

植物学报 ›› 2020, Vol. 55 ›› Issue (5): 577-587.DOI: 10.11983/CBB20100 cstr: 32102.14.CBB20100
所属专题: 粮食安全
贺闽1, 尹俊杰1, 冯志明2, 朱孝波1, 赵剑华2, 左示敏2,*( ), 陈学伟1,*(
), 陈学伟1,*( )
)
收稿日期:2020-05-28
接受日期:2020-07-21
出版日期:2020-09-01
发布日期:2020-09-03
通讯作者:
左示敏,陈学伟
作者简介:xwchen88@163.com基金资助:
Min He1, Junjie Yin1, Zhiming Feng2, Xiaobo Zhu1, Jianhua Zhao2, Shimin Zuo2,*( ), Xuewei Chen1,*(
), Xuewei Chen1,*( )
)
Received:2020-05-28
Accepted:2020-07-21
Online:2020-09-01
Published:2020-09-03
Contact:
Shimin Zuo,Xuewei Chen
摘要: 水稻(Oryza sativa)是世界上最重要的粮食作物, 但稻瘟病和纹枯病等病害严重危害水稻的产量和品质, 给我国乃至全球粮食安全带来巨大威胁。鉴定水稻抗病资源、克隆抗病基因、揭示抗性机理并在育种中加以利用, 对抵御水稻病害和保障粮食安全具有十分重要的作用。准确评价水稻资源的抗病性, 是开展抗病机理研究和育种生产应用的关键环节。该文详述了水稻幼苗期人工喷雾接种、分蘖期和孕穗期田间注射接种与离体叶片戳伤接种的稻瘟病抗性鉴定方法, 以及水稻分蘖期田间接种、孕穗期温室接种和离体茎秆接种的纹枯病抗性鉴定方法, 以期为同行鉴定水稻资源、开展抗病理论和应用研究提供参考。
贺闽, 尹俊杰, 冯志明, 朱孝波, 赵剑华, 左示敏, 陈学伟. 水稻稻瘟病和纹枯病抗性鉴定方法. 植物学报, 2020, 55(5): 577-587.
Min He, Junjie Yin, Zhiming Feng, Xiaobo Zhu, Jianhua Zhao, Shimin Zuo, Xuewei Chen. Methods for Evaluation of Rice Resistance to Blast and Sheath Blight Diseases. Chinese Bulletin of Botany, 2020, 55(5): 577-587.
| Solution | Composition | Amount |
|---|---|---|
| 20× Nitrate salts (1 L) | NaNO3 | 120 g |
| KCl | 10.4 g | |
| MgSO4.7H2O | 10.4 g | |
| KH2PO4 | 30.4 g | |
| Vitamin solution (1 L) | Biotin | 0.1 g |
| Pyridoxin | 0.1 g | |
| Thiamine | 0.1 g | |
| Riboflavin | 0.1 g | |
| p-aminobenzoic acid | 0.1 g | |
| Nicotinic acid | 0.1 g | |
| Trace elements (100 mL) | ZnSO4.7H2O | 2.2 g |
| H3BO3 | 1.1 g | |
| MnCl2.4H2O | 0.5 g | |
| FeSO4.7H2O | 0.5 g | |
| CoCl2.6H2O | 0.17 g | |
| CuSO4.5H2O | 0.16 g | |
| Na2MoO4.2H2O | 0.15 g | |
| CM agar medium (1 L, pH6.5) | Glucose | 10 g |
| Peptone | 2 g | |
| Yeast extract | 1 g | |
| Casamino acids | 1 g | |
| 20× Nitrate salts | 50 mL | |
| Vitamin solution | 1 mL | |
| Trace elements | 1 mL | |
| Agar | 15 g | |
| Oat tomato agar (1 L) | Oatmeal | 40 g, collect the liquid filter after boiling |
