

植物学报 ›› 2025, Vol. 60 ›› Issue (5): 846-853.DOI: 10.11983/CBB25102 cstr: 32102.14.CBB25102
• 技术方法 • 上一篇
收稿日期:2025-06-04
接受日期:2025-07-08
出版日期:2025-09-10
发布日期:2025-07-08
通讯作者:
*E-mail: spyan@mail.hzau.edu.cn
基金资助:Received:2025-06-04
Accepted:2025-07-08
Online:2025-09-10
Published:2025-07-08
Contact:
*E-mail: spyan@mail.hzau.edu.cn
摘要: 植物激素水杨酸(SA)促进植物的抗病性, 但抑制植物生长。植物通过动态调控SA的含量以平衡抗病与生长。高效液相色谱-荧光检测器技术是检测SA含量最常用的方法。该研究优化了流动相的成分、离子浓度、pH值以及检测波长和检测程序。优化后的流动相为10%乙腈、100 mmol∙L-1乙酸钠、pH5.2。优化后的激发光波长为300 nm, 发射光波长为405 nm。优化后的检测程序为: 进样后3.5分钟开始清洗色谱柱, 清洗时间为3.5分钟, 平衡时间为3分钟, 总时长为10分钟。优化后的检测方法显著提高了检测灵敏性、稳定性和高效性。
史世肸, 严顺平. 高效液相色谱法检测水杨酸的优化. 植物学报, 2025, 60(5): 846-853.
