

植物学报 ›› 2016, Vol. 51 ›› Issue (2): 167-174.DOI: 10.11983/CBB15048 cstr: 32102.14.CBB15048
靳振明1, 平宝哲1, 沈浩珺2, 杜淮清1, 李瑞乾1, 朱璐1, 张大兵1, 袁政1,*( )
)
收稿日期:2015-03-06
接受日期:2015-06-23
出版日期:2016-03-01
发布日期:2016-03-31
通讯作者:
E-mail: 基金资助:
Zhenming Jin1, Baozhe Ping1, Haojun Shen2, Huaiqing Du1, Ruiqian Li1, Lu Zhu1, Dabing Zhang1, Zheng Yuan1,*( )
)
Received:2015-03-06
Accepted:2015-06-23
Online:2016-03-01
Published:2016-03-31
Contact:
E-mail: 摘要: 茎秆机械强度影响植株抗倒伏能力, 是备受关注的重要农艺性状之一。与野生型相比, 水稻(Oryza sativa)脆秆隐性突变体bc-s1茎秆抗折力和抗张力分别降低31.1%和67.2%, 茎秆纤维素和木质素含量分别降低24.97%和增高38.82%。细胞学分析显示, bc-s1茎秆厚壁细胞发生不规则变化, 次生壁增厚受阻。通过图位克隆和测序分析, 初步确定bc-s1突变体中纤维素合成酶催化亚基Os09g25490/OsCesA9基因第1外显子的第28个碱基G突变为A。该等位突变体的获得为进一步揭示OsCesA9调控细胞壁建成的生物学功能提供了新的研究材料。
靳振明, 平宝哲, 沈浩珺, 杜淮清, 李瑞乾, 朱璐, 张大兵, 袁政. 水稻脆秆突变体bc-s1的表型分析和基因定位. 植物学报, 2016, 51(2): 167-174.
Zhenming Jin, Baozhe Ping, Haojun Shen, Huaiqing Du, Ruiqian Li, Lu Zhu, Dabing Zhang, Zheng Yuan. Characterisation and Gene Mapping of a Brittle Culm Mutant bc-s1 in Rice. Chinese Bulletin of Botany, 2016, 51(2): 167-174.
| Marker | Forward primer (5'-3') | Reverse primer (5'-3') | Type |
|---|---|---|---|
| Chr09_04 | GGTAATGTCACAACTCAAAAAGC | CAAGATTGTAAACCCTGTCTATTG | InDel |
| Ha1 | GCGAACCGATAAAACTGCTC | AGAGGTGTATCAAAGCAATCGAG | InDel |
| Ha4 | TCACCTCTCAACTTAATCGA | AGTCCATCAAGCCATGATGC | InDel |
| Ha6 | AGTTCGTCCGGTTTTGATCG | GTAGAATAAGCGAAACAGCA | InDel |
| Ha9 | TCGACCATCAGCGATTTGAC | TTTTCCATGCGCGGTGTTTG | InDel |
