

Chinese Bulletin of Botany ›› 2025, Vol. 60 ›› Issue (6): 944-956.DOI: 10.11983/CBB24190 cstr: 32102.14.CBB24190
• TECHNIQUES AND METHODS • Previous Articles Next Articles
Xiaoqing Ge1, Mengyao Li2, Hengyu Huang1,*( ), Aili Zhang1,*(
), Aili Zhang1,*( )
)
Received:2024-12-07
Accepted:2025-02-09
Online:2025-11-10
Published:2025-02-08
Contact:
Hengyu Huang, Aili Zhang
Xiaoqing Ge, Mengyao Li, Hengyu Huang, Aili Zhang. Rapid Propagation Technology of Microsorum punctatum in Vitro[J]. Chinese Bulletin of Botany, 2025, 60(6): 944-956.
| Levels | Factors | ||
|---|---|---|---|
| A (basic medium) | B (mg·L-1 6-BA) | C (mg·L-1 NAA) | |
| 1 | MS | 0.3 | 0.5 |
| 2 | 1/2MS | 0.6 | 1.0 |
| 3 | 1/4MS | 1.2 | 1.5 |
Table 1 The orthogonal design L9(34) of prothallus proliferation
| Levels | Factors | ||
|---|---|---|---|
| A (basic medium) | B (mg·L-1 6-BA) | C (mg·L-1 NAA) | |
| 1 | MS | 0.3 | 0.5 |
| 2 | 1/2MS | 0.6 | 1.0 |
| 3 | 1/4MS | 1.2 | 1.5 |
| Basic medium | Disinfection time (min) | Contamination rate (%) | Growth situation |
|---|---|---|---|
| MS | 5 | 100.00±0.00 a | The spore germination time was second only to 1/2MS, and the green flaky bodies were partially aggregated on the surface of the medium, and some were sparsely distributed or even blank |
| 8 | 74.67±0.04 b | ||
| 10 | 55.33±0.02 c | ||
| 12 | 32.00±0.02 d | ||
