

植物学报 ›› 2024, Vol. 59 ›› Issue (1): 10-21.DOI: 10.11983/CBB22226 cstr: 32102.14.CBB22226
收稿日期:2022-09-28
接受日期:2022-12-13
出版日期:2024-01-10
发布日期:2024-01-10
通讯作者:
*E-mail: 基金资助:
Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang*( )
)
Received:2022-09-28
Accepted:2022-12-13
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: 摘要: 叶片直立是决定作物株型、光合效率和产量的重要农艺性状之一。细胞分裂素(CTK)是调控农作物株型、抗逆和产量的重要激素, 但其在水稻(Oryza sativa)叶片直立生长中的作用仍不清晰。该文报道了水稻细胞分裂素氧化酶/脱氢酶9 (OsCKX9)影响叶枕发育并正调控叶夹角。组织学切片显示, osckx9突变体叶夹角的变化是由于叶枕近轴端和远轴端细胞的不对称分裂所致。qRT-PCR检测表明, OsCKX9在叶枕中的表达量较高。激素处理表明, OsCKX9能被tZ、iP、cZ、6-BA和eBL等诱导表达。激素测定显示, osckx9突变体的叶枕处积累了大量的CTK, 且其对eBL的敏感度显著低于野生型。综上, OsCKX9正调控水稻叶夹角, 该研究为解析水稻叶夹角的遗传基础和培育理想株型提供了基因资源。
朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育. 植物学报, 2024, 59(1): 10-21.
Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang. OsCKX9 is Involved in Regulating the Rice Lamina Joint Development and Leaf Angle. Chinese Bulletin of Botany, 2024, 59(1): 10-21.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| OsCKX9 F | CTATCCTCAGCACTTGGCCC |
| OsCKX9 R | AAATGGGACTGCCACTCCTG |
| OsUBQ5 F | GCACAAGCACAAGAAGGTGA |
| OsUBQ5 R | CCAAAGAACAGGAGCCTACG |
| OsCYC U4;1 F | CGACGACATATGCTACAACAATGC |
| OsCYC U4;1 R | CCAAAGAGGAAGTCCACCTCAAG |
表1 引物序列
Table 1 The primers used in this study
| Primer name | Primer sequence (5′-3′) |
|---|---|