| Fresh tomato juice | 150 mL | |
| Agar | 15 g | |
| PDA medium (1 L) | Potato dextrose broth | 24 g |
| Agar | 20 g |
表1 稻瘟病菌和纹枯病菌生长培养所需相关试剂配方
Table 1 Reagent formulation used for culturing Magnaporthe oryzae and Rhizoctonia solani
| Solution | Composition | Amount |
|---|---|---|
| 20× Nitrate salts (1 L) | NaNO3 | 120 g |
| KCl | 10.4 g | |
| MgSO4.7H2O | 10.4 g | |
| KH2PO4 | 30.4 g | |
| Vitamin solution (1 L) | Biotin | 0.1 g |
| Pyridoxin | 0.1 g | |
| Thiamine | 0.1 g | |
| Riboflavin | 0.1 g | |
| p-aminobenzoic acid | 0.1 g | |
| Nicotinic acid | 0.1 g | |
| Trace elements (100 mL) | ZnSO4.7H2O | 2.2 g |
| H3BO3 | 1.1 g | |
| MnCl2.4H2O | 0.5 g | |
| FeSO4.7H2O | 0.5 g | |
| CoCl2.6H2O | 0.17 g | |
| CuSO4.5H2O | 0.16 g | |
| Na2MoO4.2H2O | 0.15 g | |
| CM agar medium (1 L, pH6.5) | Glucose | 10 g |
| Peptone | 2 g | |
| Yeast extract | 1 g | |
| Casamino acids | 1 g | |
| 20× Nitrate salts | 50 mL | |
| Vitamin solution | 1 mL | |
| Trace elements | 1 mL | |
| Agar | 15 g | |
| Oat tomato agar (1 L) | Oatmeal | 40 g, collect the liquid filter after boiling |
| Fresh tomato juice | 150 mL | |
| Agar | 15 g | |
| PDA medium (1 L) | Potato dextrose broth | 24 g |
| Agar | 20 g |
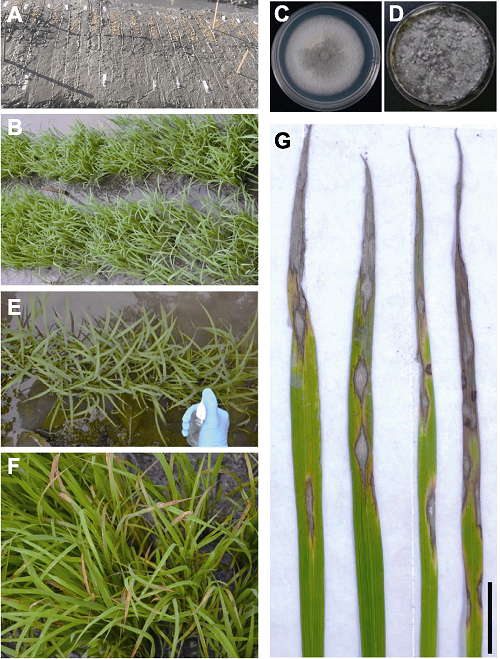
图1 水稻幼苗期接种稻瘟病菌进行抗性鉴定及典型病斑 (A) 苗床上分小区播种水稻种子; (B) 生长2周的幼苗期水稻; (C) CM平板上的稻瘟病菌菌落; (D) 番茄燕麦产孢平板上生长的稻瘟病菌; (E) 对水稻小区喷雾接种孢悬液; (F) 接种6天后稻瘟病的发病情况; (G) 水稻叶片上产生的典型纺锤形病斑(Bar=1 cm)。
Figure 1 Spraying inoculation at rice seedling stage for evaluation of blast disease resistance and typical rice blast symptom (A) Sowing rice seeds on seedbeds; (B) Rice seedlings grown for two weeks; (C) Magnaporthe oryzae colony grown on CM agar medium; (D) M. oryzae sporulation on oat tomato agar medium; (E) Inoculation of rice seedlings by spraying spore suspension; (F) Development of blast lesions six-day post inoculation; (G) Typical blast lesions formed on rice leaves (Bar=1 cm).
