

植物学报 ›› 2018, Vol. 53 ›› Issue (1): 17-26.DOI: 10.11983/CBB17135 cstr: 32102.14.CBB17135
收稿日期:2017-07-23
接受日期:2017-11-08
出版日期:2018-01-01
发布日期:2018-01-10
通讯作者:
丁兆军
基金资助:
Guangchao Liu , Zhaojun Ding*( )
)
Received:2017-07-23
Accepted:2017-11-08
Online:2018-01-01
Published:2018-01-10
Contact:
Zhaojun Ding
摘要: 植物是一类营固着生活的自养型生物, 如何更好地适应周围环境对植物的生存至关重要。生长素是调控植物生长发育的重要激素之一。近年来的研究发现, 生长素不仅能够响应内在的发育信号, 而且能够介导各种环境信号, 参与植物生长发育和生长反应的调控。该文主要从光信号、温度信号、重力信号、营养元素和金属离子信号等方面重点阐述生长素如何介导上述各种不同的环境信号, 从而调控植物的生长发育。
刘广超, 丁兆军. 生长素介导环境信号调控植物的生长发育. 植物学报, 2018, 53(1): 17-26.
Guangchao Liu , Zhaojun Ding. Auxin Regulates Plant Growth and Development by Mediating Various Environmental Cues. Chinese Bulletin of Botany, 2018, 53(1): 17-26.
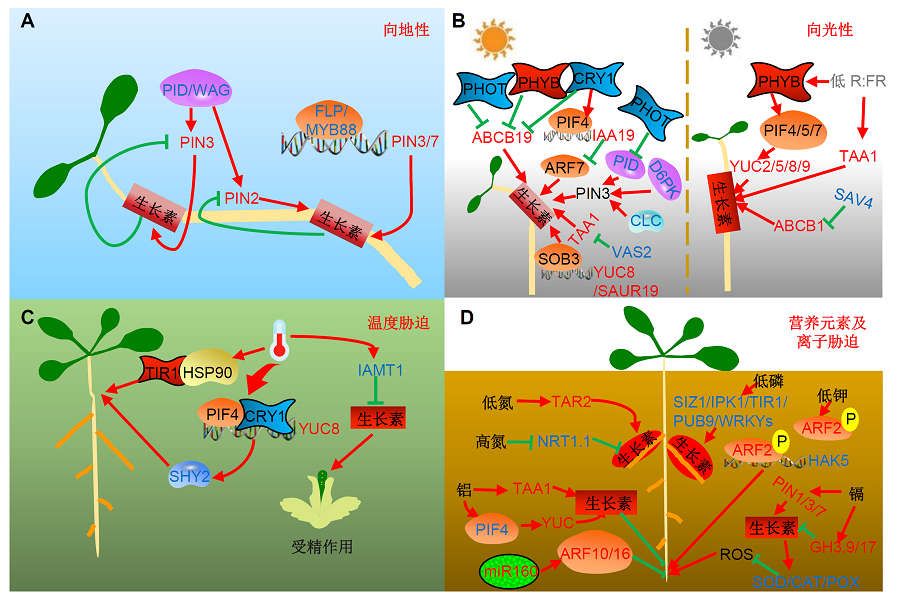
图1 生长素介导的环境信号调控植物生长发育的工作模型(A) 在重力响应过程中, 生长素的非对称分布主要是通过改变外运载体PIN的表达模式来实现的。这一调控过程一方面依赖于PID/WAG对PIN蛋白的磷酸化, 另一方面受转录因子FLP/MYB88直接的转录调节。(B) 在光信号通路过程中, 蓝光受体向光素PHOT通过抑制PID蛋白的活性, 降低对PIN3的磷酸化修饰水平进而参与生长素在下胚轴中的非对称分布。另一蓝光受体隐花色素CRY一方面和蓝光受体向光素PHOT及红光受体PHYB共同抑制ABCB19蛋白表达水平, 另一方面则通过转录因子PIF4直接结合在IAA19的启动子区并抑制下游生长素响应因子ARF7的转录活性参与下胚轴向光的弯曲生长。在植物庇荫反应中, 红光受体PHYB通过PIF家族转录因子直接调控生长素合成相关基因YUCCA的转录水平参与茎的向光弯曲; 而SAV3及SAV4同样参与了植物的庇荫反应。(C) 在响应温度信号通路中, 生长素受体TIR1通过直接与热激蛋白HSP90互作参与植物对高温环境的适应; 另一方面蓝光受体CRY1与PIF4直接互作调控YUC8的转录参与高温对下胚轴的伸长。此外, 高温诱导生长素甲基转移酶IAMT1的表达, 降低植物子房内生长素信号, 进而出现显著的雄性不育表型。(D) 在响应不同离子对植物根系发育的调控网络中, 生长素合成基因TAR2介导了低氮对植物侧根发生的调控, 而硝态氮受体基因NRT1.1通过抑制生长素的极性运输促进了高氮下植物侧根的起始。在低钾处理时, ARF2可被磷酸化, 进而解除对HAK5转录的抑制, 增强植物对钾离子的吸收能力。生长素合成基因TAA1和YUCCAs特异性地在转换区受到铝胁迫的异位诱导表达, 造成此区生长素的积累, 而ARF7可以直接转录调控细胞分裂素合成基因IPT的表达, 最终抑制主根的伸长。镉胁迫则通过维持生长素的内稳态, 进而抑制下游ROS水平, 参与对根伸长的调控。
Figure 1 An overview of plant growth and development in response to environment signal mediated by auxin (A) Nonsymmetrical of auxin in response to gravity due to altering expression pattern of auxin polar transporter PIN, which is regulated by phosphorylation of PIN through PID/WAG kinase or transcriptional regulation of FLP/MYB88. (B) In response to phototropism, blue light receptor PHOT reduced phosphorylation of PIN3 by inhibiting PID activity, thus mediated nonsymmetrical of auxin in hypocotyl. Another blue light receptor CRY inhibited ABCB919 expression together with PHOT and PHYB, and decreased ARF7 expression through binding to IAA19 promoter with PIF4. In shade condition, PHYB participated in phototropism of shoot through PIF to regulate YUCCA expression. SAV3 and SAV4 also mediate plant shade response. (C) In response to temperature signal, auxin receptor TIR1 can interact with HSP90 to participate in high temperature stress. While CRY1, together with PIF4, induced YUC8 expression to increase auxin level in hypocotyl under high temperature condition. In addition, high temperature could induce the expression of auxin transmethylase IAMT1, reduced auxin signal in ovary and led to male sterile. (D) Auxin synthesis gene TAR2 mediated lateral root development under low nitrogen condition, and nitrate