| OsCKX9 F | CTATCCTCAGCACTTGGCCC |
| OsCKX9 R | AAATGGGACTGCCACTCCTG |
| OsUBQ5 F | GCACAAGCACAAGAAGGTGA |
| OsUBQ5 R | CCAAAGAACAGGAGCCTACG |
| OsCYC U4;1 F | CGACGACATATGCTACAACAATGC |
| OsCYC U4;1 R | CCAAAGAGGAAGTCCACCTCAAG |
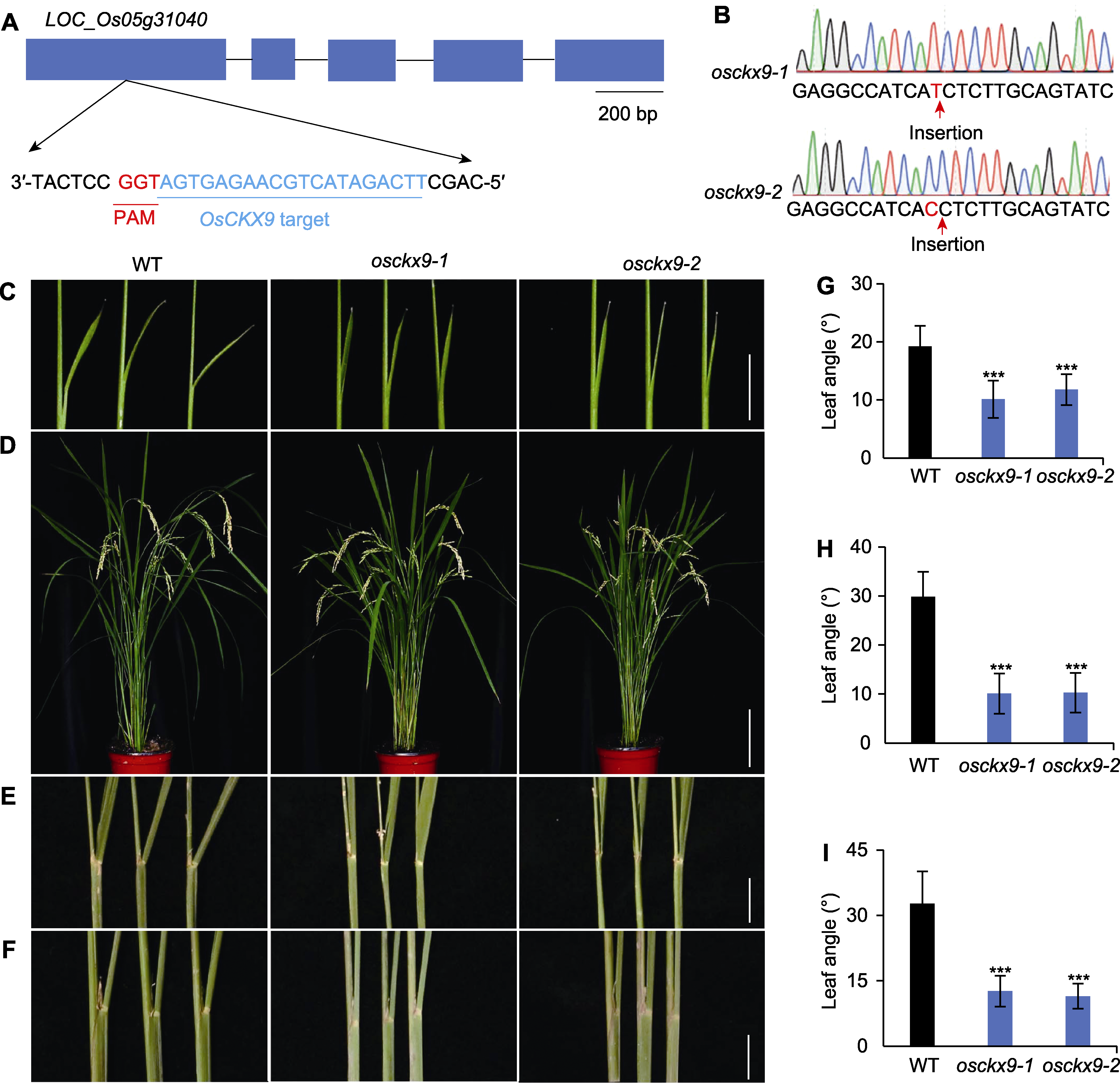
图1 osckx9突变体叶夹角变小 (A) 使用CRISPR/Cas9技术对OsCKX9进行敲除,“target”表示敲除靶点位置(蓝色方框表示外显子, 黑色横线表示非翻译区; PAM: 原间隔序列邻近基序); (B) 基因敲除株的测序验证; (C) 野生型(WT)、osckx9-1以及osckx9-2的7-DAG (萌发后天数)幼苗叶夹角表型(bar=1 cm); (D) WT、osckx9-1和osckx9-2的110-DAG植株形态表型(bar=15 cm); (E) 110-DAG的WT、osckx9-1以及osckx9-2剑叶叶夹角表型(bar=1 cm); (F) 110-DAG的WT、osckx9-1和osckx9-2第2叶叶夹角表型(bar=1 cm); (G) 量化(C)中所示植株的叶夹角(平均值±标准差, n=24); (H), (I) 量化(E), (F)中所示110-DAG植株的剑叶(H)和第2叶(I)叶夹角(平均值±标准差, n=16)。使用Student’s t-test进行统计分析。*** P<0.001
Figure 1 osckx9 mutants show smaller leaf angle (A) OsCKX9 was knocked out using CRISPR/Cas9 technique, “target” means knockout target positions (blue boxes represent exons, black horizontal lines represent untranslated regions; PAM: Primitive interval sequence adjacent to the motif); (B) Sequencing verification of gene knockout strains; (C) Morphological phenotypes of the 7-DAG (days after germination) seedlings of wild type (WT), osckx9-1 and osckx9-2 (bar=1 cm); (D) Morphological phenotypes of the adult plants of WT, osckx9-1 and osckx9-2 at 110-DAG (bar=15 cm); (E) Flag leaf phenotype of 110-DAG of