迹确认的转基因株系接种白叶枯病菌, 结果表明, 与抗病对照4021相比, 转基因株系的病斑长度增加, 说明OsWRKY42的丰度下调抑制了Xa21对白叶枯病的抗性反应。免疫印迹分析表明, 在OsWRKY42-RNAi转基因水稻中, OsPR6、OsPR15和OsPR16的蛋白质丰度降低, OsPR1A、OsPR1B、OsPR2和OsPR10A的蛋白质丰度升高, 表明这些病程相关蛋白质可能位于OsWRKY42基因的下游, 受OsWRKY42调控并参与Xa21介导的抗病性。研究结果表明, OsWRKY42是Xa21介导的抗白叶枯病途径新元件, 增进了对Xa21介导的水稻抗病机理的认识。
王田幸子, 朱峥, 陈悦, 刘玉晴, 燕高伟, 徐珊, 张彤, 马金姣, 窦世娟, 李莉云, 刘国振. 水稻OsWRKY42是Xa21介导的抗白叶枯病途径新元件. 植物学报, 2021, 56(6): 687-698.
Tianxingzi Wang, Zheng Zhu, Yue Chen, Yuqing Liu, Gaowei Yan, Shan Xu, Tong Zhang, Jinjiao Ma, Shijuan Dou, Liyun Li, Guozhen Liu. Rice OsWRKY42 is a Novel Element in Xa21-mediated Resistance Pathway Against Bacterial Leaf Blight. Chinese Bulletin of Botany, 2021, 56(6): 687-698.
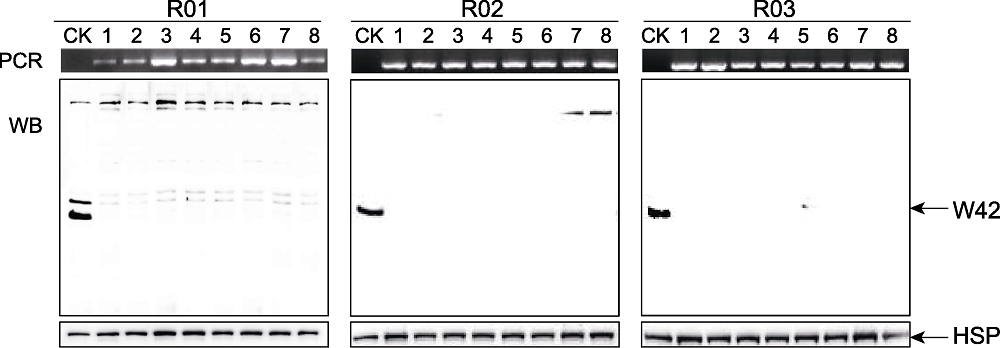
图1 OsWRKY42-RNAi转基因水稻的鉴定 将等量的4021和OsWRKY42-RNAi转基因水稻总蛋白质用Tricine-SDS-PAGE分离, 用Mini Chemiluminescent Imager and Sage Capture软件检测蛋白信号。PCR: PCR鉴定结果; WB: Western blot鉴定结果; CK: 转基因受体对照4021; R01-R03: 独立的OsWRKY42-RNAi转基因株系; 1-8: 同一转化株系中的不同转化植株; W42: 抗OsWRKY42多克隆抗体识别条带; HSP: 抗OsHSP82单克隆抗体识别条带。
Figure 1 Verification of rice OsWRKY42-RNAi transgenic lines Equal amounts of total protein from 4021 and OsWRKY42-RNAi transgenic lines were separated by Tricine-SDS-PAGE. The protein signal was detected by Mini Chemiluminescent Imager and Sage Capture software. PCR: PCR verification; WB: Western blot verification; CK: 4021 control of transgenic donor; R01-R03: Independent OsWRKY42-RNAi transgenic lines; 1-8: Individual plants within same transgenic lines; W42: Anti-OsWRKY42 polyclonal antibody detected band; HSP: Anti-OsHSP82 monoclonal antibody detected band.