| 1/2MS | 5 | 100.00±0.00 a | The flaky bodies appeared first and were widely distributed on the surface of the medium. Some of them gathered into pieces, which were green in color and stretched out in shape |
| 8 | 74.67±0.04 b | ||
| 10 | 50.67±0.02 c | ||
| 12 | 22.00±0.03 d | ||
| 1/3MS | 5 | 100.00±0.00 a | The flaky bodies were sparsely distributed on the surface of the medium |
| 8 | 80.67±0.04 b | ||
| 10 | 50.67±0.04 c | ||
| 12 | 18.67±0.02 d | ||
| 1/4MS | 5 | 100.00±0.00 a | Green spots appeared at the latest, and the flakes were scattered on the surface of the me- dium |
| 8 | 72.00±0.11 b | ||
| 10 | 53.33±0.05 b | ||
| 12 | 21.33±0.05 c |
Table 2 Spore germination and growth status
| Basic medium | Disinfection time (min) | Contamination rate (%) | Growth situation |
|---|---|---|---|
| MS | 5 | 100.00±0.00 a | The spore germination time was second only to 1/2MS, and the green flaky bodies were partially aggregated on the surface of the medium, and some were sparsely distributed or even blank |
| 8 | 74.67±0.04 b | ||
| 10 | 55.33±0.02 c | ||
| 12 | 32.00±0.02 d | ||
| 1/2MS | 5 | 100.00±0.00 a | The flaky bodies appeared first and were widely distributed on the surface of the medium. Some of them gathered into pieces, which were green in color and stretched out in shape |
| 8 | 74.67±0.04 b | ||
| 10 | 50.67±0.02 c | ||
