

植物学报 ›› 2020, Vol. 55 ›› Issue (1): 5-8.DOI: 10.11983/CBB20002 cstr: 32102.14.CBB20002
收稿日期:2020-01-07
接受日期:2020-01-13
出版日期:2020-01-01
发布日期:2020-02-07
通讯作者:
晁代印
基金资助:
Mei-ling Han1,2,Ru-jiao Tan1,3,Dai-yin Chao1,*( )
)
Received:2020-01-07
Accepted:2020-01-13
Online:2020-01-01
Published:2020-02-07
Contact:
Dai-yin Chao
摘要: 以半矮秆育种为代表的“绿色革命”极大地提高了作物产量, 但也带来氮营养利用效率降低的严重问题。“绿色革命”主要基于调控赤霉素的代谢和信号转导而实现。前期的研究发现, 赤霉素信号转导关键因子DELLA蛋白通过调控GRF4而负调控氮素的吸收利用, 为半矮秆品系氮利用效率低的问题提供了解决方案。最近的一项研究进一步揭示了GA信号途径与氮响应交叉互作的新机制。该研究发现水稻(Oryza sativa) NGR5是氮素调控分蘖数目的一个关键基因, 其表达受氮诱导。通过招募PRC2, NGR5对D14和OsSPL14等分蘖抑制基因所在位点进行H3K27me3甲基化修饰, 从而抑制其表达。而在半矮秆背景下超表达NGR5可以提高低氮水平下的水稻产量。NGR5同时也被发现为赤霉素受体GID1的一个新靶标, 受到其负调控。该研究发现了调控赤霉素信号通路的新机制, 并对高产高效的新一代“绿色革命”育种实践具有重要启示。
韩美玲,谭茹姣,晁代印. “绿色革命”新进展: 赤霉素与氮营养双重调控的表观修饰助力水稻高产高效育种. 植物学报, 2020, 55(1): 5-8.
Mei-ling Han,Ru-jiao Tan,Dai-yin Chao. A New Progress of Green Revolution: Epigenetic Modification Dual-regulated by Gibberellin and Nitrogen Supply Contributes to Breeding of High Yield and Nitrogen Use Efficiency Rice. Chinese Bulletin of Botany, 2020, 55(1): 5-8.
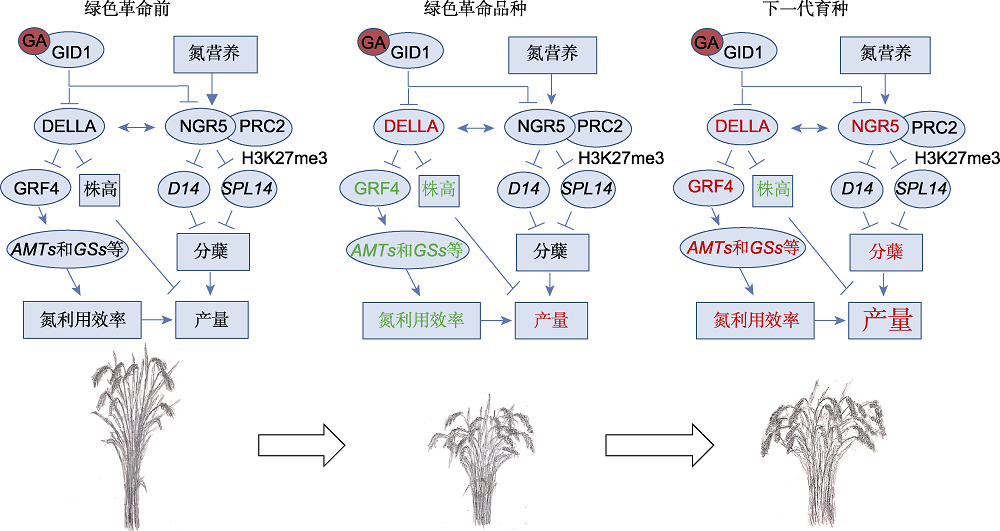
图1 “绿色革命”和下一代育种靶标及分子机制 在“绿色革命”中, 抑制GA信号通路导致DELLA蛋白积累, 产生半矮秆表型, 但同时也抑制GRF4的活性进而降低植物氮利用效率。GRF4以及NGR5为下一代高产高效的育种目标提供了优秀的靶点。增强它们的活性可以兼顾高产和营养高效, 突破以肥增产的瓶颈。红色及大字号代表增加, 而绿色和小字号代表降低。
Figure 1 The targets and molecular mechanisms of Green Revolution and next generation breeding In Green Revolution, suppression of GA signaling leads to accumulation of DELLA protein that results in semi-dwarf phenotype, but it also inhibits activity of GRF4 and subsequently decreases nitrogen use efficiency of crops. GRF4 and NGR5 provide excellent targets for next generation breeding which aims to crops with high nitrogen use efficiency and high yield. Improvement of these two genes helps to achieve yield with low nitrogen input and break through the bottle neck of fertilizer dependent yield increasing. The font size and color of the characters represent increase (red and larger font) or decrease (green and smaller font).