| Chr09_05 | GAATTTGAGTGGGTTTATACTAGC | GTTAACTTAGGCTATTTTGGCTTC | InDel |
表1 图位克隆定位引物序列
Table 1 DNA markers used for positional cloning
| Marker | Forward primer (5'-3') | Reverse primer (5'-3') | Type |
|---|---|---|---|
| Chr09_04 | GGTAATGTCACAACTCAAAAAGC | CAAGATTGTAAACCCTGTCTATTG | InDel |
| Ha1 | GCGAACCGATAAAACTGCTC | AGAGGTGTATCAAAGCAATCGAG | InDel |
| Ha4 | TCACCTCTCAACTTAATCGA | AGTCCATCAAGCCATGATGC | InDel |
| Ha6 | AGTTCGTCCGGTTTTGATCG | GTAGAATAAGCGAAACAGCA | InDel |
| Ha9 | TCGACCATCAGCGATTTGAC | TTTTCCATGCGCGGTGTTTG | InDel |
| Chr09_05 | GAATTTGAGTGGGTTTATACTAGC | GTTAACTTAGGCTATTTTGGCTTC | InDel |
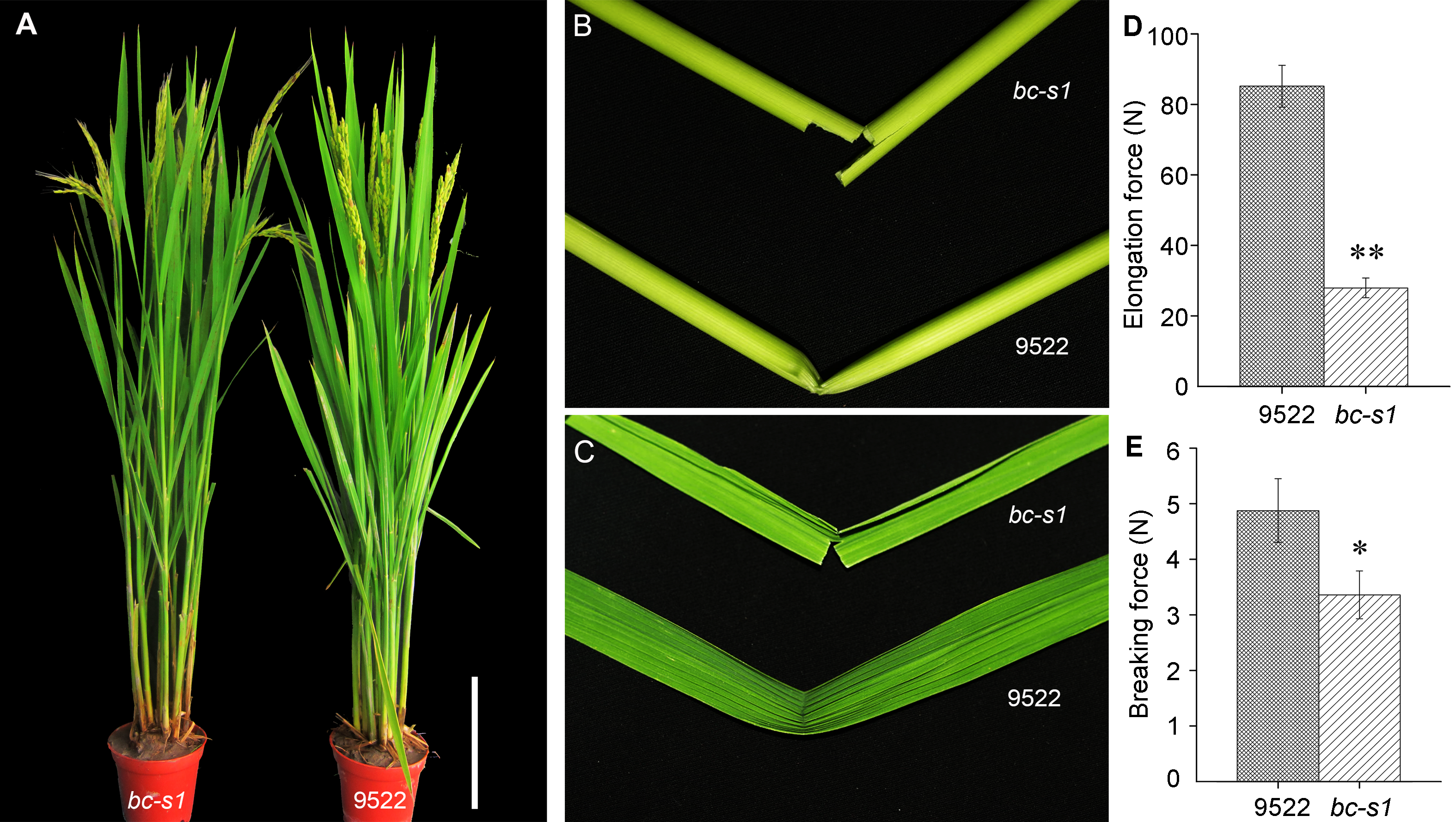
图1 水稻突变体bc-s1与野生型9522的表型与机械强度 (A) 抽穗期突变体bc-s1与野生型9522整株表型(Bar=20 cm); (B) 成熟期水稻茎秆第2节间手折脆性性状; (C) 成熟期水稻剑叶手折脆性性状; (D) 茎秆第2节间抗张力测定; (E) 茎秆第2节间抗折力测定。*和**分别表示t-检验的显著差异(P<0.05)和极显著差异(P<0.01)。
Figure 1 The phenotype and mechanical strength of bc-s1 mutant and wild-type 9522 in Oryza sativa (A) The whole plant phenotype of bc-s1 mutant and wild-type 9522 in the heading stage (Bar=20 cm); (B) An easily broken culm of bc-s1 compared with a wild-type 9522 culm in the mature stage; (C) An easily broken flag leaf of bc-s1 compared with a wild-type 9522 flag leaf in the mature stage; (D) The elongation force of the second upper internode; (E) The breaking force of the second upper internode. * and ** represent t-test at P<0.05 and P<0.01, respectively.