| Disease score | Resistance level | State of disease |
|---|---|---|
| 0 | High resistance (HR) | No blast lesion |
| 1 | Resistance (R) | Only needle-shaped brown lesion s- pots |
| 2 | R | Slightly large brown spots with diameter less than 1 mm |
| 3 | Medium re- sistance (MR) | Round or oval gray lesion which shows brown edge and appear diameter between 1-2 mm |
| 4 | Medium su- sceptibility (MS) | Typical spindle lesion which has leng- th between 1-2 cm. The lesion is us- ually confined between two veins of a leaf and the lesion area occupies less than 2.0% of a leaf |
| 5 | MS | Typical spindle lesion whose area occupies the leaf area between 2.1%- 10.0% |
| 6 | Susceptibility (S) | Typical spindle lesion whose area occupies the leaf area between 10.1%- 25.0% |
| 7 | S | Typical spindle lesion whose area occupies the leaf area between 25.1%- 50.0% |
| 8 | High sus- ceptibility (HS) | Typical spindle lesion whose area occupies the leaf area between 50.1%- 75.0% |
| 9 | HS | Typical spindle lesion whose area oc- cupies more than 75.1% of the leaf area |
表2 水稻幼苗期叶瘟调查的分级标准
Table 2 Grades of blast severity of rice seedlings
| Disease score | Resistance level | State of disease |
|---|---|---|
| 0 | High resistance (HR) | No blast lesion |
| 1 | Resistance (R) | Only needle-shaped brown lesion s- pots |
| 2 | R | Slightly large brown spots with diameter less than 1 mm |
| 3 | Medium re- sistance (MR) | Round or oval gray lesion which shows brown edge and appear diameter between 1-2 mm |
| 4 | Medium su- sceptibility (MS) | Typical spindle lesion which has leng- th between 1-2 cm. The lesion is us- ually confined between two veins of a leaf and the lesion area occupies less than 2.0% of a leaf |
| 5 | MS | Typical spindle lesion whose area occupies the leaf area between 2.1%- 10.0% |
| 6 | Susceptibility (S) | Typical spindle lesion whose area occupies the leaf area between 10.1%- 25.0% |
| 7 | S | Typical spindle lesion whose area occupies the leaf area between 25.1%- 50.0% |
| 8 | High sus- ceptibility (HS) | Typical spindle lesion whose area occupies the leaf area between 50.1%- 75.0% |
| 9 | HS | Typical spindle lesion whose area oc- cupies more than 75.1% of the leaf area |

图2 水稻分蘖盛期注射接种稻瘟病菌及叶瘟病状 (A) 分蘖盛期注射接种叶鞘; (B) 叶瘟病斑。
Figure 2 Evaluation of leaf blast disease resistance by injection inoculation at rice tillering stage (A) Inoculation of rice sheath by injection of Magnaporthe oryzae spore suspension at the stage of tillering; (B) Blast disease symptoms in the rice leaf.

图3 水稻孕穗期注射接种稻瘟病菌及穗颈瘟病状 (A) 水稻孕穗期注射接种; (B) 穗颈瘟病状。
Figure 3 Evaluation of neck blast disease resistance by injection inoculation at rice booting stage (A) Inoculation by injection of Magnaporthe oryzae spore suspension at the stage of booting; (B) Blast disease symptoms in the rice spike.
