

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (6): 957-967.DOI: 10.11983/CBB24176 cstr: 32102.14.CBB24176
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Liru Zhou1,2,3, Yan Ao1,2,3,*( ), Jing Zhong1,2,3
), Jing Zhong1,2,3
Received:2024-11-23
Accepted:2025-01-20
Online:2025-11-10
Published:2025-01-21
Contact:
Yan Ao
Liru Zhou, Yan Ao, Jing Zhong. Adventitious Bud Induction and Browning Inhibition of Xanthoceras sorbifolium Seed Kernels[J]. Chinese Bulletin of Botany, 2025, 60(6): 957-967.
| Group | Chorine disinfection time (min) | Effective chorine concentration (%) | Browning rate (%) | Death rate (%) | Contamination rate (%) |
|---|---|---|---|---|---|
| A1 | 10 | 0.1 | 22.67±3.53 d | 12.00±2.31 e | 29.33±3.53 a |
| A2 | 10 | 0.5 | 42.67±3.53 c | 12.00±2.31 e | 28.00±2.31 a |
| A3 | 10 | 1.0 | 62.60±1.33 b | 34.67±3.53 c | 21.33±3.53 ab |
| A4 | 10 | 5.0 | 42.67±3.53 c | 25.33±1.33 d | 9.33±3.53 cd |
| A5 | 20 | 0.1 | 60.00±6.93 b | 18.67±3.53 de | 21.33±3.53 ab |
| A6 | 20 | 0.5 | 61.33±3.53 b | 52.00±4.62 b | 16.00±2.31 bc |
| A7 | 20 | 1.0 | 62.67±4.81 b | 45.33±3.53 b | 4.00±2.31 d |
| A8 | 20 | 5.0 | 80.00±4.62 a | 66.67±1.33 a | 1.33±1.33 d |
Table 1 Effect of different disinfection treatments on the mature seed kernels of Xanthoceras sorbifolium
| Group | Chorine disinfection time (min) | Effective chorine concentration (%) | Browning rate (%) | Death rate (%) | Contamination rate (%) |