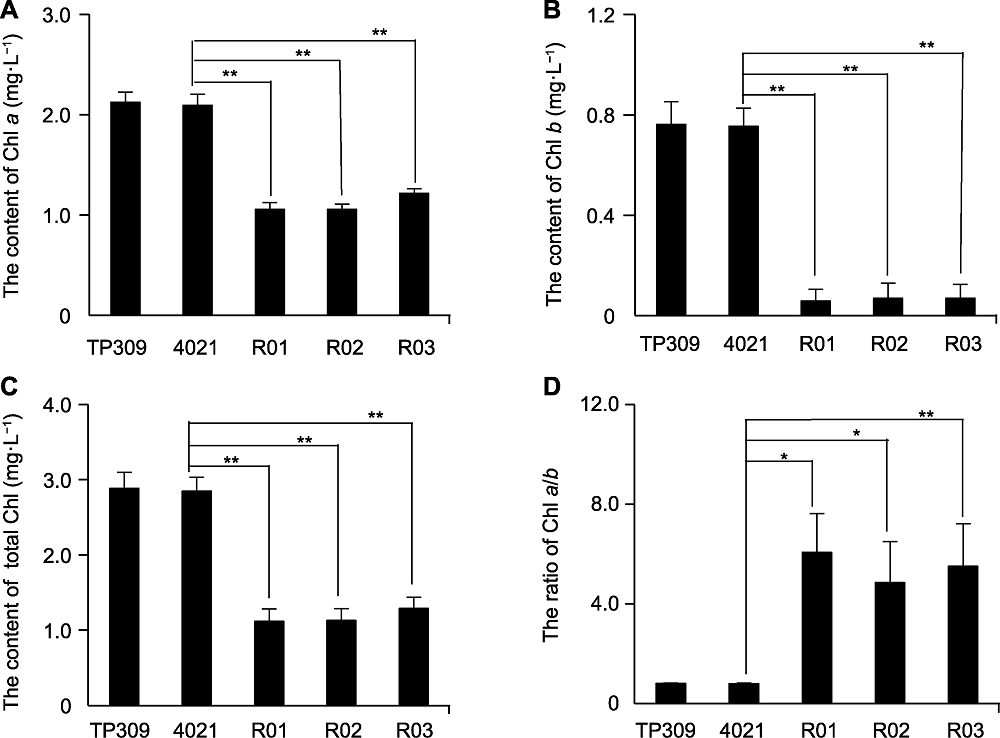
图2 OsWRKY42-RNAi转基因水稻的叶绿素含量 (A)-(D) 依次为OsWRKY42-RNAi转基因水稻中叶绿素a (Chl a)、叶绿素b (Chl b)、总叶绿素(total Chl)及叶绿素a/叶绿素b的比值(Chl a/b)。TP309: 野生型; 4021: 表达Xa21的转基因TP309纯合植株; R01-R03: OsWRKY42-RNAi转基因水稻。误差线表示标准差(SD) (n=3); *表示差异显著(P<0.05); **表示差异极显著(P<0.01) (Student's t-test)。
Figure 2 Chlorophyll contents in rice OsWRKY42-RNAi transgenic lines (A)-(D) Chlorophyll a (Chl a) content, chlorophyll b (Chl b) content, total chlorophyll (total Chl) content and the ratio of Chl a/b in OsWRKY42-RNAi transgenic lines. TP309: Wild type; 4021: Homozygous transgenic TP309 line expressing Xa21; R01-R03: OsWRKY42-RNAi transgenic lines. Error bars are standard deviation (SD) (n=3); * indicate significant differences at P<0.05; ** indicate significant differences at P<0.01 (Student's t-test).