| Disease score | State of disease |
|---|---|
| 0 | No disease symptom |
| 1 | Blast disease symptom appears in primary or secondary branch of panicle and leads to less than 5.0% yield loss in each spike |
| 3 | Blast disease symptom appears in rice rachis or spike neck which leads to 5.1%-20.0% yield loss in each spike |
| 5 | Blast disease symptom appears in rice rachis or spike neck which leads to half-shriveled grain and 20.0%-50.0% yield loss in each spike |
| 7 | Blast disease symptom appears in rice spike neck which leads to shriveled kernel and 50.0%-70.0% yield loss in each spike |
| 9 | Blast disease symptom appears in rice spike neck which leads to more than 70.0% yield loss in each spike |
表3 水稻穗颈瘟单穗损失率分级标准
Table 3 Grades for evaluation of single panicle loss by rice blast disease
| Disease score | State of disease |
|---|---|
| 0 | No disease symptom |
| 1 | Blast disease symptom appears in primary or secondary branch of panicle and leads to less than 5.0% yield loss in each spike |
| 3 | Blast disease symptom appears in rice rachis or spike neck which leads to 5.1%-20.0% yield loss in each spike |
| 5 | Blast disease symptom appears in rice rachis or spike neck which leads to half-shriveled grain and 20.0%-50.0% yield loss in each spike |
| 7 | Blast disease symptom appears in rice spike neck which leads to shriveled kernel and 50.0%-70.0% yield loss in each spike |
| 9 | Blast disease symptom appears in rice spike neck which leads to more than 70.0% yield loss in each spike |
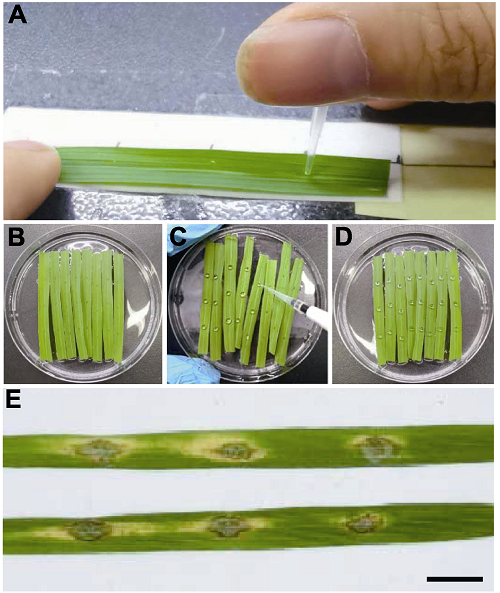
图4 水稻离体叶片的点滴接种及稻瘟病病斑 (A) 采用10 μL移液器枪头对标1.5 cm的刻度戳伤水稻叶片; (B) 戳伤之后漂浮于平皿6-BA溶液中的水稻叶片; (C) 对戳伤叶片进行点滴接种; (D) 点滴接种之后的水稻叶片; (E) 水稻叶片上的病斑(Bar=1 cm)。
Figure 4 Evaluation of blast disease resistance by inoculation of detached rice leaves (A) Punching detached rice leaves using 10 μL pipette tips with 1.5 cm scale; (B) Rice leaves put on the surface of 6-BA solution after punching; (C) Inoculation of punched leaves by spotting spore suspension; (D) Rice leaves inoculated with spore suspension of Magnaporthe oryzae; (E) Blast lesions formed on detached rice leaves (Bar=1 cm).

图5 分蘖期水稻纹枯病的田间木皮物接种鉴定 (A) 0.8 mm厚竖纹木皮; (B) 布满纹枯菌菌丝的木皮接种物(以下简称木皮接种物); (C) 木皮接种物表面菌丝的显微观测; (D) 用镊子将接种物嵌入自上而下第3叶鞘内侧; (E) 水稻分蘖期田间人工接种; (F) 高感水稻品种Lemont接种后11天的病情扩展情况; (G) 高感水稻品种Lemont抽穗后30天的病情扩展情况。
Figure 5 Evaluation of sheath blight disease resistance in field by using wood veneer inoculation method at rice tillering stage (A) Wood veneer with a thickness of 0.8 mm; (B) Wood veneer colonized with mycelia of Rhizoctonia solani (hereafter named wood inoculum); (C) Mycelia of R. solani on wood inoculum observed by micrograph; (D) Wood inoculum was put into inner side of the third leaf sheath from up to down by tweezer; (E) Rice plants in the field at tillering stage used for inoculation; (F) Sheath blight development in the highly susceptible rice variety Lemont at 11 days after inoculation in the field; (G) Disease symptoms of Lemont at 30 days after heading in the field.