|---|---|---|---|---|---|
| A1 | 10 | 0.1 | 22.67±3.53 d | 12.00±2.31 e | 29.33±3.53 a |
| A2 | 10 | 0.5 | 42.67±3.53 c | 12.00±2.31 e | 28.00±2.31 a |
| A3 | 10 | 1.0 | 62.60±1.33 b | 34.67±3.53 c | 21.33±3.53 ab |
| A4 | 10 | 5.0 | 42.67±3.53 c | 25.33±1.33 d | 9.33±3.53 cd |
| A5 | 20 | 0.1 | 60.00±6.93 b | 18.67±3.53 de | 21.33±3.53 ab |
| A6 | 20 | 0.5 | 61.33±3.53 b | 52.00±4.62 b | 16.00±2.31 bc |
| A7 | 20 | 1.0 | 62.67±4.81 b | 45.33±3.53 b | 4.00±2.31 d |
| A8 | 20 | 5.0 | 80.00±4.62 a | 66.67±1.33 a | 1.33±1.33 d |
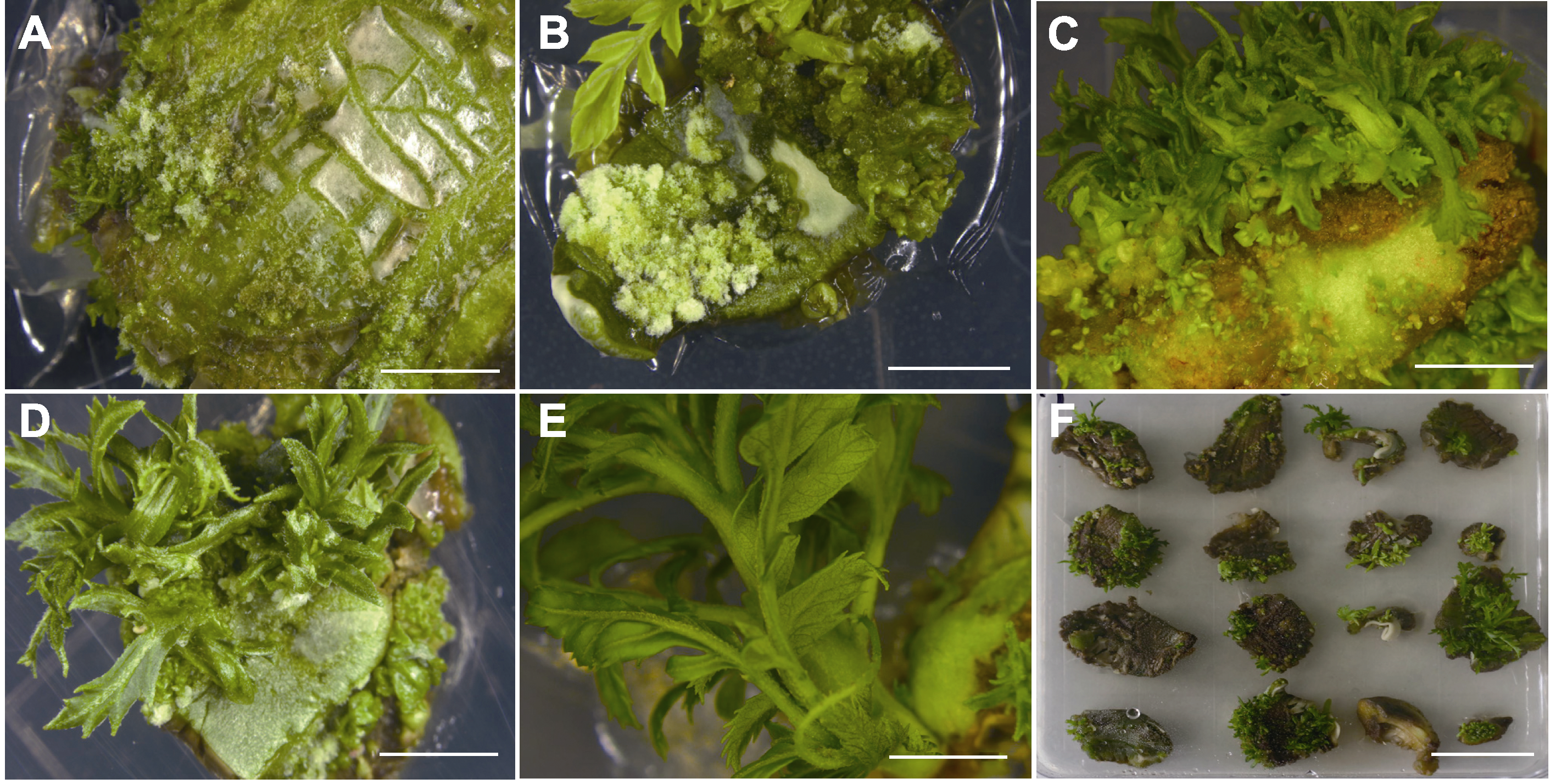
Figure 1 Different treatments of plant growth regulators on the induction of adventitious bud development for Xanthoceras sorbifolium (A)-(E) The growth of seed kernels in medium for 6, 15, 21, 28, and 35 days, respectively (bars=5 mm); (F) Browning of seed kernels inoculated into the medium for 21 days (bar=2 cm)
| Group | 6-BA (mg∙L-1) | NAA (mg∙L-1) | Germination rate (%) | Adventitious bud coefficient | Adventitious bud growth trend |
|---|---|---|---|---|---|
| B1 | 0.5 | 0.1 | 8.33±2.40 g | 2.56±0.44 b | + |
| B2 | 0.5 | 1.0 | 25.00±2.40 fg | 2.51±0.25 b | + |
| B3 | 0.5 | 1.5 | 51.38±3.67 bc | 2.02±0.22 b | ++ |
| B4 | 1.5 | 0.1 | 31.94±3.67 ef | 2.26±0.20 b | + |
| B5 | 1.5 | 1.0 | 19.45±5.01 g | 2.35±0.53 b | + |