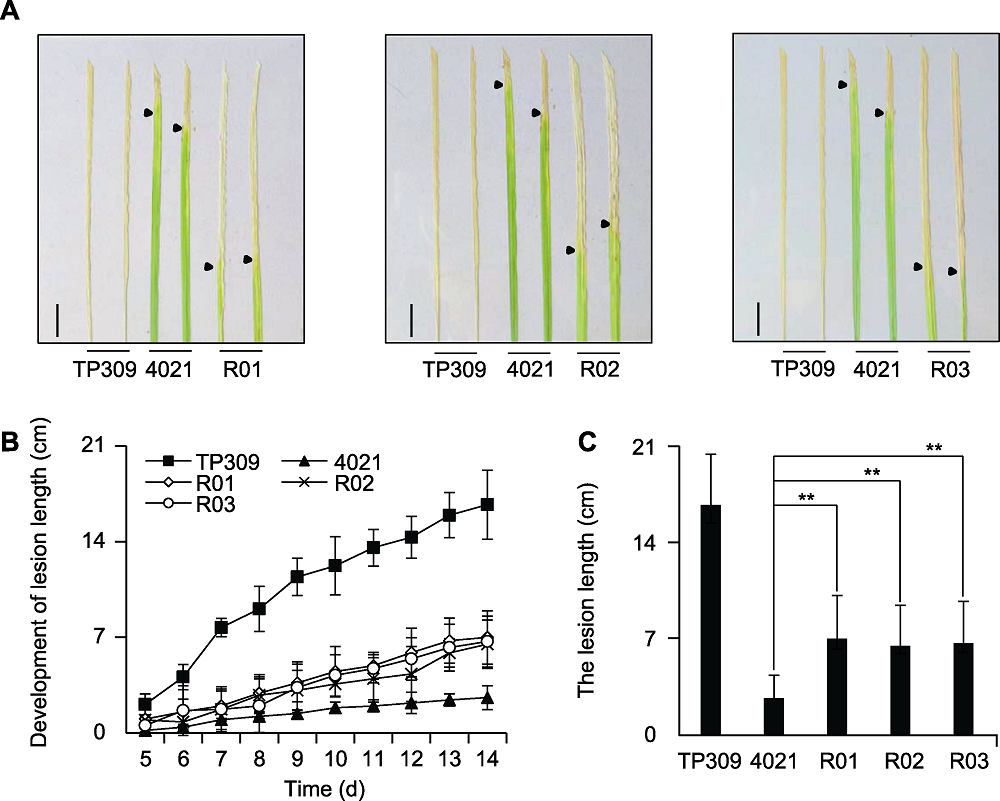
图3 OsWRKY42-RNAi转基因水稻接种Xoo的表型 (A) OsWRKY42-RNAi转基因水稻接种14天的叶片表型(bars=1 cm); (B) 病斑长度扩展曲线; (C) 接种14天病斑长度统计。误差线表示标准差SD (n>9); **差异极显著(P<0.01)。R01-R03: OsWRKY42-RNAi转基因水稻; TP309: 野生型; 4021: 表达Xa21的转基因TP309植株
Figure 3 Phenotype of rice OsWRKY42-RNAi transgenic lines inoculated with Xoo (A) The phenotype of OsWRKY42-RNAi transgenic lines at 14-day post inoculation (bars=1 cm); (B) Lesion development of inoculated plant; (C) Lesion length of inoculated plants at 14 day post inoculation. Error bars are standard deviation (SD) (n>9); ** significant differences at P<0.01 level. R01-R03: OsWRKY42-RNAi transgenic lines; TP309: Wild type; 4021: Transgenic TP309 expressing Xa21
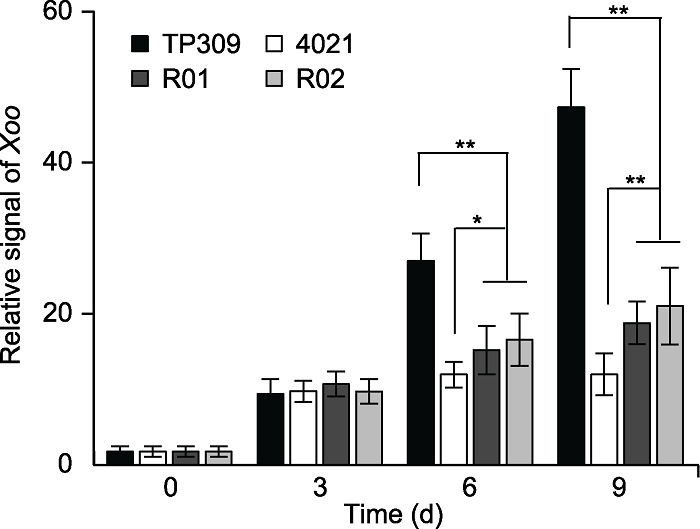
图4 水稻接种叶片中Xoo总蛋白质丰度 将等量的多个样品的总蛋白质用SDS-PAGE分离蛋白后转膜, 用抗XOO抗体检测(附图1), 抗体为anti-XOO多克隆抗体和anti-OsHSP82单克隆抗体。用Mini Chemiluminescent Imager and Sage Capture软件检测免疫印迹(WB)信号, 用Lane 1D软件对免疫印迹检测结果进行信号采集。计算3次实验的平均值和标准差, 误差线表示标准差(SD)。TP309: 野生型; 4021: 表达Xa21的转基因TP309植株; R01和R02为OsWRKY42-RNAi转基因纯合株系。
Figure 4 Expression abundance of total Xoo protein in inoculated rice leaves Equal amounts of total protein from multiple samples were resolved by SDS-PAGE (appendix figure 1), antibodies are anti-XOO polyclonal antibody and anti-OsHSP82 monoclonal antibody. Western blot (WB) signals were detected by Mini Chemiluminescent Imager and Sage Capture software. Lane 1D software was used to extract signals of Western blot. Average and standard deviation was calculated for three repeats. Error bars are standard deviation (SD). * P<0.05; ** P<0.01; TP309: Wild type; 4021: Transgenic TP309 expressing Xa21; R01 and R02 were homozygous OsWRKY42- RNAi transgenic lines.