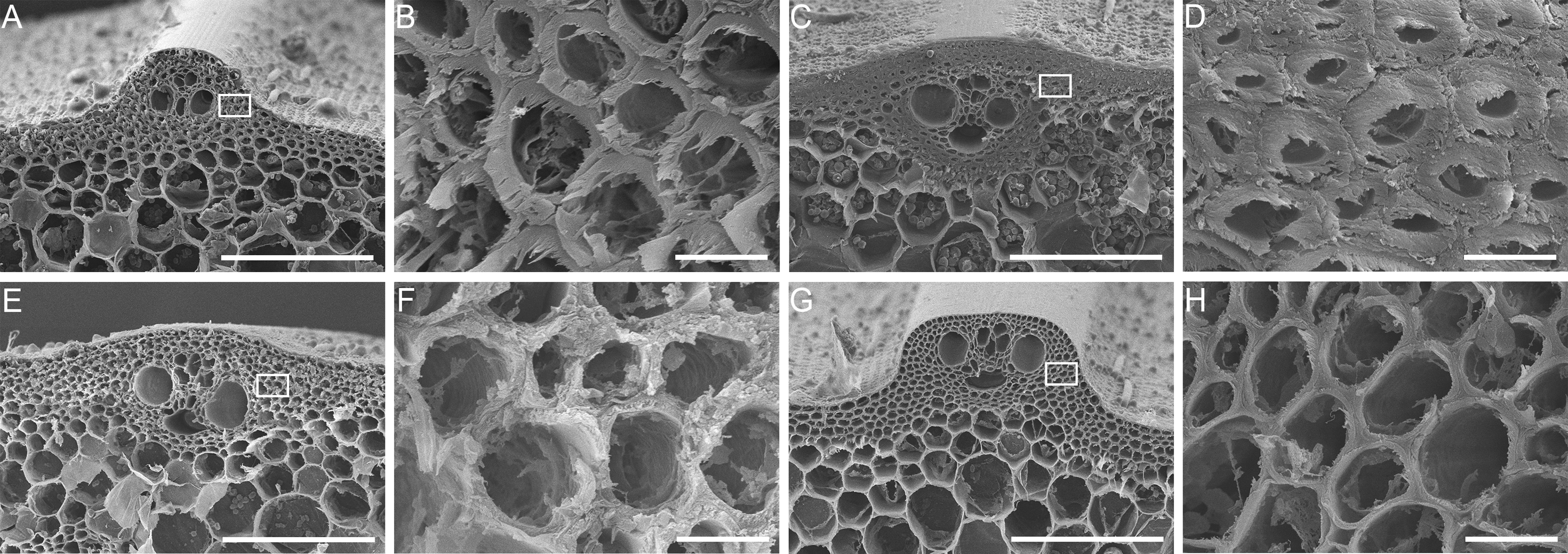
图2 水稻野生型9522与突变体bc-s1茎秆横切片扫描电镜观察 (A), (B) 野生型9522初生壁; (C), (D) 野生型9522次生壁; (E), (F) 突变体bc-s1初生壁; (G), (H) 突变体bc-s1次生壁。(A), (C), (E), (G) Bar=100 μm; (B), (D), (F), (H) Bar=5 μm
Figure 2 The cross-section of wild-type 9522 and bc-s1 mutant culms in Oryza sativa under a scanning electron microscope (A), (B) The primary wall of a wild-type 9522 culm; (C), (D) The secondary wall of a wild-type 9522 culm; (E), (F) The primary wall of a bc-s1 mutant culm; (G), (H) The secondary wall of a bc-s1 mutant culm; (A), (C), (E), (G) Bar=100 μm; (B), (D), (F), (H) Bar=5 μm
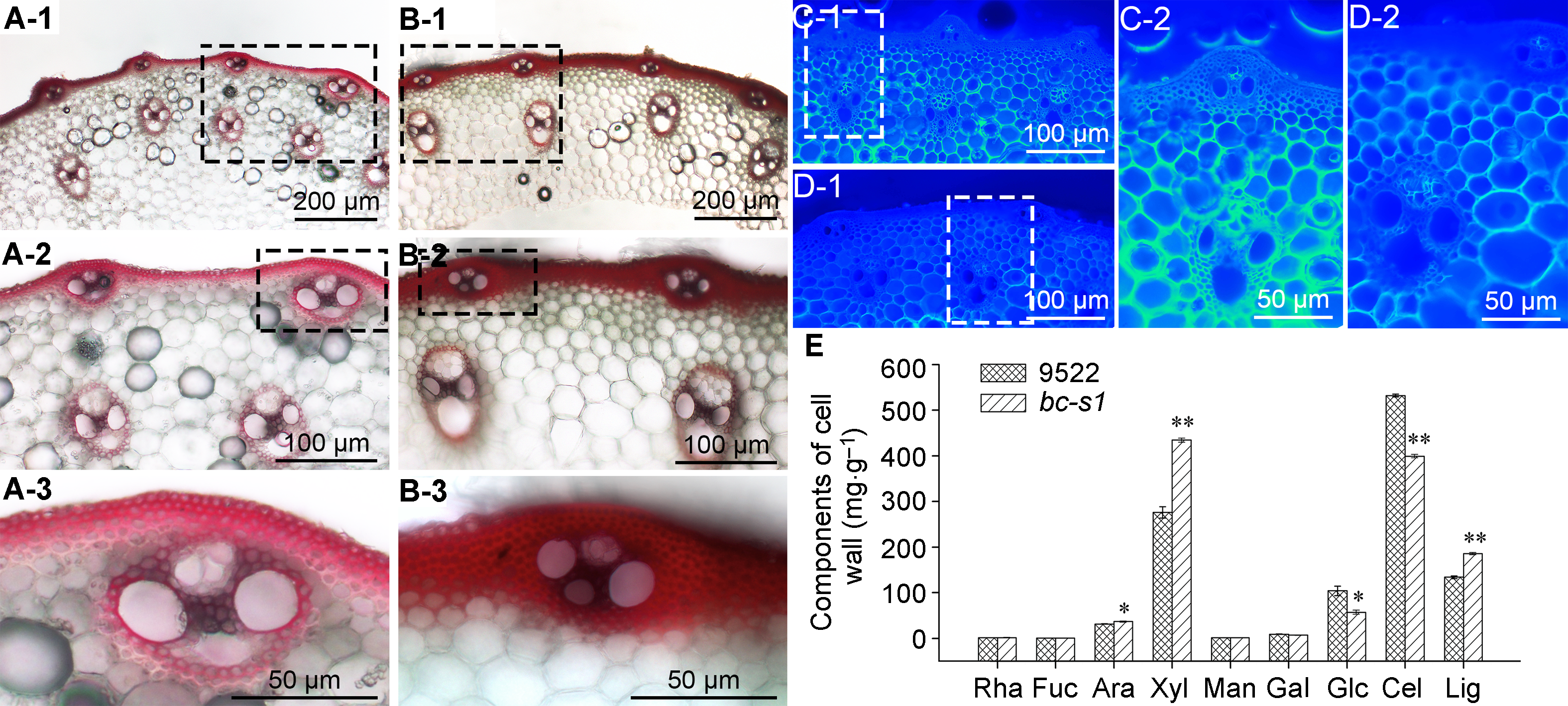
图3 水稻野生型9522与突变体bc-s1茎秆细胞壁组织切片观察与组分测定 (A), (B) 细胞壁木质素间苯三酚染色, 图(A)和(B)分别为野生型9522和脆秆突变体bc-s1第2节间茎秆组织横切片; (C), (D) 细胞壁纤维素荧光增白剂染色, 图(C)和(D)分别为野生型9522和脆秆突变体bc-s1第2节间茎秆组织横切片; (E) 野生型9522与突变体bc-s1茎秆细胞壁组分比较。*和**分别表示t-检验的显著差异(P<0.05)和极显著差异(P<0.01)。