| B6 | 1.5 | 1.5 | 40.27±2.78 de | 4.65±0.48 a | ++ |
| B7 | 2.5 | 0.1 | 43.06±3.67 cd | 3.75±0.47 a | |
| B8 | 2.5 | 1.0 | 72.22±3.68 a | 4.04±0.22 a | + |
| B9 | 2.5 | 1.5 | 59.72±3.68 b | 2.55±0.17 b |
Table 2 Effects of different plant growth regulators and their concentrations on adventitious bud induction of Xanthoceras sorbifolium mature seed kernels
| Group | 6-BA (mg∙L-1) | NAA (mg∙L-1) | Germination rate (%) | Adventitious bud coefficient | Adventitious bud growth trend |
|---|---|---|---|---|---|
| B1 | 0.5 | 0.1 | 8.33±2.40 g | 2.56±0.44 b | + |
| B2 | 0.5 | 1.0 | 25.00±2.40 fg | 2.51±0.25 b | + |
| B3 | 0.5 | 1.5 | 51.38±3.67 bc | 2.02±0.22 b | ++ |
| B4 | 1.5 | 0.1 | 31.94±3.67 ef | 2.26±0.20 b | + |
| B5 | 1.5 | 1.0 | 19.45±5.01 g | 2.35±0.53 b | + |
| B6 | 1.5 | 1.5 | 40.27±2.78 de | 4.65±0.48 a | ++ |
| B7 | 2.5 | 0.1 | 43.06±3.67 cd | 3.75±0.47 a | |
| B8 | 2.5 | 1.0 | 72.22±3.68 a | 4.04±0.22 a | + |
| B9 | 2.5 | 1.5 | 59.72±3.68 b | 2.55±0.17 b |
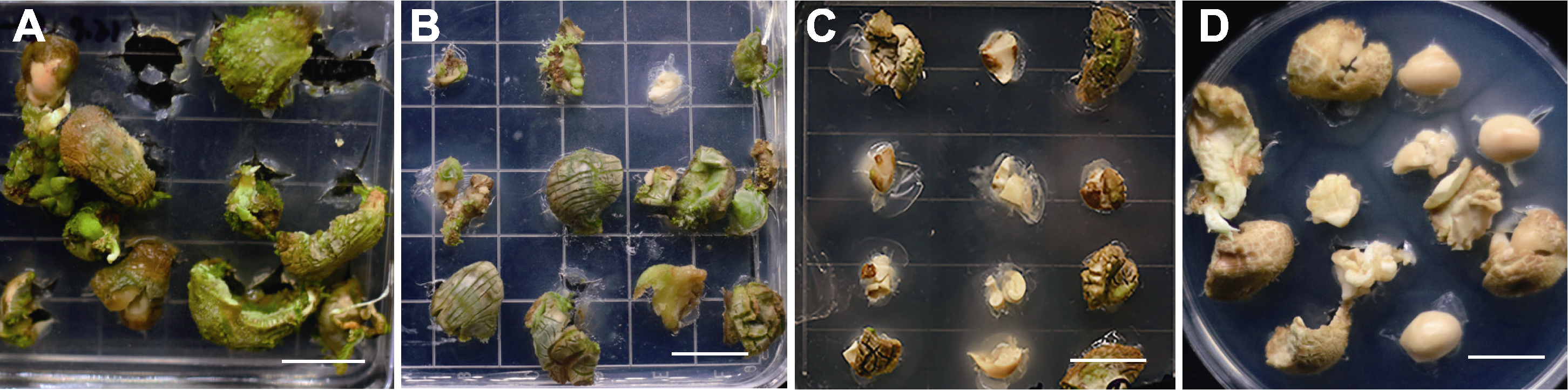
Figure 2 Browning of adventitious buds of Xanthoceras sorbifolium induced by different types of cytokinins (A)-(D) Seed kernels were grown for 20 days in medium supplemented with 2.5 mg∙L-1 6-BA, 2.5 mg∙L-1 KT, 2.5 mg∙L-1 ZT, and 2.5 mg∙L-1 TDZ, respectively. Bars=2 cm
| Group | Plant growth regulator types and concentrations (2.5 mg∙L-1) | Germination rate (%) | Adventitious bud coefficient | Death rate (%) | Browning condition |