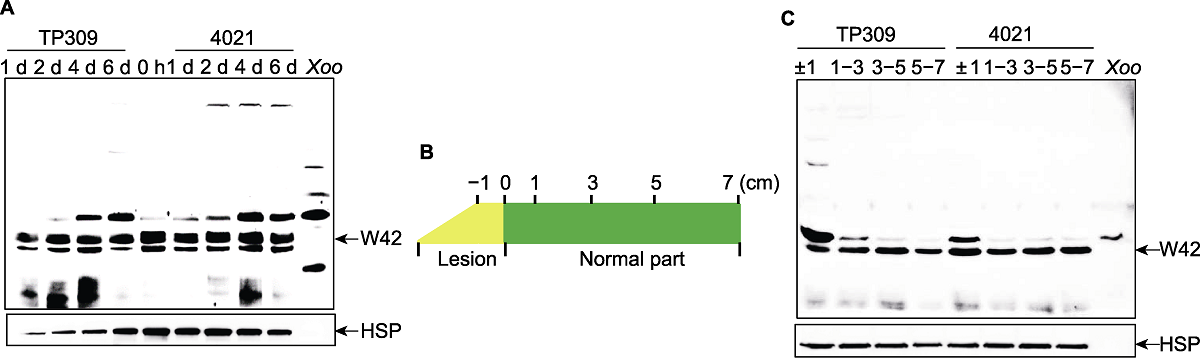
图5 OsWRKY42蛋白质在水稻-Xoo互作中的表达特征 (A) 接种后不同时间点OsWRKY42蛋白质的表达。分别在接种后0、1、2、4和6天5个时间点取材进行免疫印迹(WB)检测; (B) 接种叶片样品采集示意图。以病斑线为0点, 在接种后6天取材, 叶片取材部位分别为±1、1-3、3-5和5-7 cm; (C) 接种后叶片不同部位中OsWRKY42蛋白质的表达。以接种6天的水稻叶片为材料, 接种后的病斑线为0点, 采集接种后不同部位的叶片, 将等量的多个样品的总蛋白质用Tricine-SDS-PAGE分离, 一抗为抗OsWRKY42多克隆抗体和抗OsHSP82单克隆抗体, 用Sage Capture软件检测OsWRKY42蛋白质丰度。TP309: 野生型; 4021: 表达Xa21的转基因TP309植株; Xoo: Xoo总蛋白质; W42和HSP见图1。
Figure 5 Expression profiling of the OsWRKY42 protein in rice-Xoo interaction (A) Expression profiling of OsWRKY42 protein at different time points after inoculation. Samples were collected at 0, 1, 2, 4, 6-day post inoculation, respectively; (B) Schematic diagram for sample collection of inoculated leaves. Based on the lesion line (0), samples were taken at ±1, 1-3, 3-5, and 5-7 cm of the leaves at 6-day post inoculation, respectively; (C) Expression profiling of OsWRKY42 protein at different position of leaves at 6-day post inoculation. Based on lesion line, different portion of leaf tissues were collected after inoculation. Equal amounts of total protein from multiple samples were resolved by Tricine-SDS-PAGE. Antibodies are anti-OsWRKY42 polyclonal antibody and anti-OsHSP82 monoclonal antibody. OsWRKY42 protein signal were detected by Sage Capture software. TP309: Wild type; 4021: Transgenic TP309 expressing Xa21; Xoo: Total protein isolated from Xoo cells; W42 and HSP are shown in Figure 1.