| [1] | Crawford NM, Forde BG (2002). Molecular and developmental biology of inorganic nitrogen nutrition. Arabidopsis Book 1, e0011. |
| [2] | Gooding MJ, Addisu M, Uppal RK, Snape JW, Jones HE (2012). Effect of wheat dwarfing genes on nitrogen-use efficiency. J Agric Sci 150, 3-22. |
| [3] | Guo JH, Liu XJ, Zhang Y, Shen JL, Han WX, Zhang WF, Christie P, Goulding KWT, Vitousek PM, Zhang FS (2010). Significant acidification in major Chinese crop- lands. Science 327, 1008-1010. |
| [4] | Harberd NP, Belfield E, Yasumura Y (2009). The angiosperm gibberellin-GID1-DELLA growth regulatory mechanism: how an "inhibitor of an inhibitor" enables flexible response to fluctuating environments. Plant Cell 21, 1328-1339. |
| [5] | Khush GS (1999). Green Revolution: preparing for the 21st century. Genome 42, 646-655. |
| [6] | Kong WD, Zhu YG, Fu BJ, Han XZ, Zheng L, He JZ (2008). Effect of long-term application of chemical fertilizers on microbial biomass and functional diversity of a black soil. Pedosphere 18, 801-808. |
| [7] | Li S, Tian YH, Wu K, Ye YF, Yu JP, Zhang JQ, Liu Q, Hu MY, Li H, Tong YP, Harberd NP, Fu XD (2018). Modulating plant growth-metabolism coordination for sustainable agriculture. Nature 560, 595-600. |
| [8] | Murase K, Hirano Y, Sun TP, Hakoshima T (2008). Gibberellin-induced DELLA recognition by the gibberellin receptor GID1. Nature 456, 459-463. |
| [9] | Peng JR, Richards DE, Hartley NM, Murphy GP, Devos KM, Flintham JE, Beales J, Fish LJ, Worland AJ, Pe- lica F, Sudhakar D, Christou P, Snape JW, Gale MD, Harberd NP (1999). 'Green Revolution' genes encode mutant gibberellin response modulators. Nature 400, 256-261. |
| [10] | Pingali PL (2012). Green Revolution: impacts, limits, and the path ahead. Proc Natl Acad Sci USA 109, 12302-12308. |
| [11] | Sasaki A, Ashikari M, Ueguchi-Tanaka M, Itoh H, Nishimura A, Swapan D, Ishiyama K, Saito T, Kobayashi M, Khush GS, Kitano H, Matsuoka M (2002). Green Revolution: a mutant gibberellin-synthesis gene in rice. Nature 416, 701-702. |
| [12] | Sasaki A, Itoh H, Gomi K, Ueguchi-Tanaka M, Ishiyama K, Kobayashi M, Jeong DH, An G, Kitano H, Ashikari M, Matsuoka M (2003). Accumulation of phosphorylated repressor for gibberellin signaling in an F-box mutant. Science 299, 1896-1898. |
| [13] | Shimada A, Ueguchi-Tanaka M, Nakatsu T, Nakajima M, Naoe Y, Ohmiya H, Kato H, Matsuoka M (2008). Structural basis for gibberellin recognition by its receptor GID1. Nature 456, 520-523. |
| [14] | Spielmeyer W, Ellis MH, Chandler PM (2002). Semidwarf (sd-1), "green revolution" rice, contains a defective gibberellin 20-oxidase gene. Proc Natl Acad Sci USA 99, 9043-9048. |
| [15] | Wu K, Wang SS, Song WZ, Zhang JQ, Wang Y, Liu Q, Yu JP, Ye YF, Li S, Chen JF, Zhao Y, Wang J, Wu XK, Wang MY, Zhang YJ, Liu BM, Wu YJ, Harberd NP, Fu XD (2020). Enhanced sustainable Green Revolution yield via nitrogen-responsive chromatin modulation in rice. Science 367, eaaz2046. |
| [16] | Zhang CH, Gao LF, Sun JQ, Jia JZ, Ren ZL (2014). Haplotype variation of Green Revolution gene Rht-D1 during wheat domestication and improvement. J Integr Plant Biol 56, 774-780. |
| [1] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 赵洪贤, 刘鹏, 史曼英, 徐铭泽, 贾昕, 田赟, 查天山. 毛乌素沙地典型固沙植物黑沙蒿和赖草叶片氮分配对最大净光合速率的影响[J]. 植物生态学报, 2025, 49(3): 460-474. |
| [3] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [4] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [5] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [6] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [7] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [8] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [9] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [10] | 耿雪琪, 唐亚坤, 王丽娜, 邓旭, 张泽凌, 周莹. 氮添加增加中国陆生植物生物量并降低其氮利用效率[J]. 植物生态学报, 2024, 48(2): 147-157. |
| [11] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [12] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [13] | 朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育[J]. 植物学报, 2024, 59(1): 10-21. |
| [14] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [15] | 贾绮玮, 钟芊芊, 顾育嘉, 陆天麒, 李玮, 杨帅, 朱超宇, 胡程翔, 李三峰, 王跃星, 饶玉春. 水稻茎秆细胞壁相关组分含量QTL定位及候选基因分析[J]. 植物学报, 2023, 58(6): 882-892. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||