WT, osckx9-1 and osckx9-2 (bar=1 cm); (F) The second leaf phenotype of 110-DAG of WT, osckx9-1 and osckx9-2 (bar=1 cm); (G) Quantification of the leaf angle of the plants shown in (C) (means±SD, n=24); (H), (I) Quantification of the flag leaf angle (H) and the second leaf angle (I) of the 110-DAG plants shown in (E), (F) (means±SD, n=16). Statistical analyses were performed by Student’s t-test. *** P<0.001
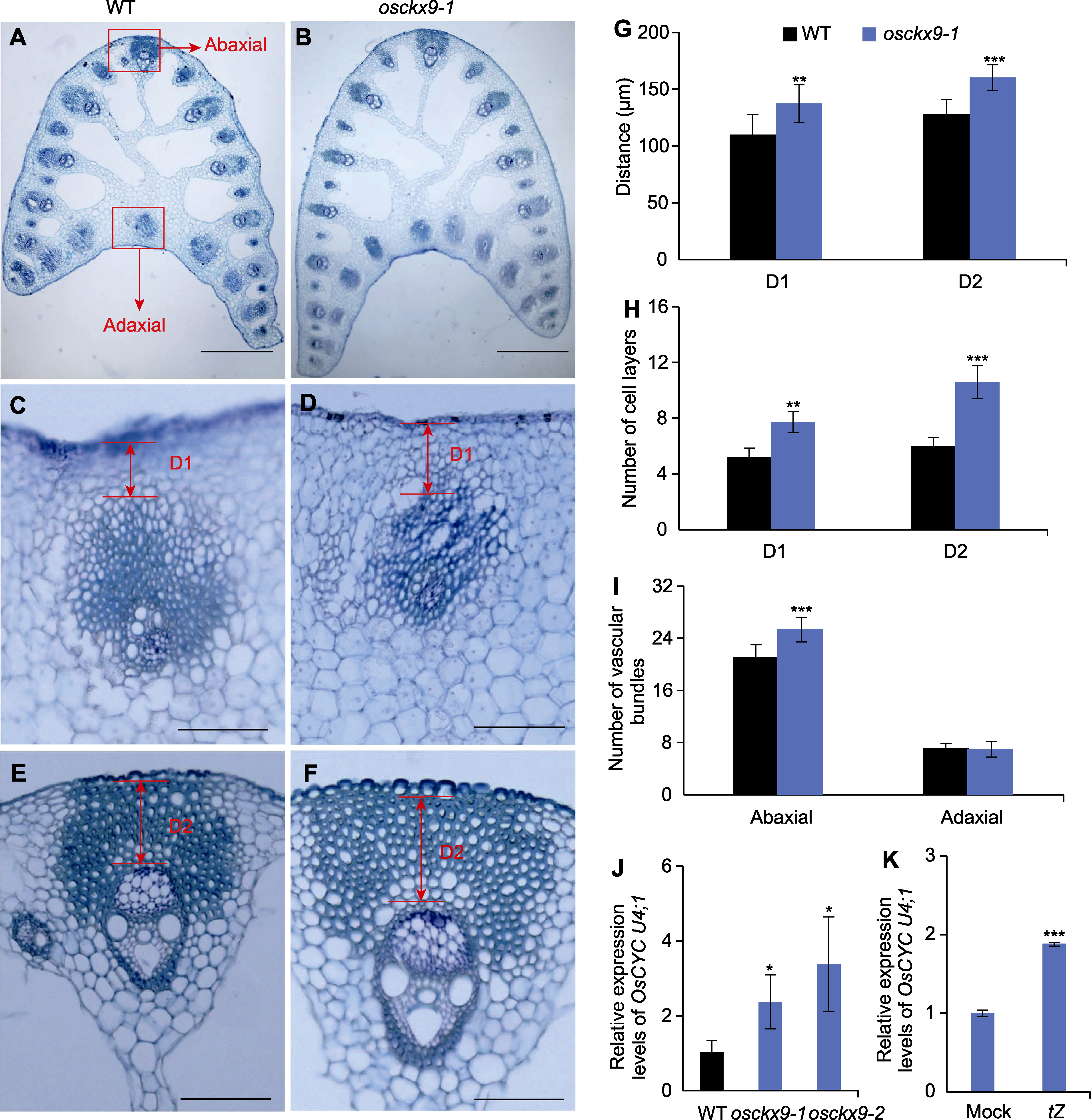
图2 OsCKX9影响叶枕远轴端细胞增殖和维管束数量 (A), (B) 野生型(WT)和osckx9-1叶枕的横截面(红框代表叶枕的近轴端和远轴端(bars=250 μm)); (C), (D) WT和osckx9-1叶枕近轴端的高放大倍率图像(bars=100 μm); (E), (F) WT和osckx9-1叶枕远轴端的高放大倍率图像(bars=250 μm); (G) 叶枕D1区和D2区长度(如(C)-(F)所示) (平均值±标准差, n=15); (H) D1区中薄壁细胞层和D2区中厚壁细胞层的定量(如(D)至(F)所示) (平均值±标准差, n=15); (I) 上下和同轴侧的维管束定量(平均值±标准差, n=15); (J) qRT-PCR检测OsCYC U4;1在WT、osckx 9-1和osckx 9-2中的表达量(平均值±标准差, n=3); (K) qRT-PCR检测OsCYC U4;1在Mock和tZ处理时的表达量(平均值±标准差, n=3)。D1: 近轴端表皮与近轴端中央维管束之间的区域; D2: 远轴端表皮与厚壁组织之间的区域; tZ: 反式玉米素。使用Student’s t-test进行统计分析。* P<0.05; ** P<0.01; *** P<0.001
Figure 2 OsCKX9 affects the cell proliferation and vascular number in the abaxial of lamina joint (A)-(B) Transverse section of the lamina joints of wild type (WT) and osckx9-1 (red box represent the adaxial and the abaxial side of the lamina joint) (bars=250 μm); (C)-(D) High magnification images of the adaxial side of the lamina joints of WT and