Figure 3 Tissue section staining and components determination in the culm cell wall of wild-type 9522 and bc-s1 mutant in Oryza sativa (A), (B) The cross section of the second upper internodes of wild-type 9522 (A) and bc-s1 mutant (B) were stained with phloroglucinol; (C), (D) The cross section of the second upper internodes of wild-type 9522 (C) and bc-s1 mutant (D) were stained with Calcofluor White; (E) The comparison of cell-wall components between bc-s1 mutant and wild-type 9522 culm. * and ** represent t-test at P<0.05 and P<0.01, respectively.
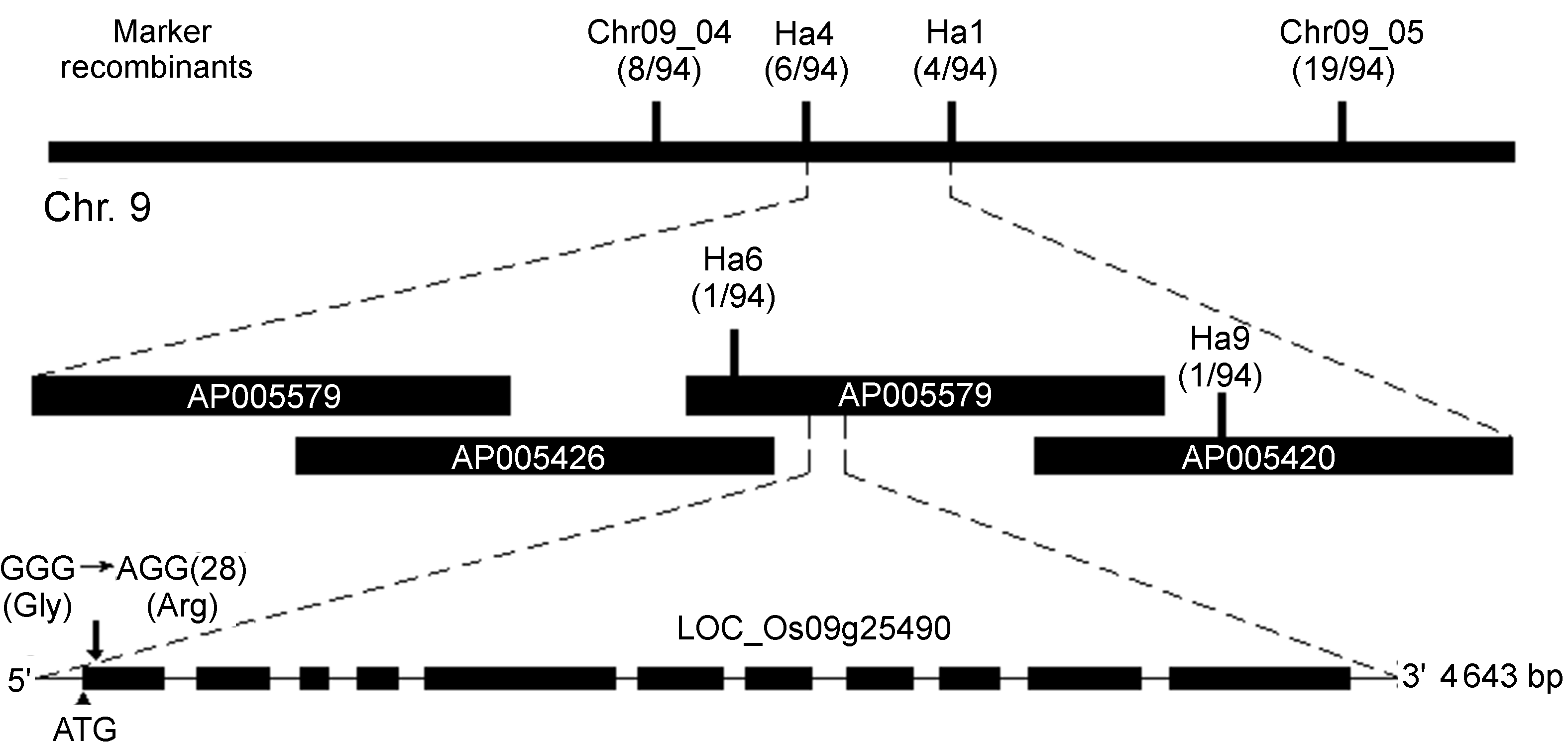
图4 BC-S1基因的图位克隆 BC-S1基因定位于水稻第9号染色体的AP005579 BAC上, 基因编号为LOC_Os09g25490, 具有编码纤维素合成酶A催化亚基9 (OsCesA9)的功能。测序分析结果显示, BC-S1基因第1外显子的第28个碱基发生G变为A的点突变。