| 12 | 22.00±0.03 d | ||
| 1/3MS | 5 | 100.00±0.00 a | The flaky bodies were sparsely distributed on the surface of the medium |
| 8 | 80.67±0.04 b | ||
| 10 | 50.67±0.04 c | ||
| 12 | 18.67±0.02 d | ||
| 1/4MS | 5 | 100.00±0.00 a | Green spots appeared at the latest, and the flakes were scattered on the surface of the me- dium |
| 8 | 72.00±0.11 b | ||
| 10 | 53.33±0.05 b | ||
| 12 | 21.33±0.05 c |
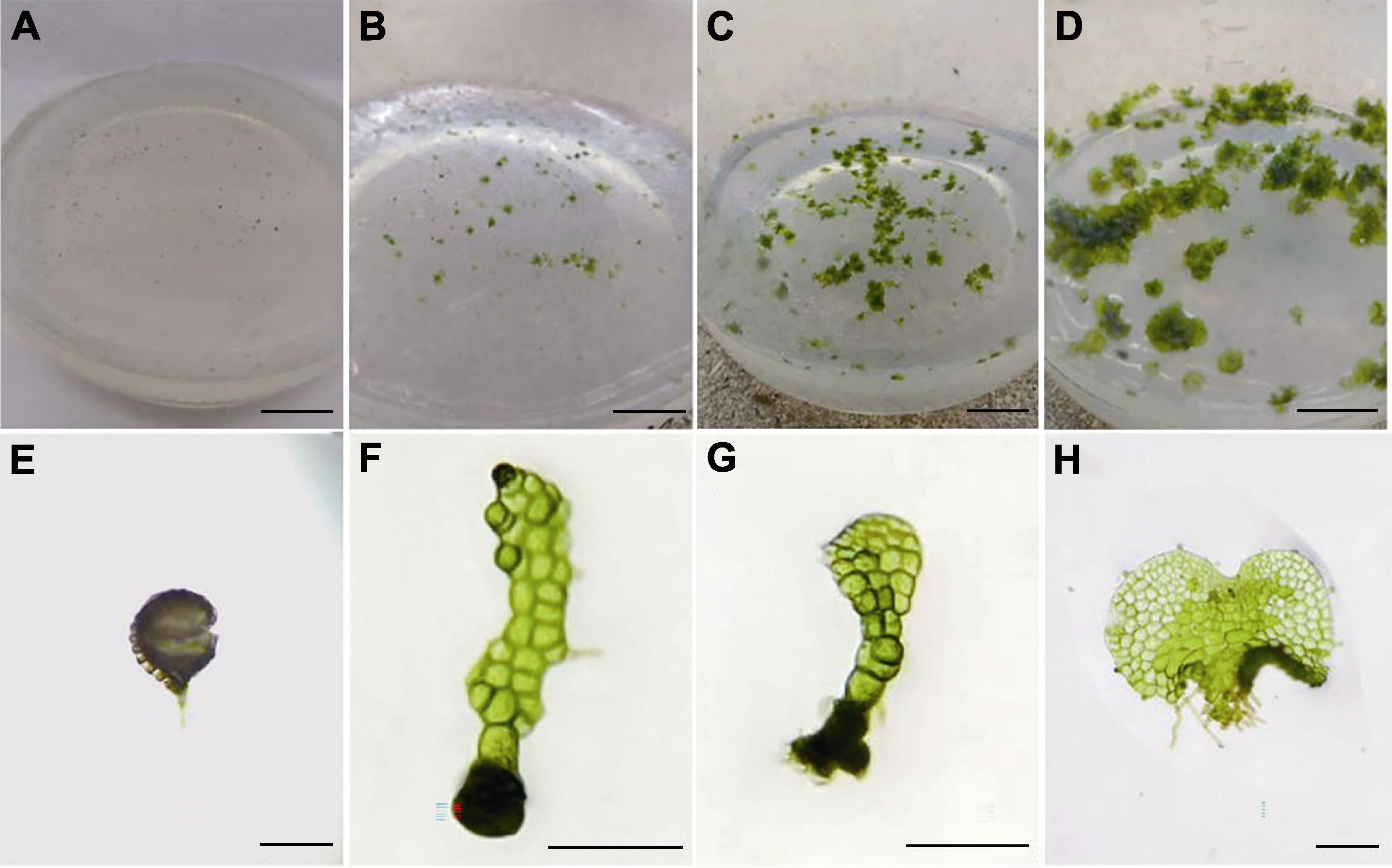
Figure 1 Spore germination process (A) Establishment of aseptic system (20 days) (bar=2 cm); (B) Germination of fertile spores (30 days) (bar=2 cm); (C) Prothallus (45 days) (bar=2 cm); (D) Prothallus (70 days) (bar=2 cm); (E) Spore germination (bar=50 μm); (F) Filamentous period (bar=100 μm); (G) Early lamellar period (bar=100 μm); (H) Cardioprothallus stage (bar=50 μm)
| Number | Factors | Multiplication coefficient | ||||
|---|---|---|---|---|---|---|
| A (basic medium) | B (6-BA) | C (NAA) | D (error) | |||
| 1 | 1 | 1 | 1 | 1 | 8.73±0.07 | |
| 2 | 1 | 2 | 2 | 2 | 8.33±0.07 | |
| 3 | 1 | 3 | 3 | 3 | 9.60±0.12 | |
| 4 | 2 | 1 | 2 | 3 | 7.53±0.18 | |
| 5 | 2 | 2 | 3 | 1 | 6.47±0.07 | |
| 6 | 2 | 3 | 1 | 2 | 6.80±0.23 | |
| 7 | 3 | 1 | 3 | 2 | 6.60±0.12 | |
| 8 | 3 | 2 | 1 | 3 | 7.00±0.12 | |
| 9 | 3 | 3 | 2 | 1 | 6.00±0.12 | |
| Multiplication coefficient | K1 | 8.89 | 7.62 | 7.51 | 7.07 | |