nitrogen receptor NRT1.1 induced lateral root initiation by inhibiting auxin polar transport. ARF2 can be phosphorylated in low potassium, thus relieve the inhibition of HAK5 and enhanced the absorbing ability of phosphorus. TAA1 and YUCCA can be specific induced in the root TZ under aluminum stress, which caused excess auxin. While ARF7 directly regulated IPT expression, leading to the inhibition of root growth. Another metal ion cadmium maintained the auxin homeostasis to reduce downstream ROS level to regulate root growth.
| [1] | Abbas M, Hernández-García J, Blanco-Touriñán N, Ali- aga N, Minguet EG, Alabadi D, Blázquez MA (2018). Reduction of indole-3-acetic acid methyltransferase activi- ty compensates for high-temperature male sterility in Ara- bidopsis.Plant Biotechnol J 16, 272-279. |
| [2] | Berleth T, Krogan NT, Scarpella E (2004). Auxin signals- turning genes on and turning cells around.Curr Opin Plant Biol 7, 553-563. |
| [3] | Bouguyon E, Brun F, Meynard D, Kubeš M, Pervent M, Leran S, Lacombe B, Krouk G, Guiderdoni E, Zaží- malová E, Hoyerová K, Nacry P, Gojon A (2015). Multiple mechanisms of nitrate sensing by Arabidopsis nitrate transceptor NRT1.1.Nat Plants 1, 15015. |
| [4] | Casal JJ (2013). Photoreceptor signaling networks in plant responses to shade.Annu Rev Plant Biol 64, 403-427. |
| [5] | Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, Adamec J, Peer WA, Murphy AS (2011). phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism.PLoS Biol 9, e1001076. |
| [6] | Deb S, Sankaranarayanan S, Wewala G, Widdup E, Sa- muel MA (2014). The S-domain receptor kinase Arabidopsis receptor kinase2 and the U box/armadillo repeat-containing E3 ubiquitin ligase9 module mediates lateral root development under phosphate starvation in Arabidopsis.Plant Physiol 165, 1647-1656. |
| [7] | Devaiah BN, Karthikeyan AS, Raghothama KG (2007). WRKY75 transcription factor is a modulator of phosphate acquisition and root development in Arabidopsis.Plant Physiol 143, 1789-1801. |
| [8] | Ding ZJ, Galván-Ampudia CS, Demarsy E, Łangowski L, Kleine-Vehn J, Fan YW, Morita MT, Tasaka M, Fankhauser C, Offringa R, Friml J (2011). Light-mediated polarization of the PIN3 auxin transporter for the phototropic response in Arabidopsis.Nat Cell Biol 13, 447-452. |
| [9] | Elobeid M, Göbel C, Feussner I, Polle A (2012). Cadmium interferes with auxin physiology and lignification in poplar.J Exp Bot 63, 1413-1421. |
| [10] | Favero DS, Jacques CN, Iwase A, Le KN, Zhao JF, Sugimoto K, Neff MM (2016). SUPPRESSOR OF PHYTOCHROME B4-#3 represses genes associated with auxin signaling to modulate hypocotyl growth.Plant Phy- siol 171, 2701-2716. |
| [11] | Folta KM, Pontin MA, Karlin-Neumann G, Bottini R, Spalding EP (2003). Genomic and physiological studies of early cryptochrome 1 action demonstrate roles for au- xin and gibberellin in the control of hypocotyl growth by blue light.Plant J 36, 203-214. |