Figure 4 Map-based cloning of BC-S1 The BC-S1 gene (Os09g25490/OsCesA9), which encodes a cellulose synthase catalytic subunit (CesA), was mapped on BAC AP005579, chromosome 9. According to sequence comparison result, the BC-S1 showed a point mutation at the 28th base of first extron (changing GGG to AGG)
| [1] | 陈亮, 储黄伟, 袁政, 潘爱虎, 梁婉琪, 黄海, 沈明山, 张大兵 (2006). 60Co γ-Ray射线诱变水稻突变体的分离和遗传学初步分析. 厦门大学学报(自然科学版) 45, 82-85. |
| [2] | 江云珠, 沈希宏, 曹立勇 (2012). 水稻茎秆性状的研究进展. 中国稻米 18, 1-7. |
| [3] | Brown Jr RM, Saxena IM (2000). Cellulose biosynthesis: a model for understanding the assembly of biopolymers. Plant Physiol Biol 38, 57-67. |
| [4] |
Doblin MS, Kurek I, Jacob-Wilk D, Delmer DP (2002). Cellulose biosynthesis in plants: from genes to rosettes. Plant Cell Physiol 43, 1407-1420.
DOI PMID |
| [5] | Freemont PS (2000). Ubiquitination: RING for destruction? Curr Biol 10, R84-R87. |
| [6] |
Harholt J, Jensen JK, Sørensen SO, Orfila C, Pauly M, Scheller HV (2006). ARABINAN DEFICIENT 1 is a putative arabinosyltransferase involved in biosynthesis of pectic arabinan in Arabidopsis. Plant Physiol 140, 49-58.
DOI PMID |
| [7] | Hoebler C, Barry L, David A, Delort-Laval J (1989). Rapid hydrolysis of plant cell wall polysaccharides by gas-liquid chromatography. J Agric Food Chem 37, 360-367. |
| [8] | Islam MS, Peng S, Visperas RM, Ereful N, Bhuiya M, Julfiquar A (2007). Lodging-related morphological traits of hybrid rice in a tropical irrigated ecosystem. Field Crops Res 101, 240-248. |
| [9] |
Joazeiro CA, Weissman AM (2000). RING finger proteins: mediators of ubiquitin ligase activity. Cell 102, 549-552.