Shi Shixi, Yan Shunping. Optimization of an High-performance Liquid Chromatography Method for the Determination of Salicylic Acid. Chinese Bulletin of Botany, 2025, 60(5): 846-853.
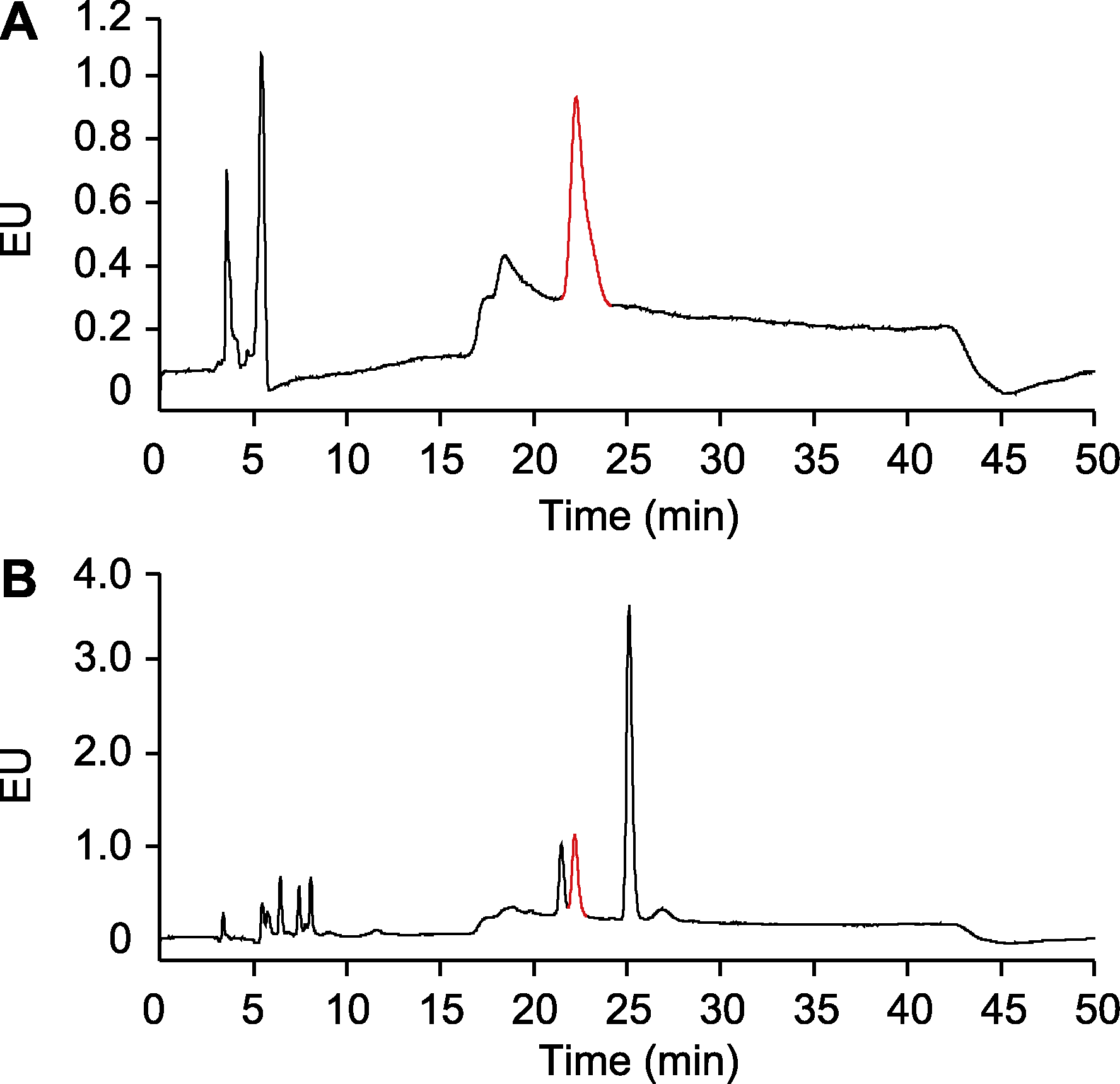
图1 使用Zhang等(2017)的方法检测水杨酸(SA) (A) 0.1 μg·mL-1 SA标准样品; (B) 样品为未被Psm ES4326侵染的拟南芥游离态SA。EU: 发射单位。红色的峰指示SA。
Figure 1 Detection of salicylic acid (SA) using methods reported in Zhang et al., 2017 (A) 0.1 μg·mL-1 SA standard sample; (B) The sample is free SA in Arabidopsis without Psm ES4326 infection. EU: Emission units. The peaks labeled in red indicate SA.
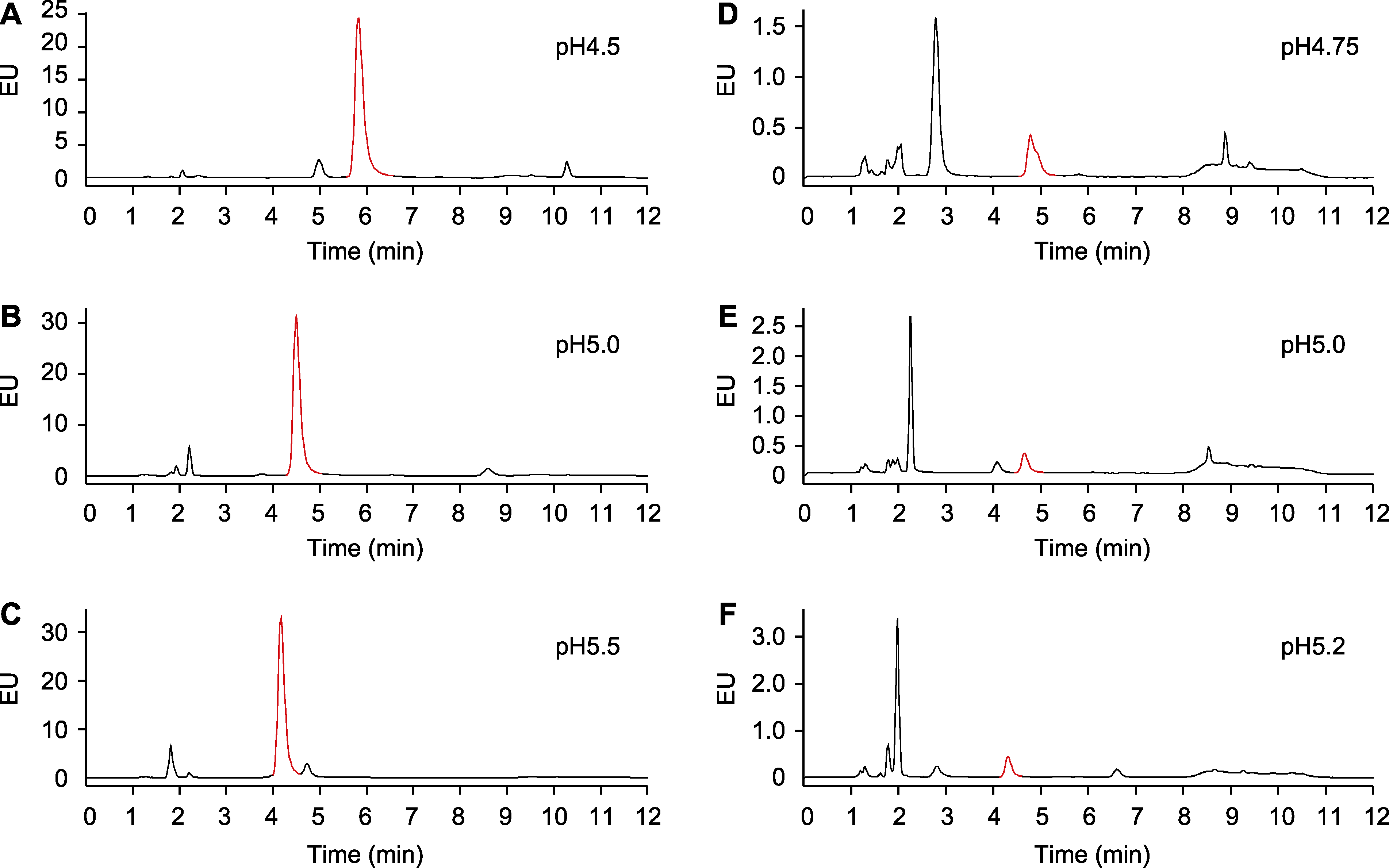
图2 流动相pH值对水杨酸(SA)分离的影响 (A)-(C) 样品为被Psm ES4326侵染24小时后的拟南芥总SA; (D)-(F) 样品为未被Psm ES4326侵染的拟南芥总SA。EU同图1。红色的峰指示SA。