| [12] | Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K (2002). Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis.Nature 415, 806-809. |
| [13] | Ge YH, Yan FL, Zourelidou M, Wang ML, Ljung K, Fastner A, Hammes UZ, Di Donato M, Geisler M, Schwe- chheimer C, Tao Y (2017). SHADE AVOIDANCE 4 is required for proper auxin distribution in the hypocotyl.Plant Physiol 173, 788-800. |
| [14] | Goyal A, Karayekov E, Galvão VC, Ren H, Casal JJ, Fankhauser C (2016). Shade promotes phototropism th- rough phytochrome B-controlled auxin production.Curr Biol 26, 3280-3287. |
| [15] | Hersch M, Lorrain S, de Wit M, Trevisan M, Ljung K, Bergmann S, Fankhauser C (2014). Light intensity modu- lates the regulatory network of the shade avoidance response in Arabidopsis.Proc Natl Acad Sci USA 111, 6515-6520. |
| [16] | Hu YF, Zhou GY, Na XF, Yang LJ, Nan WB, Liu X, Zhang YQ, Li JL, Bi YR (2013). Cadmium interferes with maintenance of auxin homeostasis in Arabidopsis seedlings.J Plant Physiol 170, 965-975. |
| [17] | Keuskamp DH, Pollmann S, Voesenek LACJ, Peeters AJM, Pierik R (2010). Auxin transport through PIN- FORMED 3 (PIN3) controls shade avoidance and fitness during competition.Proc Natl Acad Sci USA 107, 22740-22744. |
| [18] | Kollmeier M, Felle HH, Horst WJ (2000). Genotypical differences in aluminum resistance of maize are expressed in the distal part of the transition zone. Is reduced basi- petal auxin flow involved in inhibition of root elongation by aluminum?Plant Physiol 122, 945-956. |
| [19] | Krouk G, Lacombe B, Bielach A, Perrine-Walker F, Malinska K, Mounier E, Hoyerova K, Tillard P, Leon S, Ljung K, Zazimalova E, Benkova E, Nacry P, Gojon A (2010). Nitrate-regulated auxin transport by NRT1.1 defines a mechanism for nutrient sensing in plants.Dev Cell 18, 927-937. |
| [20] | Kuo HF, Chang TY, Chiang SF, Wang WD, Charng YY, Chiou TJ (2014). Arabidopsis inositol pentakisphosphate 2-kinase, AtIPK1, is required for growth and modulates phosphate homeostasis at the transcriptional level.Plant J 80, 503-515. |
| [21] | Liu GC, Gao S, Tian HY, Wu WW, Robert HS, Ding ZJ (2016). Local transcriptional control of YUCCA regulates auxin promoted root-growth inhibition in response to aluminium stress in Arabidopsis.PLoS Genet 12, e1006360. |
| [22] | Ma DB, Li X, Guo YX, Chu JF, Fang S, Yan CY, Noel JP, Liu HT (2016). Cryptochrome 1 interacts with PIF4 to regulate high temperature-mediated hypocotyl elongation in response to blue light.Proc Natl Acad Sci USA 113, 224-229. |
| [23] | Ma WY, Li JJ, Qu BY, He X, Zhao XQ, Li B, Fu XD, Tong YP (2014). Auxin biosynthetic gene TAR2 is involved in low nitrogen-mediated reprogramming of root architecture in Arabidopsis. Plant J 78, 70-79. |
| [24] | Miura K, Lee J, Gong QQ, Ma SS, Jin JB, Yoo CY, Miura T, Sato A, Bohnert HJ, Hasegawa PM (2011). SIZ1 regulation of phosphate starvation-induced root architecture remodeling involves the control of auxin accumulation.Plant Physiol 155, 1000-1012. |