| K2 | 6.93 | 7.29 | 7.29 | 7.24 | ||
| K3 | 6.53 | 7.56 | 7.56 | 7.04 | ||
| R | 2.36 | 0.33 | 0.27 | 0.20 | ||
Table 3 The orthogonal design L9(34) result of prothallus proliferation
| Number | Factors | Multiplication coefficient | ||||
|---|---|---|---|---|---|---|
| A (basic medium) | B (6-BA) | C (NAA) | D (error) | |||
| 1 | 1 | 1 | 1 | 1 | 8.73±0.07 | |
| 2 | 1 | 2 | 2 | 2 | 8.33±0.07 | |
| 3 | 1 | 3 | 3 | 3 | 9.60±0.12 | |
| 4 | 2 | 1 | 2 | 3 | 7.53±0.18 | |
| 5 | 2 | 2 | 3 | 1 | 6.47±0.07 | |
| 6 | 2 | 3 | 1 | 2 | 6.80±0.23 | |
| 7 | 3 | 1 | 3 | 2 | 6.60±0.12 | |
| 8 | 3 | 2 | 1 | 3 | 7.00±0.12 | |
| 9 | 3 | 3 | 2 | 1 | 6.00±0.12 | |
| Multiplication coefficient | K1 | 8.89 | 7.62 | 7.51 | 7.07 | |
| K2 | 6.93 | 7.29 | 7.29 | 7.24 | ||
| K3 | 6.53 | 7.56 | 7.56 | 7.04 | ||
| R | 2.36 | 0.33 | 0.27 | 0.20 | ||
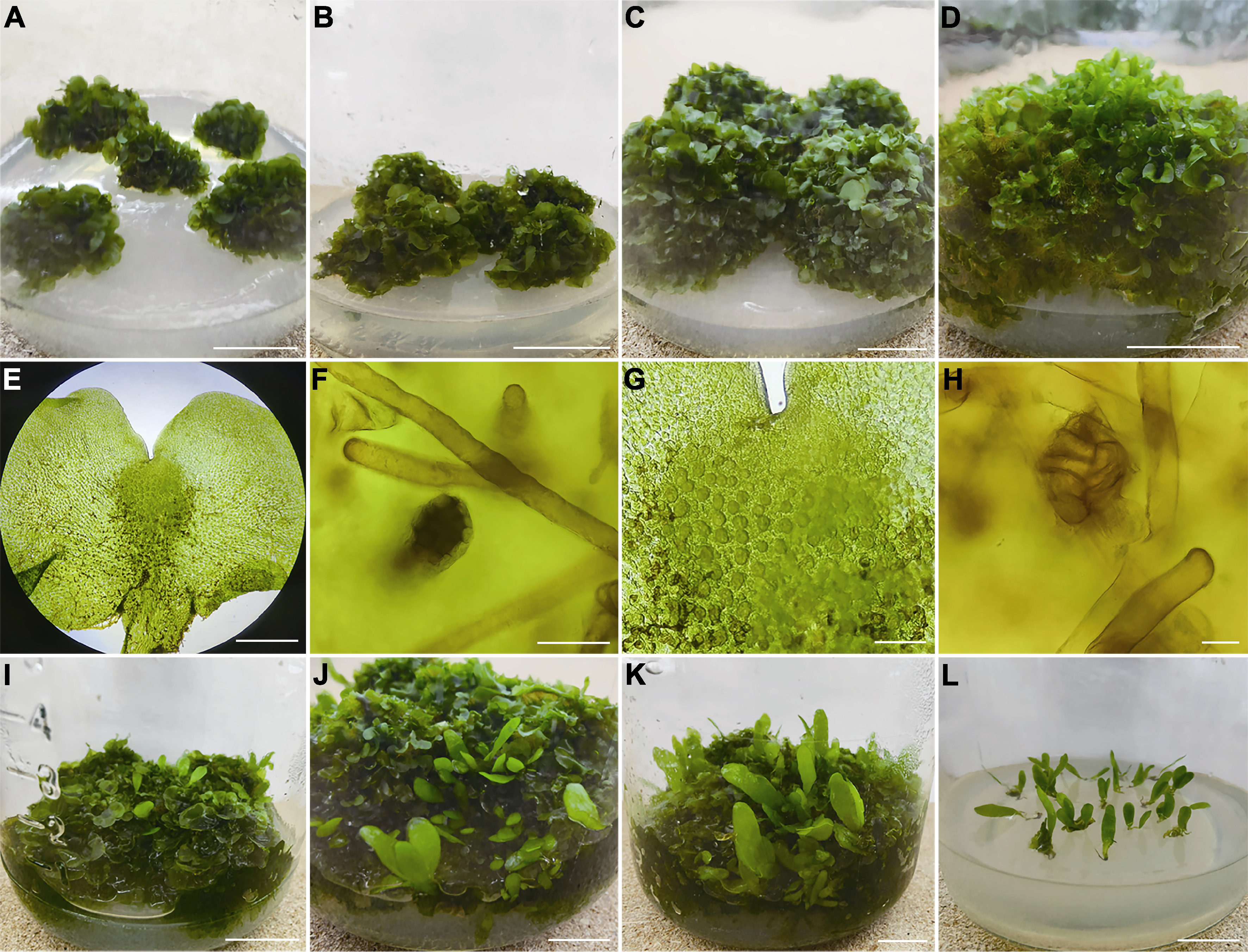
Figure 2 Proliferation of prothallus and induction of young sporophytes (A) Prolobular mass was transferred after 15 days (bar=2 cm); (B) Prolobular mass was transferred after 30 days (bar=2 cm); (C) Prolobular mass was transferred after 45 days (bar=2 cm); (D) Prolobular mass was transferred after 60 days (bar=2 cm); (E) Gametophyte (bar=500 μm); (F) Antheridium (bar=40 μm); (G) Local archegonium (bar=200 μm); (H) Archegonium (bar=40 μm); (I) Prothallus and young sporophyte