osckx9-1 (bars=100 μm); (E)-(F) High magnification images of the abaxial side of the lamina joints of WT and osckx9-1 (bars=250 μm); (G) Lengths of the D1 and D2 of the lamina joints shown in (C) to (F) (means±SD, n=15); (H) Quantification of the parenchyma cell layers in D1 and sclerenchyma cell layers in D2 shown in (D) to (F) (means±SD, n=15); (I) Quantification of vascular bundles on the abaxial and adaxial sides (means±SD, n=15). (J) Expression analysis of OsCYC U4;1 in WT, osckx9-1 and osckx9-2 by qRT-PCR (means±SD, n=3); (K) Expression analysis of OsCYC U4;1 in Mock and tZ treatment by qRT-PCR (means±SD, n=3). D1: The region between the adaxial epidermis and the adaxial central vascular bundle; D2: The region between the abaxial epidermis and the sclerenchyma; tZ: Trans-zeatin. Statistical analyses were performed by Student’s t-test. *P<0.05; **P<0.01; ***P<0.001
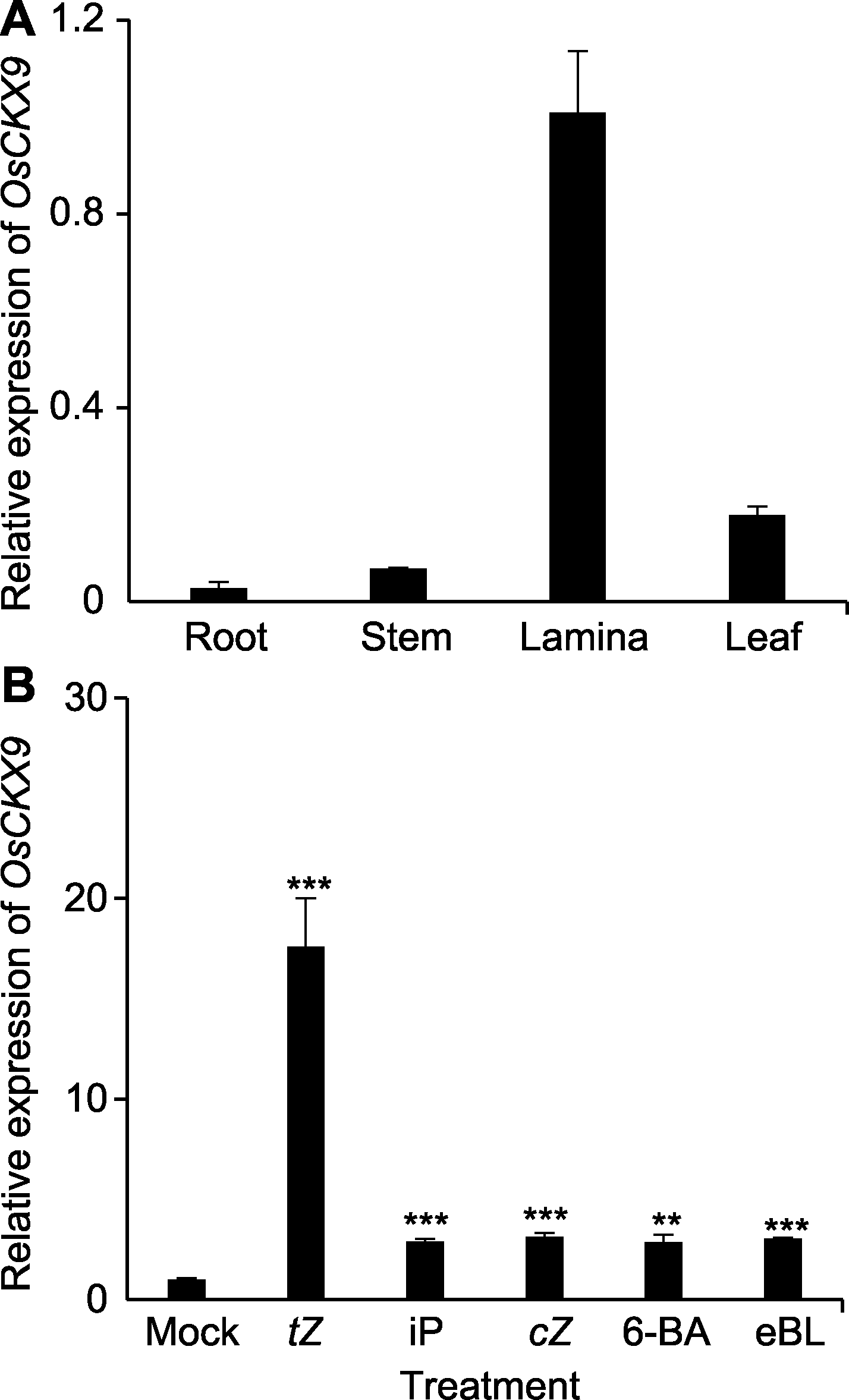
图3 OsCKX9的表达谱分析 (A) 通过qRT-PCR分析抽穗期野生型(WT)水稻根、茎、叶枕和叶中OsCKX9的表达量; (B) OsCKX9在tZ (反式玉米素)、iP (异戊烯基腺嘌呤)、cZ (顺式玉米素)、6-BA和eBL (表油菜素内酯)处理3小时的7-DAG幼苗中的相对表达量, OsUBQ5为内参基因。数值为平均值±标准差, n=3; 使用Student’s t-test进行统计分析。** P<0.01; *** P<0.001