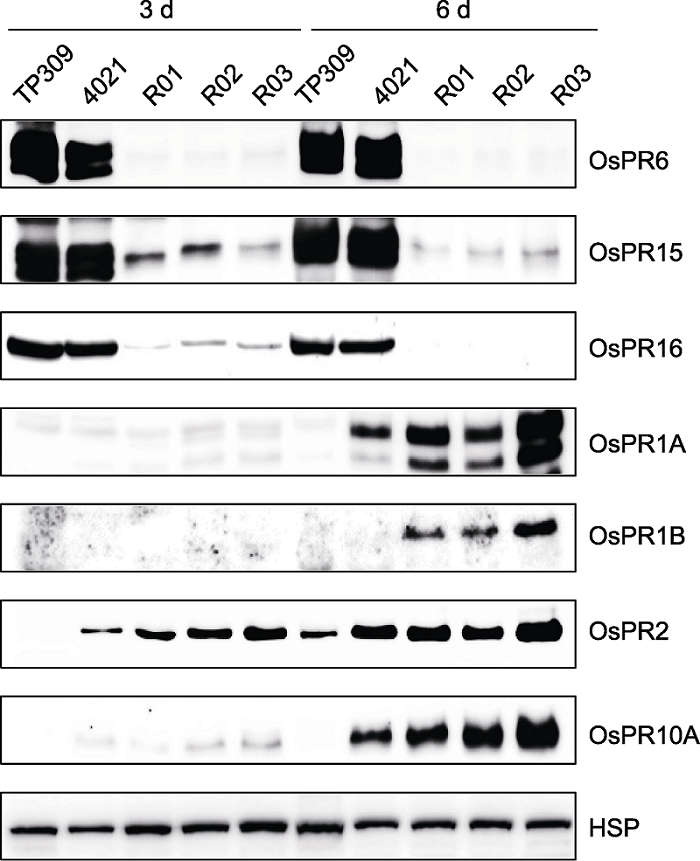
图6 OsWRKY42-RNAi水稻植株中病程相关蛋白质的表达 以接种3天和6天的叶片(病斑线±1 cm)为材料, 等量的总蛋白质样品通过SDS-PAGE或者Tricine-SDS-PAGE分离, 一抗为抗病程相关蛋白质抗体, 分别为抗OsPR1A、抗OsPR1B及抗OsPR10A多克隆抗体(Wu et al., 2011); 抗OsPR2、抗OsPR6、抗OsPR15及抗OsPR16多克隆抗体(Hou et al., 2012)和抗OsHSP82单克隆抗体(Li et al., 2011)。TP309: 野生型; 4021: 表达Xa21的转基因TP309植株; R01-R03: OsWRKY42-RNAi转基因株系; HSP见图1。
Figure 6 Expression profiling of pathogenesis-related proteins in rice OsWRKY42-RNAi transgenic lines Equal amounts of total protein, isolated from inoculated leaves (lesion line ±1 cm) of 3-day and 6-day, were resolved by SDS-PAGE or Tricine-SDS-PAGE. The antibodies were anti-OsPR1A, -OsPR1B and -OsPR10A polyclonal antibodies (Wu et al., 2011); anti-OsPR2, -OsPR6, -OsPR15 and -OsPR16 polyclonal antibodies (Hou et al., 2012) and anti-OsHSP82 monoclonal antibody (Li et al., 2011). TP309: Wild type; 4021: Transgenic TP309 individual expressing Xa21; R01-R03: OsWRKY42-RNAi transgenic lines; HSP is shown in Figure 1.
| [1] | 白辉, 王宪云, 曹英豪, 李晓明, 李莉云, 陈浩, 刘丽娟, 朱健辉, 刘国振 (2010). 水稻叶绿体蛋白质在生长发育过程中的表达研究. 生物化学与生物物理进展 37, 988-995. |
| [2] | 窦世娟, 关明俐, 李莉云, 刘国振 (2014). 水稻的病程相关基因. 科学通报 59, 245-258. |
| [3] | 李雪姣, 范伟, 牛东东, 关明俐, 缪刘杨, 史佳楠, 窦世娟, 魏健, 刘丽娟, 李莉云, 刘国振 (2014). 水稻病程相关PR1家族蛋白质在叶片生长及与白叶枯病菌互作反应中的表达. 植物学报 49, 127-138. |
| [4] | 缪刘杨, 周亮, 杨烁, 李莉云, 李雪姣, 范伟, 兰金苹, 史佳楠, 刘丽娟, 刘国振 (2014). 水稻转录因子WRKY42的转录、表达及其与W-box的结合特征分析. 生物化学与生物物理进展 41, 682-692. |
| [5] |
Chen Q, Huang XE, Chen XH, Shamsunnaher, Song WY (2019). Reversible activation of XA21-mediated resistance by temperature. Eur J Plant Pathol 153, 1177-1184.
DOI URL |
| [6] |
Chen XW, Chern M, Canlas PE, Ruan DL, Jiang CY, Ronald PC (2010). An ATPase promotes autophosphorylation of the pattern recognition receptor XA21 and inhibits XA21-mediated immunity. Proc Natl Acad Sci USA 107, 8029-8034.