| [25] | Morelli G, Ruberti I (2000). Shade avoidance responses. Driving auxin along lateral routes.Plant Physiol 122, 621-626. |
| [26] | Pérez-Torres CA, López-Bucio J, Cruz-Ramírez A, Ibarra- Laclette E, Dharmasiri S, Estelle M, Herrera-Estrella L (2008). Phosphate availability alters lateral root deve- lopment in Arabidopsis by modulating auxin sensitivity via a mechanism involving the TIR1 auxin receptor.Plant Cell 20, 3258-3272. |
| [27] | Rakusova H, Abbas M, Han H, Song S, Robert HS, Friml J (2016). Termination of shoot gravitropic responses by auxin feedback on PIN3 polarity.Curr Biol 26, 3026-3032. |
| [28] | Sato A, Yamamoto KT (2008). Overexpression of the non- canonical Aux/IAA genes causes auxin-related aberrant phenotypes in Arabidopsis. Physiol Plant 133, 397-405. |
| [29] | Sun J, Qi L, Li Y, Chu J, Li C (2012). PIF4-mediated activation of YUCCA8 expression integrates temperature into the auxin pathway in regulating Arabidopsis hypocotyl growth. PLoS Genet 8, e1002594. |
| [30] | Sun JQ, Qi LL, Li YN, Zhai QZ, Li CY (2013). PIF4 and PIF5 transcription factors link blue light and auxin to re- gulate the phototropic response in Arabidopsis.Plant Cell 25, 2102-2114. |
| [31] | Sun P, Tian QY, Chen J, Zhang WH (2010). Aluminium- induced inhibition of root elongation in Arabidopsis is mediated by ethylene and auxin.J Exp Bot 61, 347-356. |
| [32] | Svistoonoff S, Creff A, Reymond M, Sigoillot-Claude C, Ricaud L, Blanchet A, Nussaume L, Desnos T (2007). Root tip contact with low-phosphate media reprograms plant root architecture.Nat Genet 39, 792-796. |
| [33] | Swarup R, Friml J, Marchant A, Ljung K, Sandberg G, Palme K, Bennett M (2001). Localization of the auxin permease AUX1 suggests two functionally distinct hormone transport pathways operate in the Arabidopsis root apex.Genes Dev 15, 2648-2653. |
| [34] | Tao Y, Ferrer JL, Ljung K, Pojer F, Hong F, Long JA, Li L, Moreno JE, Bowman ME, Ivans LJ, Cheng Y, Lim J, Zhao YD, Ballare CL, Sandberg G, Noel JP, Chory J (2008). Rapid synthesis of auxin via a new tryptophan- dependent pathway is required for shade avoidance in plants.Cell 133, 164-176. |
| [35] | Wang HZ, Yang KZ, Zou JJ, Zhu LL, Xie ZD, Morita MT, Tasaka M, Friml J, Grotewold E, Beeckman T, Vanneste S, Sack F, Le J (2015). Transcriptional regulation of PIN genes by FOUR LIPS and MYB88 during Arabidopsis root gravitropism. Nat Commun 6, 8822. |
| [36] | Wang RH, Zhang Y, Kieffer M, Yu H, Kepinski S, Estelle M (2016). HSP90 regulates temperature-dependent seed- ling growth in Arabidopsis by stabilizing the auxin co-receptor F-box protein TIR1.Nat Commun 7, 10269. |
| [37] | Willige BC, Ahlers S, Zourelidou M, Barbosa ICR, Demarsy E, Trevisan M, Davis PA, Roelfsema MRG, Hangarter R, Fankhauser C, Schwechheimer C (2013). D6PK AGCVIII kinases are required for auxin transport and phototropic hypocotyl bending in Arabidopsis.Plant Cell 25, 1674-1688. |