Figure 3 The expression pattern of OsCKX9 (A) Expression analysis of OsCKX9 in various rice tissues (root, stem, lamina, and leaf) in the heading stage at the wild type (WT) by qRT-PCR; (B) Relative expression levels of OsCKX9 in 7-DAG seedlings treated by tZ (trans-zeatin), iP (isopentenyladenine), cZ (cis-zeatin), 6-BA and eBL (epibrassinolide) for 3 h. OsUBQ5 was used as an internal control. Values are means±SD, n=3; statistical analyses were performed by Student’s t-test. ** P<0.01; *** P<0.001
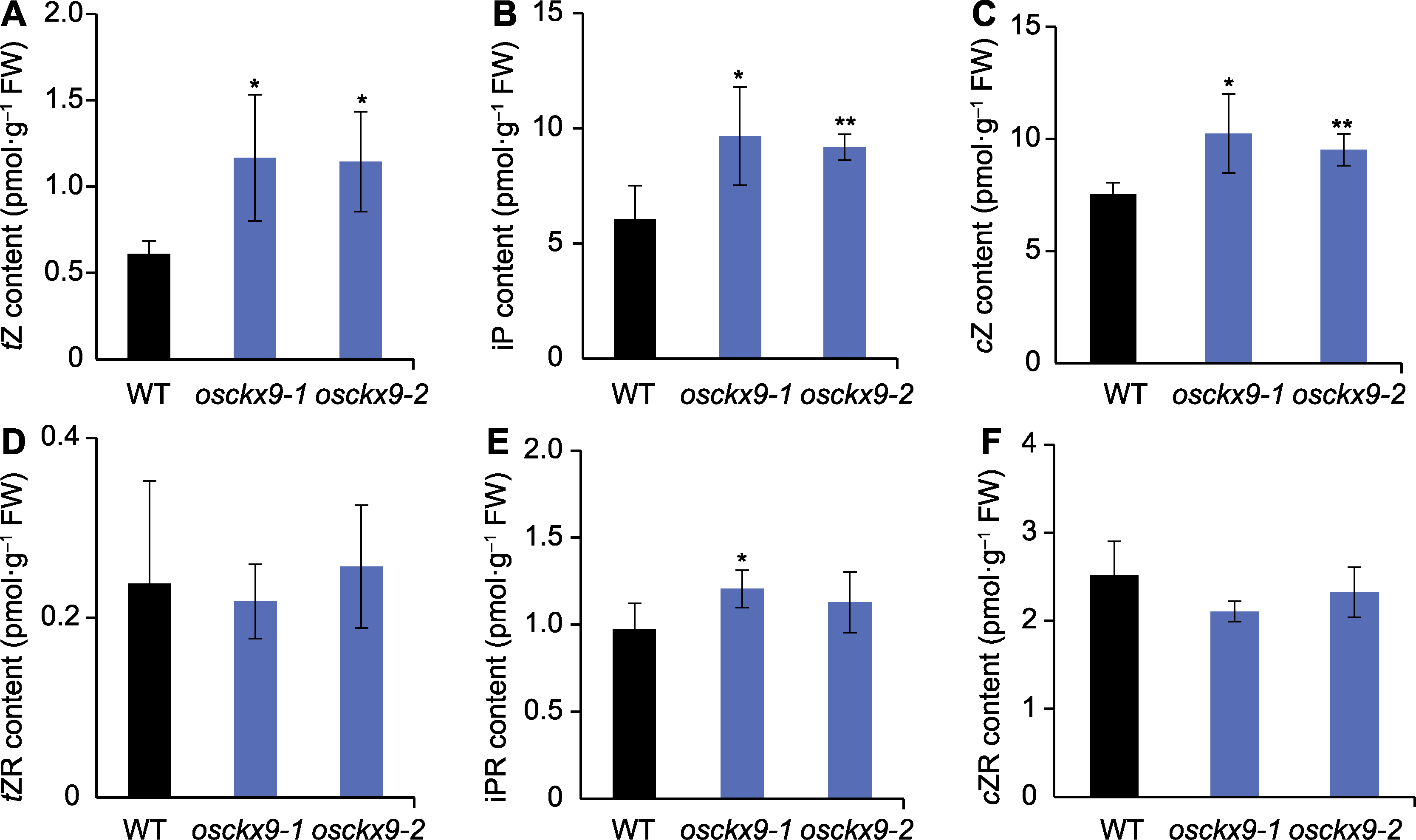
图4 osckx9叶枕处细胞分裂素(CTK)含量测定 (A)-(F) 野生型(WT)、osckx9-1和osckx9-2叶枕中内源性CTK含量定量。tZR: 反式玉米素核苷; iPR: 异戊烯基腺嘌呤核苷; cZR: 顺式玉米素核苷。tZ、iP和cZ同图3。数值为平均值±标准差, n=3; 使用Student’s t-test进行统计分析。* P<0.05; ** P<0.01
Figure 4 Quantification of cytokinin (CTK) content in osckx9 lamina joint (A)-(F) Quantification of endogenous CTK contents in the lamina joint of wild type (WT), osckx9-1 and osckx9-2. tZR: Trans-zea tin riboside; iPR: Isopentenyladenine riboside; cZR: Cis-zeatin riboside; tZ, iP, and cZ are the same as shown in Figure 3. Values are means±SD, n=3; statistical analyses were performed by Student’s t-test. * P<0.05; ** P<0.01
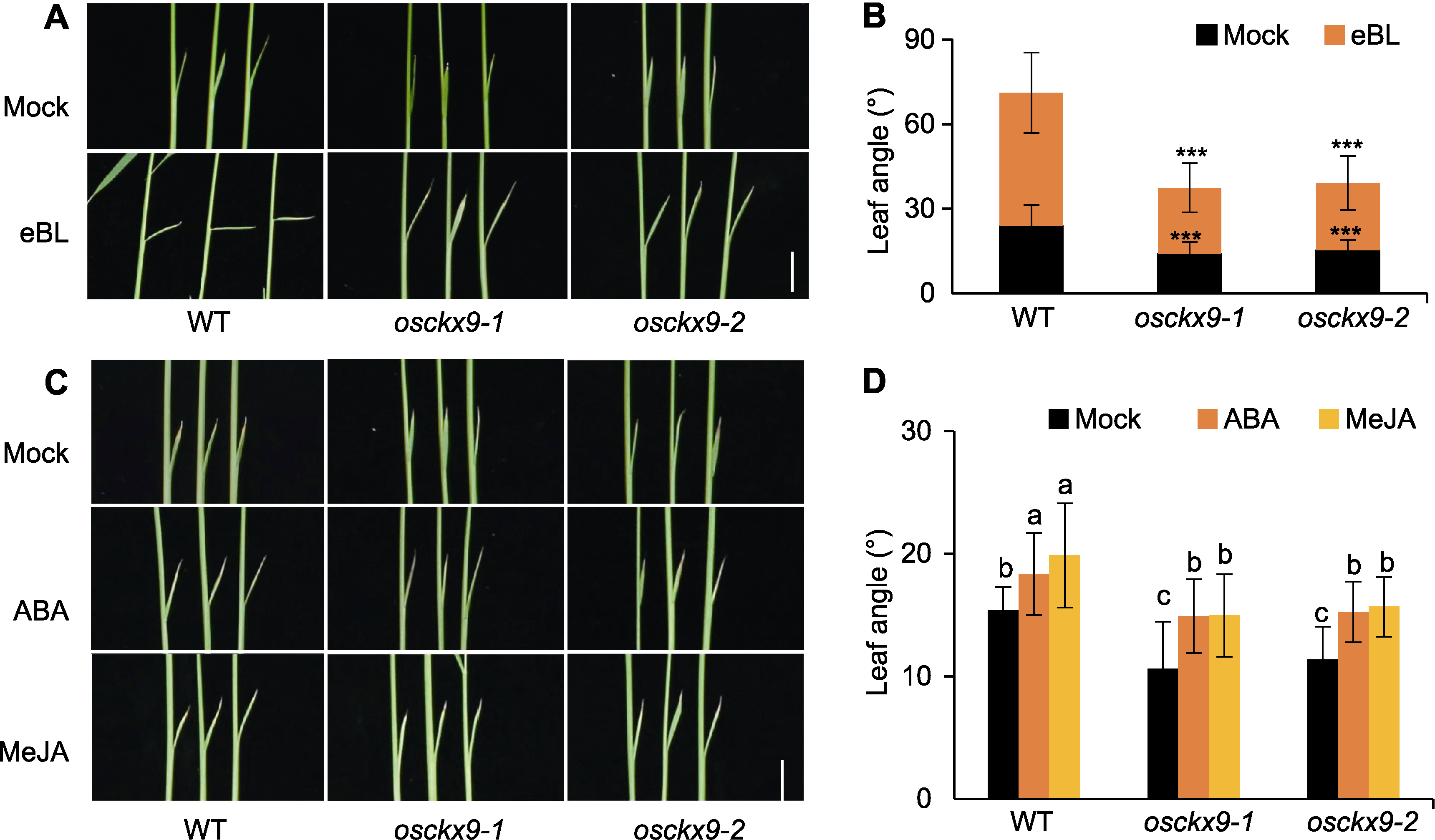
图5 osckx9对BR不敏感 (A) 野生型(WT)、osckx9-1和osckx9-2的7-DAG幼苗叶夹角对Mock和eBL (表油菜素内酯)处理的响应(bar=1 cm); (B) Mock和eBL处理的叶夹角测量(黑色为Mock叶夹角, 橙色为eBL处理后增加的角度) (数值为平均值±标准差, n=20; 使用Student’s t-test进行统计分析。*** P<0.001); (C) WT、osckx9-1和osckx9-2的7-DAG幼苗叶夹角对Mock、ABA和MeJA (茉莉酸甲酯)处理的响应(bar=1 cm); (D) Mock、ABA和MeJA处理的叶夹角测量(数值为平均值±标准差, n=20; 使用One-way ANOVAL of the LSD test对数据进行显著性分析, 不同小写字母表示对照组与处理组间在P<0.05水平差异显著)。