DOI URL |
| [7] |
Chen XW, Zuo SM, Schwessinger B, Chern M, Canlas PE, Ruan DL, Zhou XG, Wang J, Daudi A, Petzold CJ, Heazlewood JL, Ronald PC (2014). An XA21-associated kinase (OsSERK2) regulates immunity mediated by the XA21 and XA3 immune receptors. Mol Plant 7, 874-892.
DOI URL |
| [8] |
Cheng HT, Liu HB, Deng Y, Xiao JH, Li XH, Wang SP (2015). The WRKY45-2 WRKY13 WRKY42 transcriptional regulatory cascade is required for rice resistance to fungal pathogen. Plant Physiol 167, 1087-1099.
DOI URL |
| [9] |
Choi NY, Lee E, Lee SG, Choi CH, Park SR, Ahn I, Bae SC, Hwang CH, Hwang DJ (2017). Genome-wide expression profiling of OsWRKY superfamily genes during infection with Xanthomonas oryzae pv. oryzae using real- time PCR. Front Plant Sci 8, 1628.
DOI URL |
| [10] | Das A, Pramanik K, Sharma R, Gantait S, Banerjee J (2019). In-silico study of biotic and abiotic stress-related transcription factor binding sites in the promoter regions of rice germin-like protein genes. PLoS One 14, e0211887. |
| [11] |
Eulgem T, Rushton PJ, Robatzek S, Somssich IE (2000). The WRKY superfamily of plant transcription factors. Trends Plant Sci 5, 199-206.
PMID |
| [12] | Guo MC, Lan JP, Shi J, Guan ML, Wei J, Liu LJ, Li LY, Dou SJ, Liu GZ (2015). Western blot detection of Xanthomonas oryzae pv. oryzae in rice. J Plant Pathol Microbiol S4, 005. |
| [13] |
Han M, Kim CY, Lee J, Lee SK, Jeon JS (2014). OsWRKY42 represses OsMT1d and induces reactive oxygen species and leaf senescence in rice. Mol Cells 37, 532-539.
DOI URL |
| [14] | Hou MM, Xu WJ, Bai H, Liu YM, Li LY, Liu LJ, Liu B, Liu GZ (2012). Characteristic expression of rice pathogenesis-related proteins in rice leaves during interactions with Xanthomonas oryzae pv. oryzae. Plant Cell Rep 31, 895- 904. |
| [15] |
Hwang SH, Yie SW, Hwang DJ (2011). Heterologous expression of OsWRKY6 gene in Arabidopsis activates the expression of defense related genes and enhances resistance to pathogens. Plant Sci 181, 316-323.
DOI URL |
| [16] | Jiang N, Yan J, Liang Y, Shi YL, He ZZ, Wu YT, Zeng Q, Liu XL, Peng JH (2020). Resistance genes and their interactions with bacterial blight/leaf streak pathogens (Xanthomonas oryzae) in rice (Oryza sativa L.)-an updated review. Rice (NY) 13, 3. |
| [17] |
Jiang YN, Chen XH, Ding XD, Wang YS, Chen Q, Song WY (2013). The XA21 binding protein XB25 is required for maintaining XA21-mediated disease resistance. Plant J 73, 814-823.
DOI URL |
| [18] |
Jones JDG, Dangl JL (2006). The plant immune system. Nature 444, 323-329.
DOI URL |
| [19] |
Kim SG, Kim ST, Wang YM, Yu S, Choi IS, Kim YC, Kim WT, Agrawal GK, Rakwal R, Kang KY (2011). The RNase activity of rice probenazole-induced protein 1 (PBZ1) plays a key role in cell death in plants. Mol Cells 31, 25-31.
DOI URL |
| [20] |
Lamb C, Dixon RA (1997). The oxidative burst in plant disease resistance. Annu Rev Plant Physiol Plant Mol Biol 48, 251-275.
DOI URL |
| [21] |
Lee SW, Han SW, Sririyanum M, Park CJ, Seo YS, Ronald PC (2009). A type I-secreted, sulfated peptide triggers XA21-mediated innate immunity. Science 326, 850-853.
DOI URL |
| [22] |
Li XM, Bai H, Wang XY, Li LY, Cao YH, Wei J, Liu YM, Liu LJ, Gong XD, Wu L, Liu SQ, Liu GZ (2011). Identification and validation of rice reference proteins for Western blotting. J Exp Bot 62, 4763-4772.