| [38] | Wu GS, Cameron JN, Ljung K, Spalding EP (2010). A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B.Plant J 62, 179-191. |
| [39] | Xu L, Jin L, Long L, Liu LL, He X, Gao W, Zhu LF, Zhang XL (2012). Overexpression of GbWRKY1 positively regu- lates the Pi starvation response by alteration of auxin sensitivity in Arabidopsis. Plant Cell Rep 31, 2177-2188. |
| [40] | Yang ZB, Geng XY, He CM, Zhang F, Wang R, Horst WJ, Ding ZJ (2014). TAA1-regulated local auxin biosynthesis in the root-apex transition zone mediates the aluminum- induced inhibition of root growth in Arabidopsis.Plant Cell 26, 2889-2904. |
| [41] | Yu CL, Sun CD, Shen CJ, Wang SK, Liu F, Liu Y, Chen YL, Li CY, Qian Q, Aryal B, Geisler M, Jiang DA, Qi YH (2015). The auxin transporter, OsAUX1, is involved in primary root and root hair elongation and in Cd stress responses in rice ( Oryza sativa L.). Plant J 83, 818-830. |
| [42] | Yuan H, Liu D (2008). Signaling components involved in plant responses to phosphate starvation.J Integr Plant Biol 50, 849-859. |
| [43] | Zhang Y, Yu QQ, Jiang N, Yan X, Wang C, Wang QM, Liu JZ, Zhu MY, Bednarek SY, Xu J, Pan JW (2017). Clathrin regulates blue light-triggered lateral auxin distribution and hypocotyl phototropism in Arabidopsis.Plant Cell Environ 40, 165-176. |
| [44] | Zhao S, Zhang ML, Ma TL, Wang Y (2016). Phosphorylation of ARF2 relieves its repression of transcription of the K+ transporter gene HAK5 in response to low potassium stress. Plant Cell 28, 3005-3019. |
| [1] | 王亚萍, 包文泉, 白玉娥. 单细胞转录组学在植物生长发育及胁迫响应中的应用进展[J]. 植物学报, 2025, 60(1): 101-113. |
| [2] | 李青洋, 刘翠, 何李, 彭姗, 马嘉吟, 胡子祎, 刘宏波. 甘蓝型油菜BnaA02.CPSF6基因的克隆及功能分析(长英文摘要)[J]. 植物学报, 2025, 60(1): 62-73. |
| [3] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| [4] | 杜庆国, 李文学. lncRNA调控玉米生长发育和非生物胁迫研究进展[J]. 植物学报, 2024, 59(6): 950-962. |
| [5] | 闫恒宇, 李朝霞, 李玉斌. 高温对玉米生长的影响及中国耐高温玉米筛选研究进展[J]. 植物学报, 2024, 59(6): 1007-1023. |
| [6] | 路笃贤, 张严妍, 刘艳, 李岩竣, 左新秀, 林金星, 崔亚宁. 非编码RNA在植物生长发育及逆境响应中的研究进展[J]. 植物学报, 2024, 59(5): 709-725. |
| [7] | 周玉滢, 陈辉, 刘斯穆. 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024, 59(4): 651-658. |
| [8] | 曾鑫海, 陈锐, 师宇, 盖超越, 范凯, 李兆伟. 植物SPL转录因子的生物功能研究进展[J]. 植物学报, 2023, 58(6): 982-997. |
| [9] | 孔祥培, 张蒙悦, 丁兆军. 柳暗花明:胞外生长素信号感受的新突破[J]. 植物学报, 2023, 58(6): 861-865. |
| [10] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [11] | 许亚楠, 闫家榕, 孙鑫, 王晓梅, 刘玉凤, 孙周平, 齐明芳, 李天来, 王峰. 红光和远红光在调控植物生长发育及应答非生物胁迫中的作用[J]. 植物学报, 2023, 58(4): 622-637. |
| [12] | 张嘉, 李启东, 李翠, 王庆海, 侯新村, 赵春桥, 李树和, 郭强. 植物MATE转运蛋白研究进展[J]. 植物学报, 2023, 58(3): 461-474. |
| [13] | 周淑瑶, 李建明, 毛娟. AtGH3.17调控拟南芥生长素和油菜素甾醇的响应[J]. 植物学报, 2023, 58(3): 373-384. |
| [14] | 王琪, 吴允哲, 刘学英, 孙丽莉, 廖红, 傅向东. 类受体激酶调控水稻生长发育和环境适应研究进展[J]. 植物学报, 2023, 58(2): 199-213. |
| [15] | 叶青, 闫晓燕, 陈慧泽, 冯金林, 韩榕. 氮掺杂石墨烯量子点对拟南芥主根生长方向的影响[J]. 植物学报, 2022, 57(5): 623-634. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||