cultured with water after 20 days (bar=2 cm); (J) Prothallus and young sporophyte cultured with water after 60 days (bar=2 cm); (K) Prothallus and young sporophyte cultured with water after 90 days (bar=2 cm); (L) Sporophyte rooting culture (bar=2 cm)
| Basic medium | Fresh weight before multiplication (g) | Fresh weight after multiplication (g) | Multiplication weight (g) | Multiplication coefficient |
|---|---|---|---|---|
| MS | 3.92±0.54 a | 37.69±0.77 a | 33.77±0.47 a | 9.81±0.62 a |
| 1/2MS | 4.17±0.61 a | 30.72±0.98 b | 26.55±0.83 b | 6.36±0.90 b |
| 1/4MS | 4.03±0.77 a | 28.01±0.82 c | 23.98±0.37 c | 5.95±0.56 c |
Table 4 Effect of different culture media on weight increase of prothallus
| Basic medium | Fresh weight before multiplication (g) | Fresh weight after multiplication (g) | Multiplication weight (g) | Multiplication coefficient |
|---|---|---|---|---|
| MS | 3.92±0.54 a | 37.69±0.77 a | 33.77±0.47 a | 9.81±0.62 a |
| 1/2MS | 4.17±0.61 a | 30.72±0.98 b | 26.55±0.83 b | 6.36±0.90 b |
| 1/4MS | 4.03±0.77 a | 28.01±0.82 c | 23.98±0.37 c | 5.95±0.56 c |
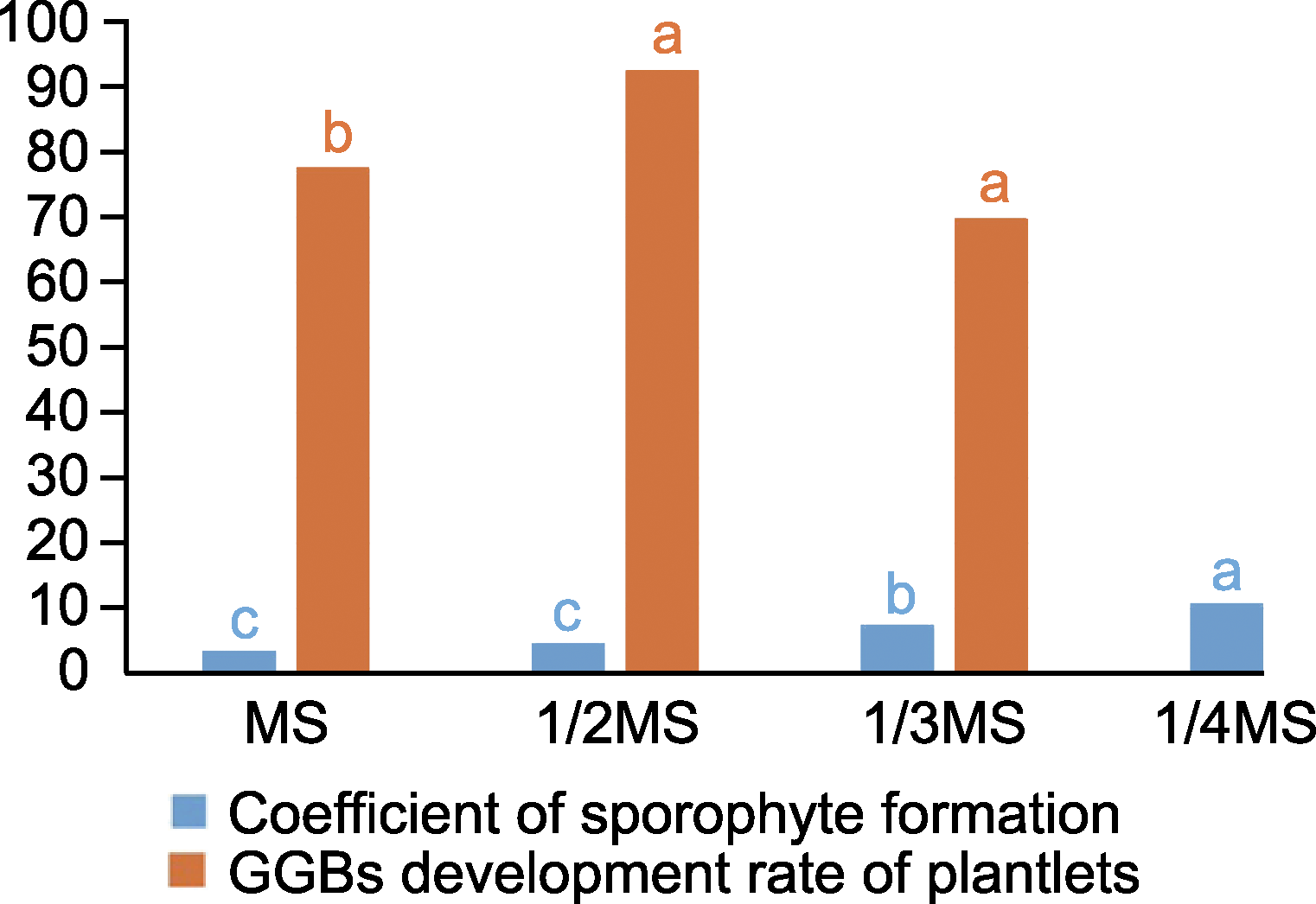
Figure 3 Effects of different inorganic salt concentrations on sporophyte induction and plantlet rate of green globular bodies (GGBs) development Different lowercase letters indicate significant differences at the 0.05 level.