DOI URL |
| [23] | Lichtenthaler HK (1987). Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Method Enzymol 148, 350-382. |
| [24] |
Liu XQ, Bai XQ, Wang XJ, Chu CC (2007). OsWRKY71, a rice transcription factor, is involved in rice defense response. J Plant Physiol 164, 969-979.
DOI URL |
| [25] | McGee JD, Hamer JE, Hodges TK (2001). Characterization of a PR-10 pathogenesis-related gene family induced in rice during infection with Magnaporthe grisea. Mol Plant Microbe Interact 14, 877-886. |
| [26] |
Pandey SP, Somssich IE (2009). The role of WRKY transcription factors in plant immunity. Plant Physiol 150, 1648- 1655.
DOI PMID |
| [27] |
Park CJ, Bart R, Chern M, Canlas PE, Bai W, Ronald PC (2010). Overexpression of the endoplasmic reticulum chaperone BiP3 regulates XA21-mediated innate immunity in rice. PLoS One 5, e9262.
DOI URL |
| [28] |
Park CJ, Peng Y, Chen XW, Dardick C, Ruan DL, Bart R, Canlas PE, Ronald PC (2008). Rice XB15, a protein phosphatase 2C, negatively regulates cell death and XA21- mediated innate immunity. PLoS Biol 6, e282.
DOI URL |
| [29] |
Park CJ, Sharma R, Lefebvre B, Canlas PE, Ronald PC (2013). The endoplasmic reticulum-quality control component SDF2 is essential for XA21-mediated immunity in rice. Plant Sci 210, 53-60.
DOI URL |
| [30] | Park CJ, Wei T, Sharma R, Ronald PC (2017). Overexpression of rice auxilin-like protein, XB21, induces necrotic lesions, up-regulates endocytosis-related genes, and confers enhanced resistance to Xanthomonas oryzae pv. oryzae. Rice (NY) 10, 27. |
| [31] |
Peng Y, Bartley LE, Canlas P, Ronald PC (2010). OsWRKY IIa transcription factors modulate rice innate immunity. Rice (NY) 3, 36-42.
DOI URL |
| [32] |
Peng Y, Bartley LE, Chen XW, Dardick C, Chern M, Ruan R, Canlas PE, Ronald PC (2008). OsWRKY62 is a negative regulator of basal and Xa21-mediated defense against Xanthomonas oryzae pv. oryzae in rice. Mol Plant 1, 446-458.
DOI URL |
| [33] |
Pillai SE, Kumar C, Patel HK, Sonti RV (2018). Overexpression of a cell wall damage induced transcription factor, OsWRKY42, leads to enhanced callose deposition and tolerance to salt stress but does not enhance tolerance to bacterial infection. BMC Plant Biol 18, 177.
DOI URL |
| [34] |
Qiu DY, Xiao J, Ding XH, Xiong M, Cai M, Cao YL, Li XH, Xu CG, Wang SP (2007). OsWRKY13mediates rice disease resistance by regulating defense-related genes in salicylate- and jasmonate-dependent signaling. Mol Plant Microbe Interact 20, 492-499.
DOI URL |
| [35] | Rice WRKY Working Group (2012). Nomenclature report on rice WRKY's-conflict regarding gene names and its solution. Rice (NY) 5, 3. |
| [36] |
Ryu HS, Han M, Lee SK, Cho JI, Ryoo N, Heu S, Lee YH, Bhoo SH, Wang GL, Hahn TR, Jeon JS (2006). A comprehensive expression analysis of the WRKY gene superfamily in rice plants during defense response. Plant Cell Rep 25, 836-847.
DOI URL |
| [37] |
Sahu A, Das A, Saikia K, Barah P (2020). Temperature differentially modulates the transcriptome response in Oryza sativa to Xanthomonas oryzae pv. oryzae infection. Genomics 112, 4842-4852.
DOI URL |
| [38] |
Schwessinger B, Zipfel C (2008). News from the frontline: recent insights into PAMP-triggered immunity in plants. Curr Opin Plant Biol 11, 389-395.
DOI PMID |
| [39] |
Seo YS, Chern M, Bartley LE, Han M, Jung KH, Lee I, Walia H, Richter T, Xu X, Cao PJ, Bai W, Ramanan R, Amonpant F, Arul L, Canlas PE, Ruan R, Park CJ, Chen XW, Hwang S, Jeon JS, Ronald PC (2011). Towards establishment of a rice stress response interactome. PLoS Genet 7, e1002020.