Figure 5 osckx9 shows insensitivity to BR (A) Wild type (WT), osckx9-1 and osckx9-2 plants responded with 7-DAG seedling leaf angles using Mock and eBL (epibrassinolide) treatment (bar=1 cm); (B) Measurement of leaf angles treated with Mock or eBL (black is the angle of the Mock leaf, orange is the angle added after eBL treatment (values are means±SD, n=20; statistical analyses were performed by Student’s t-test. *** P<0.001); (C) WT, osckx9-1 and osckx9-2 plants responded with 7-DAG seedling leaf angles using Mock, ABA and MeJA (jasmonates) treatments (bar=1 cm); (D) Mock-, ABA- and MeJA-treated measurement of leaf angles (values are means±SD, n=20; data were analyzed statistically using the One-way ANOVAL of the LSD test, and the different lowercase letters indicate significant differences in the Mock group and the treatment group at P<0.05 level).
| [1] | 宋松泉, 刘军, 杨华, 张文虎, 张琪, 高家东 (2021). 细胞分裂素调控种子发育、休眠与萌发的研究进展. 植物学报 56, 218-231. |
| [2] | 俞启璐, 赵江哲, 朱晓仙, 张可伟 (2021). 水稻根分泌激素调节生长速度. 植物学报 56, 175-182. |
| [3] | 张芬, 郭得平, 林明丽, 闫淼淼 (2008). 细胞分裂素氧化酶/脱氢酶的生理生化和分子特性. 植物生理学通讯 44, 797-803. |
| [4] |
Allen M, Qin WS, Moreau F, Moffatt B (2002). Adenine phosphoribosyltransferase isoforms of Arabidopsis and their potential contributions to adenine and cytokinin metabolism. Physiol Plant 115, 56-68.