| Disease sore | Sheath blight severity |
|---|---|
| 0 | Plant healthy, no symptoms |
| 1 | Lesions mainly restricted to the emerged portion of sixth sheath from top on the culm |
| 1.5 | Lesions extending to lower 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2 | Lesions extending to upper 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2.5 | Lesions extending to lower 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3 | Lesions extending to upper 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3.5 | Lesions extending to lower 1/2 of the emerged portion of third sheath from top on the culm |
| 4 | Lesions extending to upper 1/2 of the emerged portion of third sheath from top on the culm |
| 4.5 | Lesions extending to lower 1/2 of the emerged portion of second sheath from top on the culm |
| 5 | Lesions extending to upper 1/2 of the emerged portion of second sheath from top on the culm |
| 5.5 | Lesions extending to lower 1/4 of the emerged portion of flag leaf sheath from top on the culm |
| 6 | Lesions usually coalescing and reaching to lower 1/4 to 1/2 of the emerged portion of flag leaf sheath on the culm |
| 6.5 | Lesions usually coalescing and reaching to lower 1/2 to 3/4 of the emerged portion of flag leaf sheath on the culm |
| 7 | Lesions usually coalescing and reaching to upper 1/4 of the emerged portion of flag leaf sheath on the culm |
| 7.5 | Lesions usually coalescing and reaching to lower 1/2 of flag leaf on the culm, the flag leaf presenting semi-rolling or <50% flag leaf tissues affected |
| 8 | Lesions usually coalescing and reaching to upper 1/2 of flag leaf on the culm, more than 50% flag leaf tissues affected |
| 8.5 | Panicle rachis and culm with brown streak and becoming light brown, all sheath and leaf tissues dead and drying on the culms, florets in lower 1/3 of panicle often not filling on the culm |
| 9 | Panicle rachis and culm dead and becoming dry, severely affected culms lodging, florets in lower 1/3 to 1/2 or more of panicle not filling on the culm |
表4 田间纹枯病病级的评价标准
Table 4 Rating scales for determining sheath blight disease severity on rice using field inoculation method
| Disease sore | Sheath blight severity |
|---|---|
| 0 | Plant healthy, no symptoms |
| 1 | Lesions mainly restricted to the emerged portion of sixth sheath from top on the culm |
| 1.5 | Lesions extending to lower 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2 | Lesions extending to upper 1/2 of the emerged portion of fifth sheath from top on the culm |
| 2.5 | Lesions extending to lower 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3 | Lesions extending to upper 1/2 of the emerged portion of fourth sheath from top on the culm |
| 3.5 | Lesions extending to lower 1/2 of the emerged portion of third sheath from top on the culm |
| 4 | Lesions extending to upper 1/2 of the emerged portion of third sheath from top on the culm |