DOI PMID |
| [10] | Kirk TK, Obst JR (Part b. Lignin, Pectin, Chitin. Inc. 1988). Lignin determination. In: Wood WA, Kellogg ST, eds. Methods in Enzymology-Biomass, and New York: Academic Press. pp. 87-101. |
| [11] | Kotake T, Aohara T, Hirano K, Sato A, Kaneko Y, Tsumuraya Y, Takatsuji H, Kawasaki S (2011). Rice Brittle culm 6 encodes a dominant-negative form of CesA protein that perturbs cellulose synthesis in secondary cell walls. J Exp Bot 62, 2053-2062. |
| [12] |
Kurek I, Kawagoe Y, Jacob-Wilk D, Doblin M, Delmer D (2002). Dimerization of cotton fiber cellulose synthase catalytic subunits occurs via oxidation of the zinc-binding domains. Proc Natl Acad Sci USA 99, 11109-11114.
PMID |
| [13] |
Li Y, Qian Q, Zhou Y, Yan M, Sun L, Zhang M, Fu Z, Wang Y, Han B, Pang X (2003). BRITTLE CULM1, which encodes a COBRA-like protein, affects the mechanical properties of rice plants. Plant Cell 15, 2020-2031.
DOI PMID |
| [14] |
Murray M, Thompson WF (1980). Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8, 4321-4326.
DOI PMID |
| [15] |
Scheible WR, Eshed R, Richmond T, Delmer D, Somerville C (2001). Modifications of cellulose synthase confer resistance to isoxaben and thiazolidinone herbicides in Arabidopsis Ixr1 mutants. Proc Natl Acad Sci USA 98, 10079-10084.
DOI PMID |
| [16] | Song XQ, Liu LF, Jiang YJ, Zhang BC, Gao YP, Liu XL, Lin QS, Ling HQ, Zhou YH (2013). Disruption of secondary wall cellulose biosynthesis alters cadmium translocation and tolerance in rice plants. Mol Plant 6, 768-780. |
| [17] |
Tanaka K, Murata K, Yamazaki M, Onosato K, Miyao A, Hirochika H (2003). Three distinct rice cellulose synthase catalytic subunit genes required for cellulose synthesis in the secondary wall. Plant Physiol 133, 73-83.
DOI PMID |
| [18] |
Timmers J, Vernhettes S, Desprez T, Vincken JP, Visser RG, Trindade LM (2009). Interactions between membrane-bound cellulose synthases involved in the synthesis of the secondary cell wall. FEBS Lett 583, 978-982.
DOI PMID |
| [19] |
Updegraff DM (1969). Semimicro determination of cellulose in biological materials. Anal Biochem 32, 420-424.
DOI PMID |
| [20] |
Wang D, Yuan S, Yin L, Zhao J, Guo B, Lan J, Li X (2012). A missense mutation in the transmembrane domain of CESA9 affects cell wall biosynthesis and plant growth in rice. Plant Sci 196, 117-124.
DOI PMID |
| [21] | Zhang B, Zhou Y (2011). Rice brittleness mutants: a way to open the 'black box' of monocot cell wall biosynthesis. J Integr Plant Biol 53, 136-142. |
| [22] |
Zhong R, Ye ZH (2007). Regulation of cell wall biosynthesis. Curr Opin Plant Biol 10, 564-572.
DOI PMID |
| [1] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [3] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [4] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [5] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [6] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [7] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [8] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [9] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [10] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [11] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [12] | 朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育[J]. 植物学报, 2024, 59(1): 10-21. |
| [13] | 贾绮玮, 钟芊芊, 顾育嘉, 陆天麒, 李玮, 杨帅, 朱超宇, 胡程翔, 李三峰, 王跃星, 饶玉春. 水稻茎秆细胞壁相关组分含量QTL定位及候选基因分析[J]. 植物学报, 2023, 58(6): 882-892. |
| [14] | 田传玉, 方妍力, 沈晴, 王宏杰, 陈析丰, 郭威, 赵开军, 王春连, 纪志远. 2019-2021年我国南方稻区白叶枯病菌的毒力与遗传多样性调查研究[J]. 植物学报, 2023, 58(5): 743-749. |
| [15] | 戴若惠, 钱心妤, 孙静蕾, 芦涛, 贾绮玮, 陆天麒, 路梅, 饶玉春. 水稻叶色调控机制及相关基因研究进展[J]. 植物学报, 2023, 58(5): 799-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||