| No. | Plant growth regulators | Induction rate (%) | Multiplication coefficient | |
|---|---|---|---|---|
| 6-BA (mg·L-1) | NAA (mg·L-1) | |||
| CK | 0 | 0 | 21.33±0.03 g | 2.63±1.43 d |
| R1 | 0.5 | 0.1 | 76.67±0.03 cd | 18.73±3.40 c |
| R2 | 0.5 | 0.6 | 64.67±0.08 de | 20.60±3.64 bc |
| R3 | 0.5 | 1.2 | 49.33±0.01 f | 19.40±2.50 bc |
| R4 | 1.0 | 0.1 | 80.00±0.04 bc | 19.87±1.65 bc |
| R5 | 1.0 | 0.6 | 70.67±0.05 cde | 13.73±2.32 c |
| R6 | 1.0 | 1.2 | 63.33±0.05 e | 19.13±1.92 bc |
| R7 | 1.5 | 0.1 | 93.33±0.03 a | 32.00±3.30 a |
| R8 | 1.5 | 0.6 | 89.33±0.03 ab | 28.20±2.66 ab |
| R9 | 1.5 | 1.2 | 65.33±0.04 de | 16.60±3.17 c |
Table 5 Effects of different plant growth regulators concentrations on green globular bodies (GGBs) induction and proliferation
| No. | Plant growth regulators | Induction rate (%) | Multiplication coefficient | |
|---|---|---|---|---|
| 6-BA (mg·L-1) | NAA (mg·L-1) | |||
| CK | 0 | 0 | 21.33±0.03 g | 2.63±1.43 d |
| R1 | 0.5 | 0.1 | 76.67±0.03 cd | 18.73±3.40 c |
| R2 | 0.5 | 0.6 | 64.67±0.08 de | 20.60±3.64 bc |
| R3 | 0.5 | 1.2 | 49.33±0.01 f | 19.40±2.50 bc |
| R4 | 1.0 | 0.1 | 80.00±0.04 bc | 19.87±1.65 bc |
| R5 | 1.0 | 0.6 | 70.67±0.05 cde | 13.73±2.32 c |
| R6 | 1.0 | 1.2 | 63.33±0.05 e | 19.13±1.92 bc |
| R7 | 1.5 | 0.1 | 93.33±0.03 a | 32.00±3.30 a |
| R8 | 1.5 | 0.6 | 89.33±0.03 ab | 28.20±2.66 ab |
| R9 | 1.5 | 1.2 | 65.33±0.04 de | 16.60±3.17 c |

Figure 4 Green globular induction of sporophytes and regeneration and domestication of green globular body (GGB) plants of Microsorum punctatum (A) GGBs induced by sporophyte (bar=1 cm); (B) Spore grows green globular bodies (bar=1 mm); (C) Rhizomes isolated green globules (bar=1 mm); (D), (E) GGBs polymer cultivated from e-green spheroids ((D) bar=1 mm; (E) bar=1 cm); (F) A single GGB was isolated from GGBs polymer (bar=500 μm); (G), (H) GGBs differentiated into young sporophytes ((G) bar=5 mm; (H) bar=1 mm); (I) Sporophyte rhizomes grow GGB (bar=1 cm); (J) GGB multiplication (bar=1 cm); (K) GGB differentiate into plantlets (bar=1 cm); (L) The sporophyte took root after 60 days of culture (bar=1 cm); (M)-(P) Sporophytic domestication ((M) bar=5 cm; (N)-(P) bars=3 cm). Due to limited space for the domestication and growth of spores (M), they were later transferred to larger compartments for domestication (N)-(P).
| [1] | Aldea F, Banciu C, Brezeanu A, Helepciuc FE, Soare LC (2016). In vitro micropropagation of fern species (Pteridophyta) of biotechnological interest, for ex situ conservation. Oltenia Studii Si Comunicări Ştiinţele Naturii 32, 27-35. |
| [2] | Banks JA (1999). Gametophyte development in ferns. Annu Rev Plant Biol 50, 163-186. |
| [3] |
Bertrand AM, Albuerne MA, Fernández H, González A, Sánchez-Tamés R (1999). In vitro organogenesis of Polypodium cambricum. Plant Cell Tissue Organ Cult 57, 65-69.
DOI |
| [4] | Chen P, Yang LL, Peng Y, Zhang SZ, Xu GH, Yang JF, Zhang SZ (2021). In vitro regeneration of Osmunda mildei. Guihaia 41, 301-307. (in Chinese) |
| 陈朋, 杨蕾蕾, 彭杨, 张苏州, 徐桂红, 杨建芬, 张寿洲 (2021). 粤紫萁离体再生技术研究. 广西植物 41, 301-307. | |
| [5] | Cho JS, Lee CH (2017). Several factors affecting mass production of Microlepia strigosa (Thunb.) C. Presl sporophytes. Hortic Sci Technol 35, 46-58. |
| [6] | Dela Cruz RY, Ang AMG, Doblas GZ, Librando IL, Porquis HC, Batoctoy BCLS, Cabresos CC, Jacalan DRY, Amoroso VB (2017). Phytochemical screening, antioxidant and anti-inflammatory activities of the three fern (poly- podiaceae) species in Bukidnon, Philippines. Bull Environ Pharmacol Life Sci 6, 28-33. |
| [7] | Duan JQ, Sun DK, Xiang JY, Tang JR (2021). Study on tissue culture and rapid propagation technology of Asplenium nidus. Seed 40(5), 84-90. (in Chinese) |
| 段杰秋, 孙大宽, 向建英, 唐军荣 (2021). 巢蕨组培快繁技术研究. 种子 40(5), 84-90. | |
| [8] |
Fernández H, Revilla MA (2003). In vitro culture of ornamental ferns. Plant Cell Tissue Organ Cult 73, 1-13.