DOI URL |
| [5] |
Ashikari M, Sakakibara H, Lin SY, Yamamoto T, Takashi T, Nishimura A, Angeles ER, Qian Q, Kitano H, Matsuoka M (2005). Cytokinin oxidase regulates rice grain pro- duction. Science 309, 741-745.
DOI URL |
| [6] |
Bai MY, Zhang LY, GampalaSS, Zhu SW, Song WY, Chong K, Wang ZY (2007). Functions of OsBZR1 and 14-3-3 proteins in brassinosteroid signaling in rice. Proc Natl Acad Sci USA 104, 13839-13844.
DOI URL |
| [7] |
Cao HP, Chen SK (1995). Brassinosteroid-induced rice lamina joint inclination and its relation to indole-3-acetic acid and ethylene. Plant Growth Regul 16, 189-196.
DOI URL |
| [8] |
Cao YY, Zhong ZJ, Wang HY, Shen RX (2022). Leaf angle: a target of genetic improvement in cereal crops tailored for high-density planting. Plant Biotechnol J 20, 426-436.
DOI PMID |
| [9] |
Duan JB, Yu H, Yuan K, Liao ZG, Meng XB, Jing YH, Liu GF, Chu JF, Li JY (2019). Strigolactone promotes cytokinin degradation through transcriptional activation of CYTO- KININ OXIDASE/DEHYDROGENASE 9in rice. Proc Natl Acad Sci USA 116, 14319-14324.
DOI URL |
| [10] |
Frébort I, Kowalska M, Hluska T, Frébortová J, Galuszka P (2011). Evolution of cytokinin biosynthesis and degradation. J Exp Bot 62, 2431-2452.
DOI PMID |
| [11] |
Gan LJ, Wu H, Wu DP, Zhang ZF, Guo ZF, Yang N, Xia K, Zhou X, Oh K, Matsuoka M, Ng D, Zhu CH (2015). Methyl jasmonate inhibits lamina joint inclination by repressing brassinosteroid biosynthesis and signaling in rice. Plant Sci 241, 238-245.
DOI PMID |
| [12] |
Gao SP, Fang J, Xu F, Wang W, Sun XH, Chu JF, Cai BD, Feng YQ, Chu CC (2014). CYTOKININ OXIDASE/DEHYDROGENASE4integrates cytokinin and auxin signaling to control rice crown root formation. Plant Physiol 165, 1035-1046.
DOI URL |
| [13] |
Golovko A, Sitbon F, Tillberg E, Nicander B (2002). Identification of a tRNA isopentenyltransferase gene from Ara- bidopsis thaliana. Plant Mol Biol 49, 161-169.
DOI PMID |
| [14] |
Guo JF, Li W, Shang LG, Wang YG, Yan P, Bai YH, Da XW, Wang K, Guo QQ, Jiang RR, Mao CZ, Mo XR (2021). OsbHLH98 regulates leaf angle in rice through transcriptional repression of OsBUL1. New Phytol 230, 1953-1966.
DOI URL |
| [15] |
Hothorn M, Dabi T, Chory J (2011). Structural basis for cytokinin recognition by Arabidopsis thaliana histidine kinase 4. Nat Chem Biol 7, 766-768.
DOI |
| [16] |
Huang GQ, Hu H, van de Meene A, Zhang J, Dong L, Zheng S, Zhang FL, Betts NS, Liang WQ, Bennett MJ, Persson S, Zhang DB (2021). AUXIN RESPONSE FACTORS 6and 17 control the flag leaf angle in rice by regulating secondary cell wall biosynthesis of lamina joints. Plant Cell 33, 3120-3133.
DOI URL |
| [17] |
Huang P, Zhao JZ, Hong JL, Zhu B, Xia S, Zhu EG, Han PF, Zhang KW (2023). Cytokinins regulate rice lamina joint development and leaf angle. Plant Physiol 191, 56-69.
DOI URL |
| [18] |
Hwang I, Sheen J, Müller B (2012). Cytokinin signaling networks. Annu Rev Plant Biol 63, 353-380.
DOI PMID |
| [19] |
Kiba T, Takei K, Kojima M, Sakakibara H (2013). Side- chain modification of cytokinins controls shoot growth in Arabidopsis. Dev Cell 27, 452-461.
DOI URL |
| [20] |
Kuroha T, Tokunaga H, Kojima M, Ueda N, Ishida T, Nagawa S, Fukuda H, Sugimoto K, Sakakibara H (2009). Functional analyses of LONELY GUY cytokinin-activating enzymes reveal the importance of the direct activation pathway in Arabidopsis. Plant Cell 21, 3152-3169.
DOI URL |
| [21] |
Lee J, Park JJ, Kim SL, Yim J, An G (2007). Mutations in the rice liguleless gene result in a complete loss of the auricle, ligule, and laminar joint. Plant Mol Biol 65, 487-499.
DOI PMID |
| [22] |
Li QQ, Xu F, Chen Z, Teng ZF, Sun K, Li XC, Yu JY, Zhang GX, Liang Y, Huang XH, Du L, Qian YW, Wang YC, Chu CC, Tang JY (2021). Synergistic interplay of ABA and BR signal in regulating plant growth and adaptation. Nat Plants 7, 1108-1118.