Figure 2 Effects of pH of the mobile phase on salicylic acid (SA) separation (A)-(C) The samples are total SA extracted from Arabidopsis treated with Psm ES4326 for 24 h; (D)-(F) The samples are total SA in Arabidopsis without Psm ES4326 infection. EU is the same as shown in Figure 1. The peaks labeled in red indicate SA.
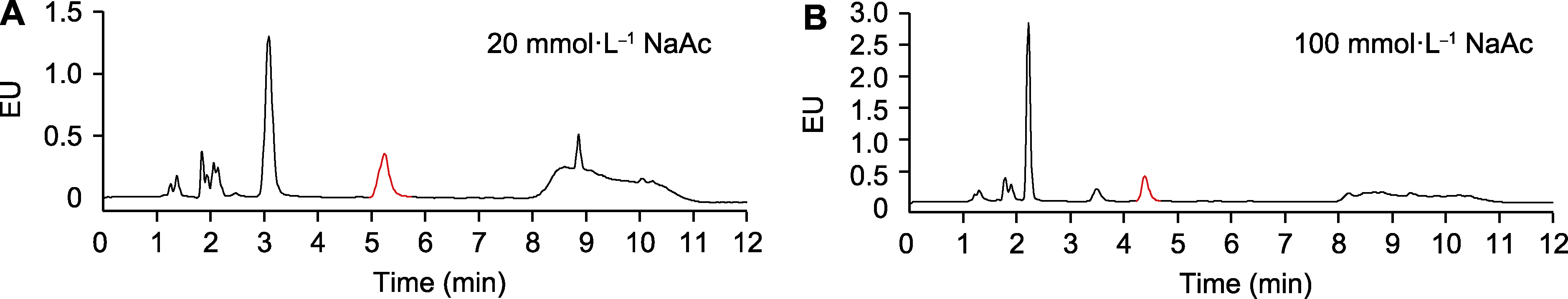
图3 流动相中乙酸钠浓度对水杨酸(SA)分离的影响 (A) 20 mmol∙L-1乙酸钠; (B) 100 mmol∙L-1乙酸钠。样品为未被Psm ES4326侵染的拟南芥总SA。EU同图1。红色的峰指示SA。
Figure 3 Effects of the concentration of sodium acetate in the mobile phase on salicylic acid (SA) separation (A) 20 mmol∙L-1 NaAc; (B) 100 mmol∙L-1 NaAc. The samples are total SA in Arabidopsis without Psm ES4326 infection. EU is the same as shown in Figure 1. The peaks labeled in red indicate SA.