|---|---|---|---|---|---|
| C1 | 6-BA | 73.61±3.67 a | 7.09±0.33 a | 12.50±2.41 d | ↑↑↑ |
| C2 | KT | 13.89±3.67 c | 2.97±0.32 b | 38.89±1.39 a | ↑ |
| C3 | ZT | 23.61±3.67 bc | 0.84±0.10 d | 22.22±1.39 c | ↑↑↑↑ |
| C4 | TDZ | 27.70±2.79 b | 1.90±0.05 c | 30.56±3.67 b | ↑↑↑ |
Table 3 Effects of different types of cytokinins on browning of seed kernel explants of Xanthoceras sorbifolium
| Group | Plant growth regulator types and concentrations (2.5 mg∙L-1) | Germination rate (%) | Adventitious bud coefficient | Death rate (%) | Browning condition |
|---|---|---|---|---|---|
| C1 | 6-BA | 73.61±3.67 a | 7.09±0.33 a | 12.50±2.41 d | ↑↑↑ |
| C2 | KT | 13.89±3.67 c | 2.97±0.32 b | 38.89±1.39 a | ↑ |
| C3 | ZT | 23.61±3.67 bc | 0.84±0.10 d | 22.22±1.39 c | ↑↑↑↑ |
| C4 | TDZ | 27.70±2.79 b | 1.90±0.05 c | 30.56±3.67 b | ↑↑↑ |
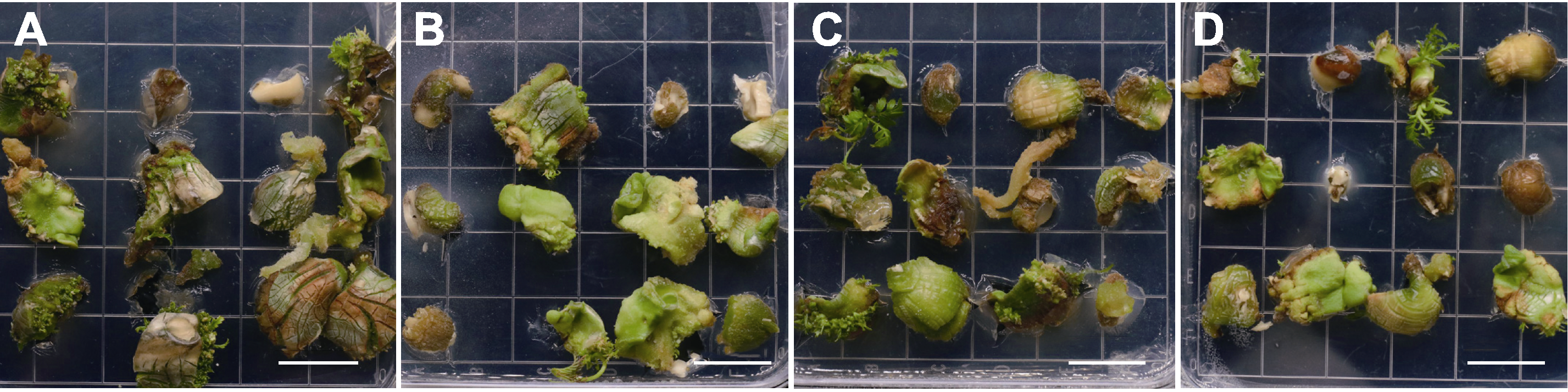
Figure 3 Effects of temperature and light on browning of Xanthoceras sorbifolium explants (A) Growing seed kernels under light (19.5 µmol∙m-2∙s-1) at normal temperature (26°C); (B) Growing seed kernels under darkness (0 µmol∙m-2∙s-1) at normal temperature (26°C); (C) Growing seed kernels under light (19.5 µmol∙m-2∙s-1) at low temperature (20°C); (D) Growing seed kernels under darkness (0 µmol∙m-2∙s-1) at low temperature (20°C). Bars=2 cm