DOI |
| [9] | Flora of China Editorial Committee (2000). Flora of China (vol. 6). Beijing: Science Press. pp. 226. (in Chinese) |
| 中国科学院中国植物志编辑委员会 (2000). 中国植物志(第6卷, 第2分册). 北京: 科学出版社. pp. 226. | |
| [10] | Gao XW, Zhou SY, Bian ZJ, Wang QH, Dai XL (2018). Effects on morphological development of gametophytes of three fern species under different moisture conditions. Jo urnal of Shanghai Normal University (Nat Sci) 47, 726-733. (in Chinese) |
| 高晓雯, 周施雨, 卞竹箐, 王清华, 戴锡玲 (2018). 不同水分条件对3种蕨类植物配子体形态发育的影响. 上海师范大学学报(自然科学版) 47, 726-733. | |
| [11] |
Greer GK, McCarthy BC (2000). Patterns of growth and reproduction in a natural population of the fern Polystichum acrostichoides. Am Fern J 90, 60-76.
DOI URL |
| [12] | Hegde S, D’Souza L (2000). Recent advances in biotechnology of ferns. In: Trivedi PC, ed. Plant Biotechnology—Recent Advances. New Delhi: Panima Publishing Corporation. pp. 213-237. |
| [13] | IUCN (2024). The IUCN Red List of Threatened Species. https://www.iucnredlist.org. 2024-03-09. |
| [14] | Jang BK, Cho JS, Lee KC, Lee CH (2017). Culture conditions affecting spore germination, prothallus propagation and sporophyte formation of Dryopteris nipponensis Koidz. Hortic Sci Technol 35, 480-489. |
| [15] |
Jang BK, Cho JS, Park K, Lee CH (2019). A methodology for large-scale Athyrium sheareri gametophyte proliferation and sporophyte production using tissue culture. In Vitro Cell Dev Biol Plant 55, 519-526.
DOI |
| [16] |
Korpelainen H (1994). Growth, sex determination and reproduction of Dryopteris filix-mas (L.) Schott gametophytes under varying nutritional conditions. Bot J Linn Soc 114, 357-366.
DOI URL |
| [17] |
Korpelainen H (1995). Growth and reproductive characteristics in artificially formed clonal gametophytes of Dryopteris filix-mas (Dryopteridaceae). Plant Syst Evol 196, 195-206.
DOI URL |
| [18] | Krieg CP, Chambers SM (2022). The ecology and physiology of fern gametophytes: a methodological synthesis. Appl Plant Sci 10, e11464. |
| [19] | Lin CW, Shih CH, Chang HC, Kao CY (2024). Regeneration of endangered fern Adiantum reniforme var. sinense. Indian J Agric Res 58, 56-62. |
| [20] |
Murashige T, Skoog F (1962). A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol Plant 15, 473-497.
DOI URL |
| [21] |
Park K, Jang BK, Lee CH (2019). Culture condition for gametophyte and sporophyte masspropagation of bamboo fern (Coniogramme japonica) using tissue culture. J Plant Biotechnol 46, 119-126.
DOI URL |
| [22] |
Park K, Jang BK, Lee HM, Cho JS, Lee CH (2020). An efficient method for in vitro shoot-tip culture and sporophyte production using Selaginella martensii Spring sporophyte. Plants 9, 235.
DOI URL |
| [23] |
Petchsri S, Boonkerd T, Baum BR, Karladee D, Suriyong S, Lungkaphin A (2012). Phenetic study of the Microsorum punctatum complex (Polypodiaceae). Sci Asia 38, 1-12.
DOI URL |
| [24] |
Ravi BX, Robert J, Gabriel M (2014). In vitro spore germination and gametophytic growth development of a critically endangered fern Pteris tripartita Sw. Afr J Biotechnol 13, 2350-2358.