DOI PMID |
| [23] |
Li XM, Sun SY, Li CX, Qiao SL, Wang T, Leng LN, Shen HY, Wang XL (2014). The strigolactone-related mutants have enhanced lamina joint inclination phenotype at the seedling stage. J Genet Genomics 41, 605-608.
DOI PMID |
| [24] |
Sakakibara H (2006). Cytokinins: activity, biosynthesis, and translocation. Annu Rev Plant Biol 57, 431-449.
PMID |
| [25] |
Sakamoto T, Morinaka Y, Ohnishi T, Sunohara H, Fujioka S, Ueguchi-Tanaka M, Mizutani M, Sakata K, Takatsuto S, Yoshida S, Tanaka H, Kitano H, Matsuoka M (2006). Erect leaves caused by brassinosteroid deficiency increase biomass production and grain yield in rice. Nat Biotechnol 24, 105-109.
DOI PMID |
| [26] |
Schmülling T, Werner T, Riefler M, Krupková E, Bartrinay Manns I (2003). Structure and function of cytokinin oxidase/dehydrogenase genes of maize, rice, Arabidopsis and other species. J Plant Res 116, 241-252.
DOI PMID |
| [27] |
Šimášková M, O'Brien JA, Khan M, Van Noorden G, Ötvös K, Vieten A, De Clercq I, Van Haperen JMA, Cuesta C, Hoyerová K, Vanneste S, Marhavý P, Wabnik K, Van Breusegem F, Nowack M, Murphy A, Friml J, Weijers D, Beeckman T, Benková E (2015). Cytokinin response factors regulate PIN-FORMED auxin transporters. Nat Commun 6, 8717.
DOI PMID |
| [28] | Sun SY, Chen DH, Li XM, Qiao SL, Shi CN, Li CX, Shen HY, Wang XL (2015). Brassinosteroid signaling regulates leaf erectness in Oryza sativa via the control of a specific U-type cyclin and cell proliferation. Dev Cell 34, 220-228. |
| [29] |
Tong HN, Chu CC (2018). Functional specificities of brassinosteroid and potential utilization for crop improvement. Trends Plant Sci 23, 1016-1028.
DOI PMID |
| [30] |
Wang B, Smith SM, Li JY (2018). Genetic regulation of shoot architecture. Annu Rev Plant Biol 69, 437-468.
DOI PMID |
| [31] |
Yang WB, Cortijo S, Korsbo N, Roszak P, Schiessl K, Gurzadyan A, Wightman R, Jönsson H, Meyerowitz E (2021). Molecular mechanism of cytokinin-activated cell division in Arabidopsis. Science 371, 1350-1355.
DOI URL |
| [32] |
Zhang SN, Wang SK, Xu YX, Yu CL, Shen CJ, Qian Q, Geisler M, Jiang DA, Qi YH (2015). The auxin response factor, OsARF19, controls rice leaf angles through positively regulating OsGH3-5 and OsBRI1. Plant Cell Environ 38, 638-654.
DOI URL |
| [33] |
Zhang W, Peng KX, Cui FB, Wang DL, Zhao JZ, Zhang YJ, Yu NN, Wang YY, Zeng DL, Wang YH, Cheng ZK, Zhang KW (2021). Cytokinin oxidase/dehydrogenase OsCKX11 coordinates source and sink relationship in rice by simultaneous regulation of leaf senescence and grain number. Plant Biotechnol J 19, 335-350.
DOI PMID |
| [34] |
Zhang XY, Chen YT, Lin X, Hong XY, Zhu Y, Li WY, He WR, An FY, Guo HW (2013). Adenine phosphoribosyl transferase 1 is a key enzyme catalyzing cytokinin conversion from nucleobases to nucleotides in Arabidopsis. Mol Plant 6, 1661-1672.
DOI URL |
| [35] |
Zhao JZ, Yu NN, Ju M, Fan B, Zhang YJ, Zhu EG, Zhang MY, Zhang KW (2019). ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot 70, 6277-6291.
DOI PMID |
| [1] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [3] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [4] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [5] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [6] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [7] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [8] | 孙苗苗, 张蔚, 张林霞, 霍竣涛, 李志能, 刘国锋. 矮牵牛花朵大小遗传规律及相关基因的表达分析[J]. 植物学报, 2024, 59(3): 422-432. |
| [9] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [10] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [11] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [12] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [13] | 贾绮玮, 钟芊芊, 顾育嘉, 陆天麒, 李玮, 杨帅, 朱超宇, 胡程翔, 李三峰, 王跃星, 饶玉春. 水稻茎秆细胞壁相关组分含量QTL定位及候选基因分析[J]. 植物学报, 2023, 58(6): 882-892. |
| [14] | 田传玉, 方妍力, 沈晴, 王宏杰, 陈析丰, 郭威, 赵开军, 王春连, 纪志远. 2019-2021年我国南方稻区白叶枯病菌的毒力与遗传多样性调查研究[J]. 植物学报, 2023, 58(5): 743-749. |
| [15] | 戴若惠, 钱心妤, 孙静蕾, 芦涛, 贾绮玮, 陆天麒, 路梅, 饶玉春. 水稻叶色调控机制及相关基因研究进展[J]. 植物学报, 2023, 58(5): 799-812. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||