| Group | Light and temperature combinations | Germination rate (%) | Browning rate (%) | Death rate (%) |
|---|---|---|---|---|
| D1 | Light (19.5 µmol∙m-2∙s-1)+normal temperature (26°C) | 72.22±3.68 a | 100.00±0.00 a | 18.05±1.39 b |
| D2 | Darkness (0 µmol∙m-2∙s-1)+normal temperature (26°C) | 26.39±1.39 c | 51.39±3.67 c | 30.55±2.78 a |
| D3 | Light (19.5 µmol∙m-2∙s-1)+low temperature (20°C) | 38.89±3.67 b | 65.28±2.77 b | 26.39±3.67 a |
| D4 | Darkness (0 µmol∙m-2∙s-1)+low temperature (20°C) | 26.39±2.78 c | 47.22±3.67 c | 30.56±1.38 a |
Table 4 Effects of light and temperature on browning of Xanthoceras sorbifolium explants
| Group | Light and temperature combinations | Germination rate (%) | Browning rate (%) | Death rate (%) |
|---|---|---|---|---|
| D1 | Light (19.5 µmol∙m-2∙s-1)+normal temperature (26°C) | 72.22±3.68 a | 100.00±0.00 a | 18.05±1.39 b |
| D2 | Darkness (0 µmol∙m-2∙s-1)+normal temperature (26°C) | 26.39±1.39 c | 51.39±3.67 c | 30.55±2.78 a |
| D3 | Light (19.5 µmol∙m-2∙s-1)+low temperature (20°C) | 38.89±3.67 b | 65.28±2.77 b | 26.39±3.67 a |
| D4 | Darkness (0 µmol∙m-2∙s-1)+low temperature (20°C) | 26.39±2.78 c | 47.22±3.67 c | 30.56±1.38 a |
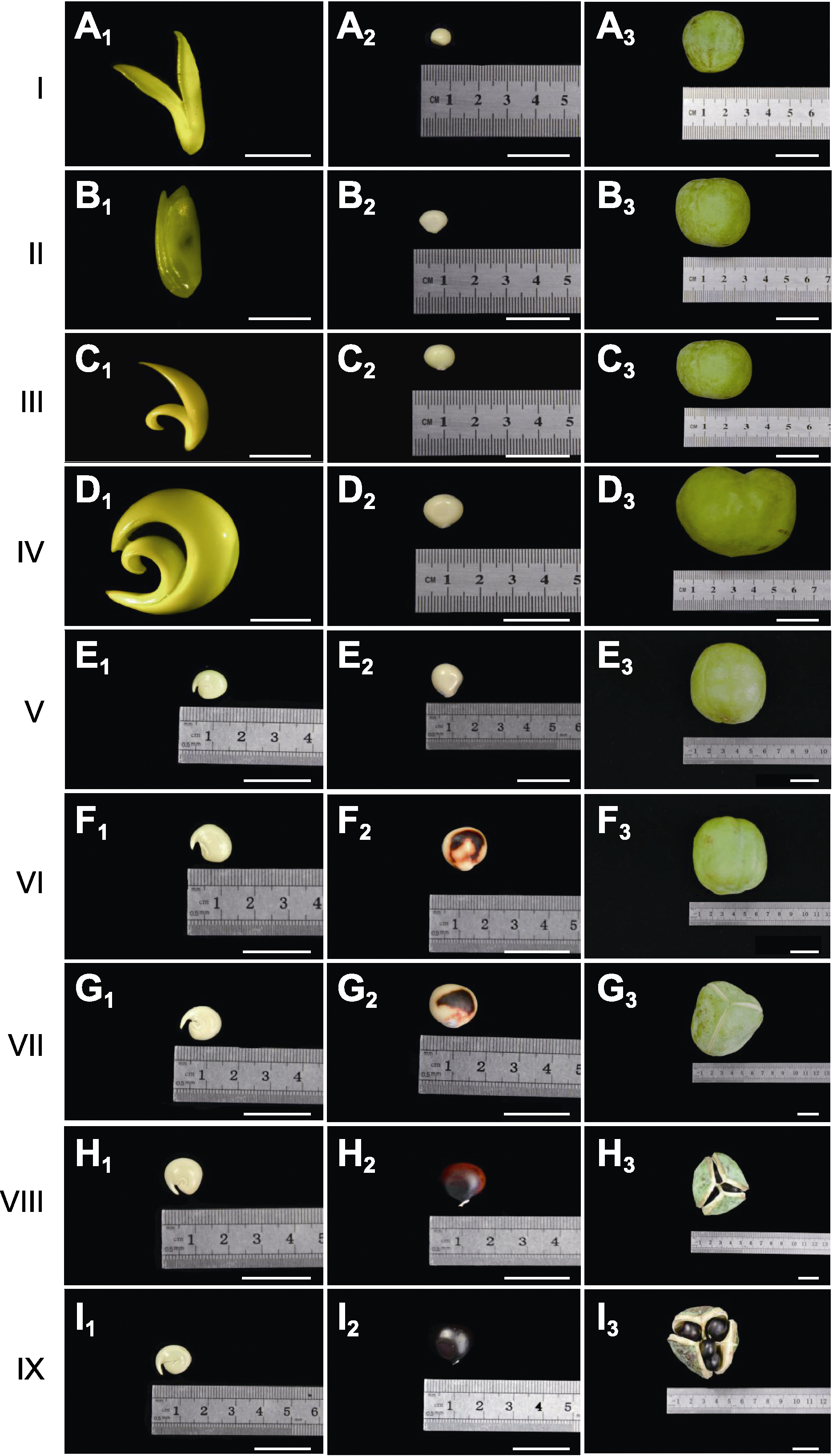
Figure 4 The growth of the I-IX stages of Xanthoceras sorbi- folium seeds (A1)-(I1) The changes in seed kernel growth process; (A2)-(I2) The changes in seed growth process; (A3)-(I3) The changes in fruit growth process. (A1)-(D1) Bars=5 mm; (E1)-(I3) Bars=2 cm