| 4.5 | Lesions extending to lower 1/2 of the emerged portion of second sheath from top on the culm |
| 5 | Lesions extending to upper 1/2 of the emerged portion of second sheath from top on the culm |
| 5.5 | Lesions extending to lower 1/4 of the emerged portion of flag leaf sheath from top on the culm |
| 6 | Lesions usually coalescing and reaching to lower 1/4 to 1/2 of the emerged portion of flag leaf sheath on the culm |
| 6.5 | Lesions usually coalescing and reaching to lower 1/2 to 3/4 of the emerged portion of flag leaf sheath on the culm |
| 7 | Lesions usually coalescing and reaching to upper 1/4 of the emerged portion of flag leaf sheath on the culm |
| 7.5 | Lesions usually coalescing and reaching to lower 1/2 of flag leaf on the culm, the flag leaf presenting semi-rolling or <50% flag leaf tissues affected |
| 8 | Lesions usually coalescing and reaching to upper 1/2 of flag leaf on the culm, more than 50% flag leaf tissues affected |
| 8.5 | Panicle rachis and culm with brown streak and becoming light brown, all sheath and leaf tissues dead and drying on the culms, florets in lower 1/3 of panicle often not filling on the culm |
| 9 | Panicle rachis and culm dead and becoming dry, severely affected culms lodging, florets in lower 1/3 to 1/2 or more of panicle not filling on the culm |

图6 水稻孕穗期温室木皮物接种的纹枯病抗性鉴定 (A) 生长于花盆中的孕穗期水稻植株; (B) 对孕穗期水稻的叶鞘接种纹枯病菌木皮; (C) 接种后置于温室中的水稻植株; (D) 感病品种接种21天后的纹枯病病状。
Figure 6 Evaluation of sheath blight disease resistance in greenhouse by using wood veneer inoculation method at rice booting stage (A) Rice plants at booting stage growing in flowerpots; (B) Inoculation of rice sheath with Rhizoctonia solani wood inoculum at booting stage; (C) Rice plants growth in greenhouse after inoculation; (D) Disease symptoms of susceptible rice variety at 21 days after inoculation.
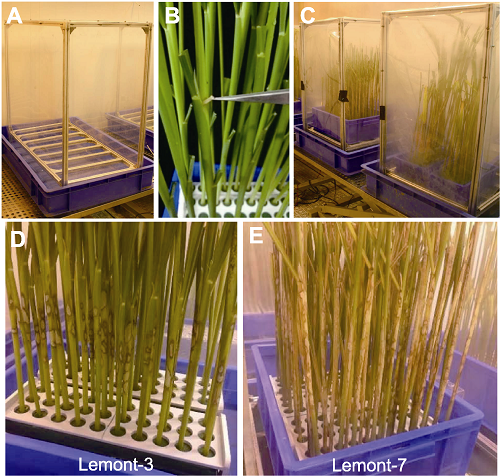
图7 水稻离体茎秆纹枯病接种鉴定方法 (A) 接种框架; (B) 使用嵌入法进行木皮接种物接种; (C) 接种后的培养室; (D) 高感品种Lemont接种后3天病斑的扩展情况; (E) Lemont接种7天后病斑的扩展情况。
Figure 7 Methods for evaluation of sheath blight disease resistance by using detached stems of rice (A) Inoculation shelves; (B) Inoculation of detached rice stem by using wood inoculum; (C) Growth chamber containing inoculated plants; (D) Sheath blight disease symptoms of the highly susceptible cultivar Lemont at three days after inoculation; (E) Sheath blight disease symptoms of the highly susceptible cultivar Lemont at seven days after inoculation.
| Relative lesion length (%) | 0-0.15 | 0.16-0.3 | 0.31-0.45 | 0.46-0.6 | 0.61-0.75 | 0.76-1.0 |
|---|---|---|---|---|---|---|
| Resistant level | HR | R | MR | MS | S | HS |
表5 离体茎秆接种鉴定法的纹枯病抗感分类标准
Table 5 Grades of sheath blight resistance levels of rice varieties using detached-stem inoculation method
| Relative lesion length (%) | 0-0.15 | 0.16-0.3 | 0.31-0.45 | 0.46-0.6 | 0.61-0.75 | 0.76-1.0 |
|---|---|---|---|---|---|---|
| Resistant level | HR | R | MR | MS | S | HS |