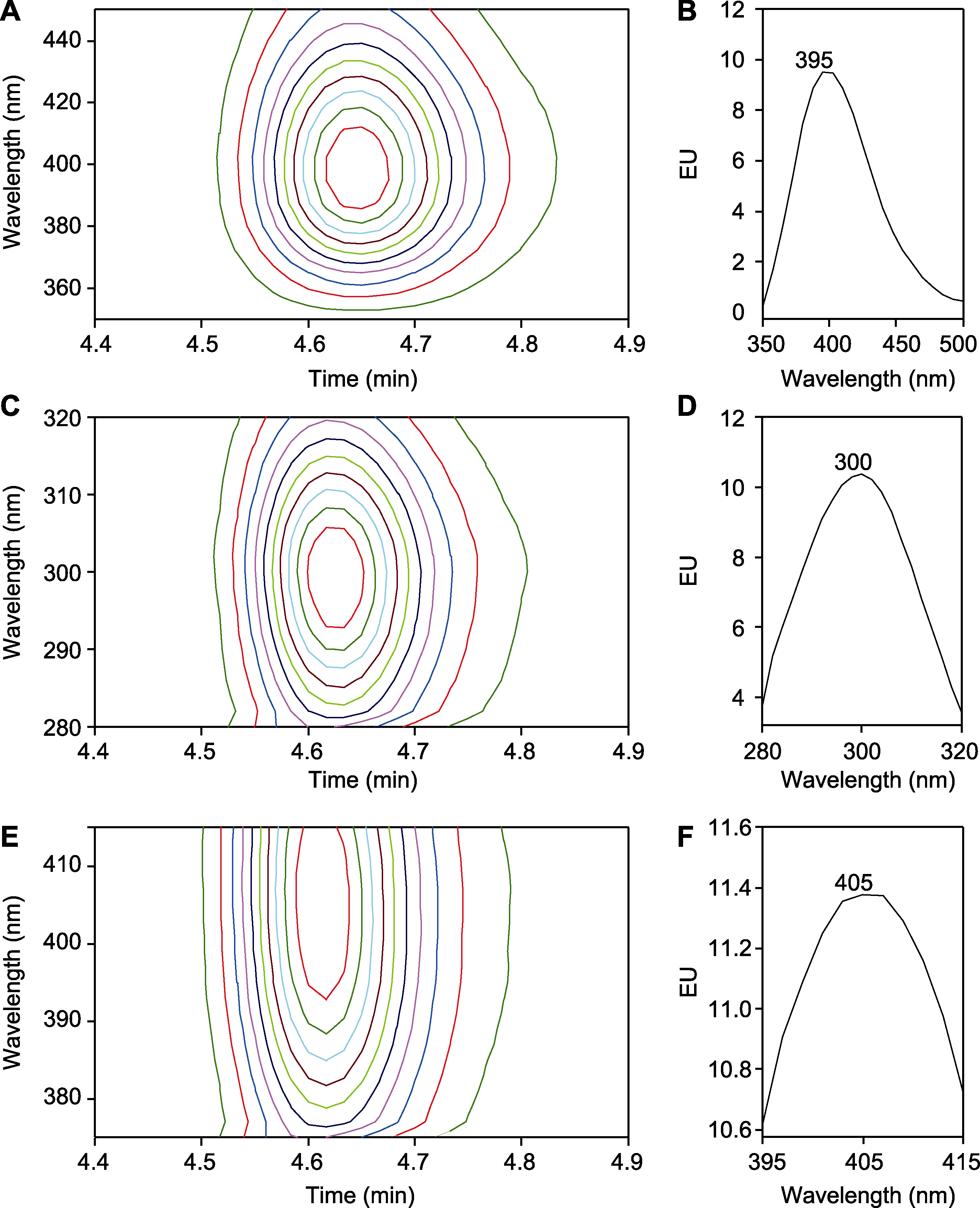
图4 水杨酸(SA)检测波长的优化 (A), (B) 激发光波长为296 nm, 发射光波长为350-450 nm; (C), (D) 激发光波长为280-320 nm, 发射光波长为395 nm; (E), (F) 激发光波长为300 nm, 发射光波长为375-415 nm。(A), (C), (E) 二维信号图; (B), (D), (F) 荧光波长-荧光能量积分图。样品均为1 µg∙mL-1 SA标准样品。EU同图1。
Figure 4 Optimization of detection wavelength for salicylic acid (SA) (A), (B) The excitation wavelength is 296 nm and the emission wavelength is 350-450 nm; (C), (D) The excitation wavelength is 280-320 nm and the emission wavelength is 395 nm; (E), (F) The excitation wavelength is 300 nm and the emission wavelength is 375-415 nm. (A), (C), (E) Two-dimensional signal plots; (B), (D), (F) Fluorescence wavelength-fluorescence energy integration plots. All samples are 1 µg∙mL-1 SA standards. EU is the same as shown in Figure 1.