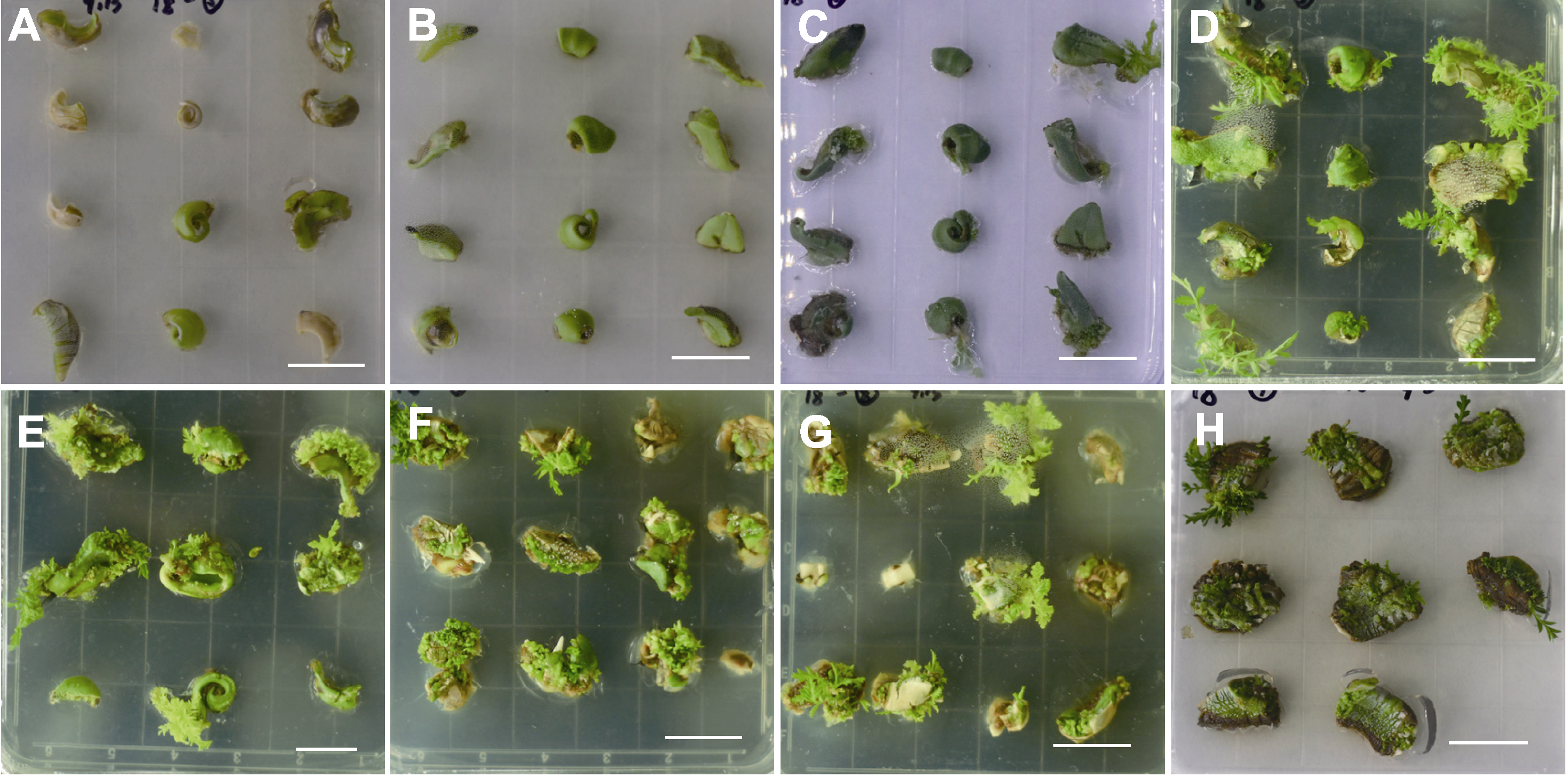
Figure 5 Induction and growth of adventitious bud in different development stages of Xanthoceras sorbifolium seed kernels (A)-(D) The growth status of the seed kernels at stage V after 7, 14, 21, and 30 days of inoculation; (E)-(H) The growth status of the seed kernels at stages VI, VII, VIII, and IX after 30 days of inoculation. Bars=2 cm
| Group | Seed development stage | Germination rate (%) | Adventitious bud coefficient | Browning condition |
|---|---|---|---|---|
| E1 | V | 94.44±5.55 a | 3.68±0.13 c | ↑ |
| E2 | VI | 97.22±2.78 a | 5.72±0.24 a | ↑ |
| E3 | VII | 83.33±4.81 a | 4.29±0.15 b | ↑↑ |
| E4 | VIII | 66.67±4.81 b | 4.37±0.46 b | ↑↑ |
| E5 | IX | 44.45±2.78 c | 3.38±0.12 c | ↑↑↑↑ |
Table 5 Effect of explant tenderness on tissue culture browning in Xanthoceras sorbifolium
| Group | Seed development stage | Germination rate (%) | Adventitious bud coefficient | Browning condition |
|---|---|---|---|---|
| E1 | V | 94.44±5.55 a | 3.68±0.13 c | ↑ |
| E2 | VI | 97.22±2.78 a | 5.72±0.24 a | ↑ |
| E3 | VII | 83.33±4.81 a | 4.29±0.15 b | ↑↑ |
| E4 | VIII | 66.67±4.81 b | 4.37±0.46 b | ↑↑ |
| E5 | IX | 44.45±2.78 c | 3.38±0.12 c | ↑↑↑↑ |
| [1] |
陈彩霞, 黄敏, 赵怡, 曹传取, 王吉升, 史志话, 汪葛峰, 高俊山 (2023). 大花海棠‘比哥’离体快繁再生体系的初步建立. 中国农学通报 39(34), 86-91.