| [1] | 曹妮, 陈渊, 季芝娟, 曾宇翔, 杨长登, 梁燕 ( 2019). 水稻抗稻瘟病分子机制研究进展. 中国水稻科学 33, 489-498. |
| [2] | 陈锦文, 谢旺有, 王天生, 陈惠清, 黄荣裕, 谢少和 ( 2015). 人工注射接种及田间病圃鉴定水稻对稻瘟病的抗病性. 广西植保 28(4), 6-10. |
| [3] | 高杜娟, 唐善军, 陈友德, 周斌 ( 2019). 水稻主要病害生物防治的研究进展. 中国农学通报 35(26), 140-147. |
| [4] | 许有嫔, 廖海澄, 陈金华, 罗櫞, 宿加, 邹成东, 李伟滔, 王静, 马炳田, 贺闽, 陈学伟 ( 2017). 利用绿色荧光蛋白GFP研究稻瘟病菌与水稻的互作. 植物保护 43(6), 53-61. |
| [5] | 杨德卫, 王莫, 韩利波, 唐定中, 李生平 ( 2019). 水稻稻瘟病抗性基因的克隆、育种利用及稻瘟菌无毒基因研究进展. 植物学报 54, 265-276. |
| [6] | 左示敏, 张亚芳, 陈宗祥, 陈夕军, 潘学彪 ( 2010). 水稻抗纹枯病遗传育种研究进展. 中国科学: 生命科学 40, 1014-1023. |
| [7] |
Dean R, van Kan JAL, Pretorius ZA, Hammond-Kosack KE, Di Pietro A, Spanu PD, Rudd JJ, Dickman M, Kahmann R, Ellis J, Foster GD ( 2012). The top 10 fungal pathogens in molecular plant pathology. Mol Plant Pathol 13, 804.
DOI URL |
| [8] |
Li WT, Chern M, Yin JJ, Wang J, Chen XW ( 2019). Recent advances in broad-spectrum resistance to the rice blast disease. Curr Opin Plant Biol 50, 114-120.
DOI URL PMID |
| [9] |
Wang L, Liu LM, Hou YX, Li L, Huang SW ( 2015). Pathotypic and genetic diversity in the population of Rhizoctonia solani AG1-IA causing rice sheath blight in China. Plant Pathol 64, 718-728.
DOI URL |
| [10] |
Wilson RA, Talbot NJ ( 2009). Under pressure: investigating the biology of plant infection by Magnaporthe oryzae. Nat Rev Microbiol 7, 185-195.
DOI URL PMID |
| [11] | Yoshida S, Fonao DA, Cock JH, Gomez KA (1976). Laboratory manual for physiological studies of rice. In: International Rice Research Institute, 3rd edn. Manila: The International Rice Research Institute. pp. 61-66. |
| [12] |
Zheng AP, Lin RM, Zhang DH, Qin PG, Xu LZ, Ai P, Ding L, Wang YR, Chen Y, Liu Y, Sun ZG, Feng HT, Liang XX, Fu RT, Tang CQ, Li Q, Zhang J, Xie ZL, Deng QM, Li SC, Wang SQ, Zhu J, Wang LX, Liu HN, Li P ( 2013). The evolution and pathogenic mechanisms of the rice sheath blight pathogen. Nat Commun 4, 1424.
DOI URL PMID |
| [1] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [3] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [4] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [5] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [6] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [7] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [8] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [9] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [10] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [11] | 朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育[J]. 植物学报, 2024, 59(1): 10-21. |
| [12] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [13] | 贾绮玮, 钟芊芊, 顾育嘉, 陆天麒, 李玮, 杨帅, 朱超宇, 胡程翔, 李三峰, 王跃星, 饶玉春. 水稻茎秆细胞壁相关组分含量QTL定位及候选基因分析[J]. 植物学报, 2023, 58(6): 882-892. |
| [14] | 田传玉, 方妍力, 沈晴, 王宏杰, 陈析丰, 郭威, 赵开军, 王春连, 纪志远. 2019-2021年我国南方稻区白叶枯病菌的毒力与遗传多样性调查研究[J]. 植物学报, 2023, 58(5): 743-749. |
| [15] | 戴若惠, 钱心妤, 孙静蕾, 芦涛, 贾绮玮, 陆天麒, 路梅, 饶玉春. 水稻叶色调控机制及相关基因研究进展[J]. 植物学报, 2023, 58(5): 799-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||