DOI URL |
| [25] |
Ravi BX, Varuvel GVA, Kilimas R, Robert J (2015). Apogamous sporophyte development through spore reproduction of a South Asia’s critically endangered fern: Pteris tripartita Sw. Asian Pac J Reprod 4, 135-139.
DOI URL |
| [26] | Rybczyński JJ, Tomiczak K, Grzyb M, Mikuła A (2018). Morphogenic events in ferns:single and multicellular explants in vitro. In: In: Fernández H, ed. Current Advances in Fern Research. Cham: Springer. pp. 99-120. |
| [27] |
Sanders HL, Darrah PR, Langdale JA (2011). Sector analysis and predictive modelling reveal iterative shoot-like development in fern fronds. Development 138, 2925-2934.
DOI PMID |
| [28] |
Somer M, Arbesú R, Menéndez V, Revilla MA, Fernández H (2010). Sporophyte induction studies in ferns in vitro. Euphytica 171, 203-210.
DOI URL |
| [29] |
Vasco A, Moran RC, Ambrose BA (2013). The evolution, morphology, and development of fern leaves. Front Plant Sci 4, 345.
DOI PMID |
| [30] | Yang FC (2009). Special topics on tropical pteridophyte (XIII) cultivation and management of Microsorum punctatum. China Flowers and Horticulture (24), 20-21. (in Chinese) |
| 杨逢春 (2009). 热带蕨类植物专题(十三) 星蕨的栽培管理. 中国花卉园艺 (24), 20-21. | |
| [31] |
Yu RP, Li F, Wang GX, Ruan JW, Wu LF, Wu M, Yang CM, Shan QL (2021). In vitro regeneration of the colorful fern Pteris aspericaulis var. tricolor via green globular bodies system. In Vitro Cell Dev Biol Plant 57, 225-234.
DOI |
| [32] |
Yu RP, Zhang GF, Li H, Cao H, Mo XJ, Gui M, Zhou XH, Jiang YL, Li SC, Wang JH (2017). In vitro propagation of the endangered tree fern Cibotium barometz through formation of green globular bodies. Plant Cell Tissue Organ Cult 128, 369-379.
DOI URL |
| [33] | Zhang DR, Bu ZG, Chen LL, Chang Y (2020). Establishment of a tissue culture and rapid propagation system of Dryopteris fragrans. Chin Bull Bot 55, 760-767. (in Chinese) |
| 张冬瑞, 卜志刚, 陈玲玲, 常缨 (2020). 香鳞毛蕨的组织培养和快速繁殖体系构建. 植物学报 55, 760-767. | |
| [34] |
Zhang W, Wang Q, Chen GH, Chen HF (2020). Partial tissue culture and rapid propagation of Phymatosorus scolopendria spores. Plant Physiology Journal 56, 856-862. (in Chinese)
DOI URL |
| 张薇, 王强, 陈国华, 陈红锋 (2020). 瘤蕨孢子的不完全组织培养与快速繁殖. 植物生理学报 56, 856-862. |
| [1] | Rongpei Yu, Yang Li, Dong Li, Xuanhuai Zhan, Lei Shi. Radiosensitivity of Green Globular Bodies of Matteuccia struthiopteris Exposed to 60Coγ Radiation [J]. Chinese Bulletin of Botany, 2015, 50(5): 565-572. |
| [2] | Xiaofeng Wu, Ruoyang Hu, Xuedong Li. Spore Germination and Callus Induction of Atrichum undulatum [J]. Chinese Bulletin of Botany, 2013, 48(6): 651-657. |
| [3] | . Gametophyte Development of Pteris fauriei [J]. Chinese Bulletin of Botany, 2005, 22(增刊): 50-56. |
| [4] | YI Yan-Jun QIANG Sheng. Study of Spore Germination and Protonema Development of Five Species of Mosses [J]. Chinese Bulletin of Botany, 2005, 22(06): 708-714. |
| [5] | ZHANG Kai-Mei SHI Lei LI Dong. The Gametophyte Development in Pteris ensiformis [J]. Chinese Bulletin of Botany, 2005, 22(05): 566-571. |
| [6] | . Observation on the Gametophyte Development of Microsorium fortunei (Moore) Ching [J]. Chinese Bulletin of Botany, 2004, 21(06): 660-666. |
| [7] | FAN Qing-Shu ZHAO Jian-Cheng YU Shu-Hong LI Xiu-Qin. Progress in Study on Spore Germination and Protonema Development of the Bryophytes [J]. Chinese Bulletin of Botany, 2003, 20(03): 280-286. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||