DOI |
| [2] |
陈双双, 秦紫艺, 邱帅, 冯景, 高凯, 齐香玉, 陈慧杰, 邓衍明 (2024). 大叶绣球艾薇塔组培快繁技术. 浙江农业科学 65, 1627-1633.
DOI |
| [3] | 冯蔚, 李镇刚, 冉瑞法, 肖圣燕 (2018). 不同消毒药剂及激素对桑树冬芽组织培养的影响. 中国蚕业 39(4), 10-13. |
| [4] | 高述民, 马凯, 杜希华, 李凤兰 (2002). 文冠果(Xanthoceras sorbifolia)研究进展. 植物学通报 19, 296-301, 289. |
| [5] | 葛朝红, 李梦喆, 张洪伟, 闵卓, 蕾晓鹏, 刘旭, 李伟明 (2023). 文冠果种质资源种仁主要营养成分检测及相关分析. 特产研究 45(3), 30-37. |
| [6] | 黄敏敏, 李贵花 (2022). 中国文冠果植物化学成分及药理作用研究进展. 山东化工 51(4), 86-88, 91. |
| [7] | 黄卫丽, 刘平生, 赵颖 (2019). 文冠果组培中抑制嫩茎褐化措施的研究. 内蒙古林业科技 45(4), 53-55. |
| [8] | 姬媛媛 (2021). 文冠果的组织培养技术研究. 硕士论文. 呼和浩特: 内蒙古农业大学. pp. 1-43. |
| [9] | 李凯泉, 陕林康, 丁立军 (2024). 响应面优化文冠果油基生物柴油制备工艺. 山东工业技术 (2), 3-11. |
| [10] | 李晓玲, 丛娟, 于晓明, 董英山 (2008). 植物体细胞无性系变异研究进展. 植物学报 25, 121-128. |
| [11] | 刘文诗 (2023).‘沃柑’组培再生体系及遗传转化体系的建立. 硕士论文. 雅安: 四川农业大学. pp. 1-41. |
| [12] | 陆璐, 吴丹, 王磊, 王震, 张鑫洋, 徐海涛, 解孝满, 赵永军 (2024). 5个文冠果新品种的选育. 中国果树 (1), 118-121. |
| [13] | 强淑君, 靳磊 (2024). 北方地区不同种质文冠果观赏性综合评价. 农业灾害研究 14(3), 67-69. |
| [14] | 秦宇 (2012). 红豆杉组织培养体系建立与优化. 硕士论文. 长沙: 湖南农业大学. pp. 1-47. |
| [15] | 檀苏红, 邓少宁, 程梦叶, 刘博威, 张驰, 李双, 朱陈宇, 耿晶晶, 王文江 (2024). 柿砧木优系L938离体快繁技术建立. 果树学报 41, 1875-1884. |
| [16] | 王蓓 (2024). 文冠果生态特性与营林措施探究. 中国林业产业 (3), 40-42. |
| [17] | 王媚, 赵慧, 王淼, 刘智强 (2023). 文冠果活性成分与药理作用的研究进展. 园艺与种苗 43(9), 39-41. |
| [18] | 王瑞, 陈永忠, 王湘南, 彭邵锋, 陈隆升, 马力, 罗健, 杨小胡 (2015). 油茶组培苗高效增殖体系的建立. 中南林业科技大学学报 35(10), 40-43. |
| [19] | 王姗, 付复兴, 鲍华鹏, 马星宇 (2024). 木兰科植物组织培养技术研究进展. 安徽农业科学 52(5), 11-13, 27. |
| [20] | 王永明, 赵静茹, 陈颖 (1986). 文冠果的组织培养. 植物生理学通讯 (1), 42. |
| [21] | 韦凤娟, 廖克波, 杨梅, 戴勤, 俞建妹, 梁机, 梁晓春 (2011). 擎天树组培外植体消毒与褐化抑制的研究. 中国农学通报 27(31), 18-22. |
| [22] |
杨越 (2019). 文冠果育种研究现状与展望. 林业与生态科学 34, 363-368.
DOI |
| [23] | 苑福林 (2020). 文冠果扦插和组织培养技术研究. 硕士论文. 泰安: 山东农业大学. pp. 1-46. |
| [24] | 臧国忠, 陈尚武, 张文, 马会勤 (2008). 文冠果子叶同步胚的高效诱导及植株再生. 西北林学院学报 (5), 91-94. |
| [25] | 张东, 薛雅琳, 段章群, 李秀娟, 朱琳, 何少卿 (2017). 文冠果油脂肪酸、甘油三酯组成及其相关性分析研究. 中国油脂 42(2), 26-29. |
| [26] | 张桂琴, 徐祥龄, 赵志学 (1980). 文冠果嫩茎组织诱导植株移栽初获成功. 林业科技通讯 (7), 4-5. |
| [27] |
赵美, 刘一玮, 石翔天, 李亚俐, 申树林, 尹能文, 赵会彦, 傅鹰, 曲存民 (2024). 以甘蓝下胚轴为外植体的再生体系优化. 作物学报 50, 2408-2414.