DOI URL |
| [40] |
Shimono M, Koga H, Akagi A, Hayashi N, Goto S, Sawada M, Kurihara T, Matsushita A, Sugano S, Jiang CJ, Kaku H, Inoue H, Takatsuji H (2012). Rice WRKY45plays important roles in fungal and bacterial disease resistance. Mol Plant Pathol 13, 83-94.
DOI PMID |
| [41] |
Son S, An HK, Seol YJ, Park SR, Im JH (2020). Rice transcription factor WRKY114 directly regulates the expression of OsPR1a and Chitinase to enhance resistance against Xanthomonas oryzae pv. oryzae. Biochem Biophys Res Commun 533, 1262-1268.
DOI URL |
| [42] |
Song WY, Wang GL, Chen LL, Kim HS, Pi LY, Holsten T, Gardner J, Wang B, Zhai WX, Zhu LH, Fauquet C, Ronald P (1995). A receptor kinase-like protein encoded by the rice disease resistance gene, Xa21. Science 270, 1804-1806.
PMID |
| [43] |
Tanaka A, Christensen MJ, Takemoto D, Park P, Scott B (2006). Reactive oxygen species play a role in regulating a fungus-perennial ryegrass mutualistic interaction. Plant Cell 18, 1052-1066.
PMID |
| [44] |
Vo KTX, Kim CY, Hoang TV, Lee SK, Shirsekar G, Seo YS, Lee SW, Wang GL, Jeon JS (2017). OsWRKY67 plays a positive role in basal and XA21-mediated resistance in rice. Front Plant Sci 8, 2220.
DOI URL |
| [45] |
Wang X, Li BB, Ma TT, Sun LY, Tai L, Hu CH, Liu WT, Li WQ, Chen KM (2020). The NAD kinase OsNADK1 affects the intracellular redox balance and enhances the tolerance of rice to drought. BMC Plant Biol 20, 11.
DOI URL |
| [46] |
Wang YS, Pi LY, Chen XH, Chakrabarty PK, Jiang JD, De Leon AL, Liu GZ, Li LC, Benny U, Oard J, Ronald PC, Song WY (2006). Rice XA21 binding protein 3 is a ubiquitin ligase required for full Xa21-mediated disease resistance. Plant Cell 18, 3635-3646.
DOI URL |
| [47] | Wu Q, Hou MM, Li LY, Liu LJ, Hou YX, Liu GZ (2011). Induction of pathogenesis-related proteins in rice bacterial blight resistant gene XA21-mediated interactions with Xanthomonas oryzae pv. oryzae. J Plant Pathol 93, 455- 459. |
| [48] |
Yang S, Zhou L, Miao LY, Shi JN, Sun CQ, Fan W, Lan JP, Chen H, Liu LJ, Dou SJ, Liu GZ, Li LY (2016). The expression and binding properties of the rice WRKY68 protein in the Xa21-mediated resistance response to Xanthomonas oryzae pv. oryzae. J Integr Agr 15, 2451- 2460.
DOI URL |
| [49] |
Zhang XC, Li DY, Zhang HJ, Wang XE, Zheng Z, Song FM (2010). Molecular characterization of rice OsBIANK1, encoding a plasma membrane-anchored ankyrin repeat protein, and its inducible expression in defense responses. Mol Biol Rep 37, 653-660.
DOI URL |
| [1] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [3] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [4] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [5] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [6] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [7] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [8] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [9] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [10] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [11] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [12] | 朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育[J]. 植物学报, 2024, 59(1): 10-21. |
| [13] | 贾绮玮, 钟芊芊, 顾育嘉, 陆天麒, 李玮, 杨帅, 朱超宇, 胡程翔, 李三峰, 王跃星, 饶玉春. 水稻茎秆细胞壁相关组分含量QTL定位及候选基因分析[J]. 植物学报, 2023, 58(6): 882-892. |
| [14] | 田传玉, 方妍力, 沈晴, 王宏杰, 陈析丰, 郭威, 赵开军, 王春连, 纪志远. 2019-2021年我国南方稻区白叶枯病菌的毒力与遗传多样性调查研究[J]. 植物学报, 2023, 58(5): 743-749. |
| [15] | 戴若惠, 钱心妤, 孙静蕾, 芦涛, 贾绮玮, 陆天麒, 路梅, 饶玉春. 水稻叶色调控机制及相关基因研究进展[J]. 植物学报, 2023, 58(5): 799-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||