DOI |
| [28] | 周颖怡, 王景飞, 钟建立, 李栋梁 (2024). 茶树组织培养技术与应用研究进展. 分子植物育种 22, 4733-4746. |
| [29] |
Ashraf MA, Nan Q (2022). Evolutionarily conserved shoot- borne root developmental circuit. Mol Plant 15, 783.
DOI URL |
| [30] |
Bian Z, Wang XL, Lu JK, Wang DL, Zhou YY, Liu YS, Wang SK, Yu ZQ, Xu DP, Meng S (2022). The yellowhorn AGL transcription factor gene XsAGL22 contributes to ABA biosynthesis and drought tolerance in poplar. Tree Physiol 42, 1296-1309.
DOI URL |
| [31] |
Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2019). Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ 42, 998-1018.
DOI |
| [32] | Liang Q, Li HY, Li SK, Yuan FL, Sun JF, Duan QC, Li QY, Zhang R, Sang YL, Wang N, Hou XW, Yang KQ, Liu JN, Yang L (2019). The genome assembly and annotation of yellowhorn (Xanthoceras sorbifolium Bunge). GigaScience 8, giz071. |
| [33] |
Long Y, Yang Y, Pan GT, Shen YO (2022). New insights into tissue culture plant-regeneration mechanisms. Front Plant Sci 13, 926752.
DOI URL |
| [34] | Omary M, Gil-Yarom N, Yahav C, Steiner E, Hendelman A, Efroni I (2022). A conserved superlocus regulates above- and belowground root initiation. Science 375, eabf4368. |
| [35] |
Shi TL, Ma HY, Wang XR, Liu H, Yan XM, Tian XC, Li ZC, Bao YT, Chen ZY, Zhao SW, Xiang QH, Jia KH, Nie S, Guan WB, Mao JF (2024). Differential gene expression and potential regulatory network of fatty acid biosynthesis during fruit and leaf development in yellowhorn (Xanthoceras sorbifolium), an oil-producing tree with significant deployment values. Front Plant Sci 14, 1297817.
DOI URL |
| [36] |
Su YH, Liu YB, Zhang XS (2011). Auxin-cytokinin interaction regulates meristem development. Mol Plant 4, 616-625.
DOI URL |
| [37] |
Yang XM, Wang Y, Yang YW, Shareng T, Xing YK, Bai GW, Xing ZY, Ji YY, Liu LL, Cao GX (2024). Genome- wide characterization and development of simple sequence repeat markers for molecular diversity analyses in yellowhorn (Xanthoceras sorbifolium Bunge). Plants 13, 2794.
DOI URL |
| [38] |
Zhao YX, Luo YL, Chen YX, Ao Y (2024). Integration of miRNA and ARF gene analysis provides a reference for the pistil abortion mechanism in Xanthoceras sorbifolium. Ind Crops Prod 212, 118289.
DOI URL |
| [1] | Liangliang Zhang, Xianting Wang, Yong Chen, Yifan Zhu, Xinyuan Lu, Zaitseva Svetlana Mikhailovna, Haiyun Yang. Establishment of Tissue Culture and Rapid Propagation System of Wild Plant Parrotia subaequalis Under National First Class Protection [J]. Chinese Bulletin of Botany, 2026, 61(1): 1-0. |
| [2] | Feng Shuaishuai, Qiao Feng, Li Aihua, He Xuan, Jiang Tingting, Han Wei , Yang Zong, Huang Ailing, Li Quanxi, Liu jin, Tan Deyun. Establishment of an In Vitro Regeneration System for Stem Segments of Blackberry ‘APF - 190T’ [J]. Chinese Bulletin of Botany, 2026, 61(1): 1-0. |
| [3] | Ruxin Zhang, Chenrong Li, Tongxin Wang, Jie Li, Tingge Li, Huixian Xu, Meier Li, Ying Zhao, Ting Peng, Jian Wang. Establishment of a Regeneration System for Viola × wittrockiana [J]. Chinese Bulletin of Botany, 2025, 60(6): 968-977. |
| [4] | Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao. A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 611-620. |
| [5] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [6] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [7] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [8] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [9] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [10] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [11] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [12] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [13] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [14] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [15] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||