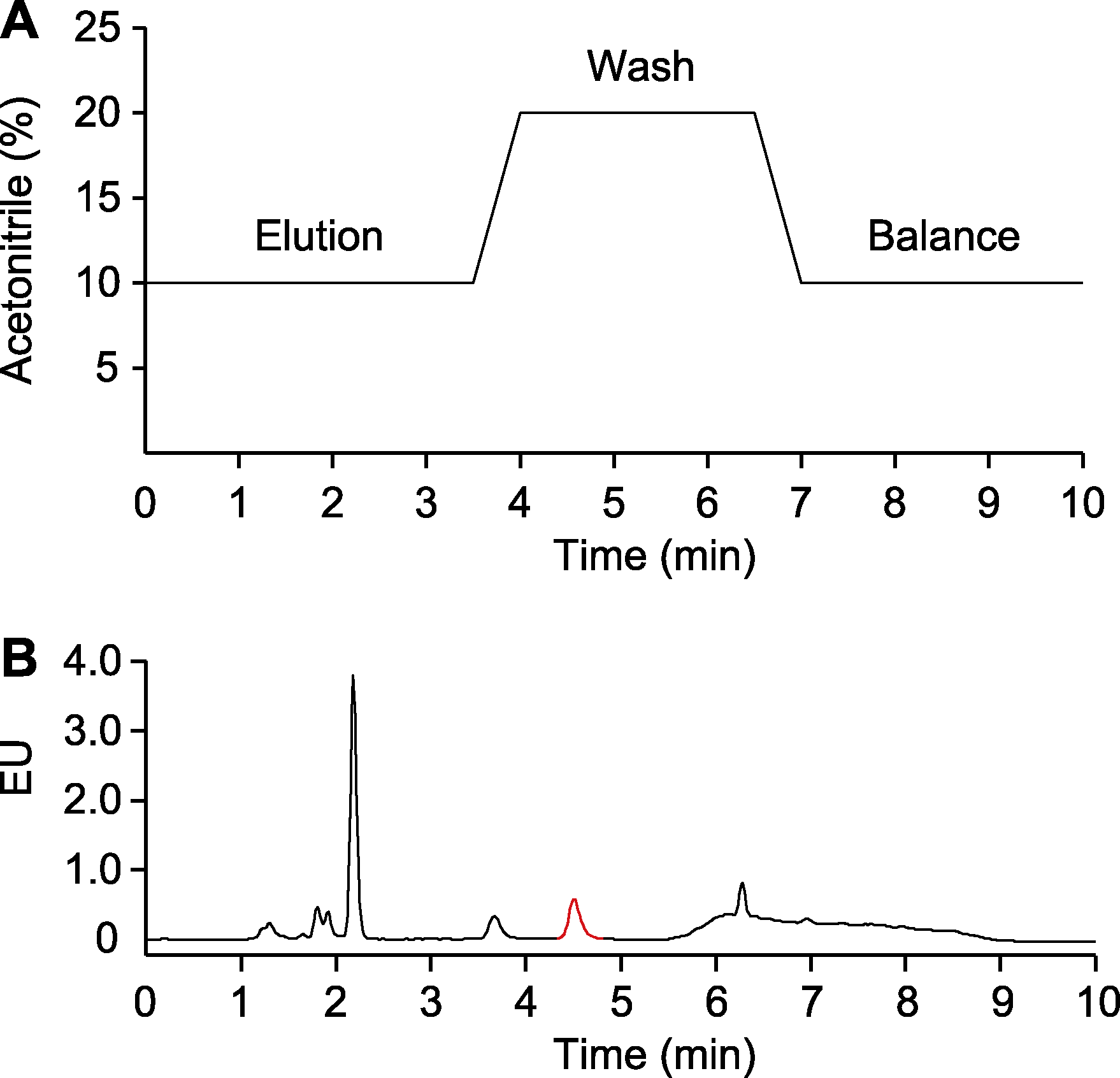
图5 水杨酸(SA)检测程序的优化 (A) 检测程序图; (B) 检测结果。样品为未被Psm ES4326侵染的拟南芥总SA。EU同图1。红色的峰指示SA。
Figure 5 Optimization of salicylic acid (SA) detection procedure (A) Graph of detection procedure; (B) Detection results. The samples are total SA in Arabidopsis without Psm ES4326 infection. EU is the same as shown in Figure 1. The peak labeled in red indicates SA.
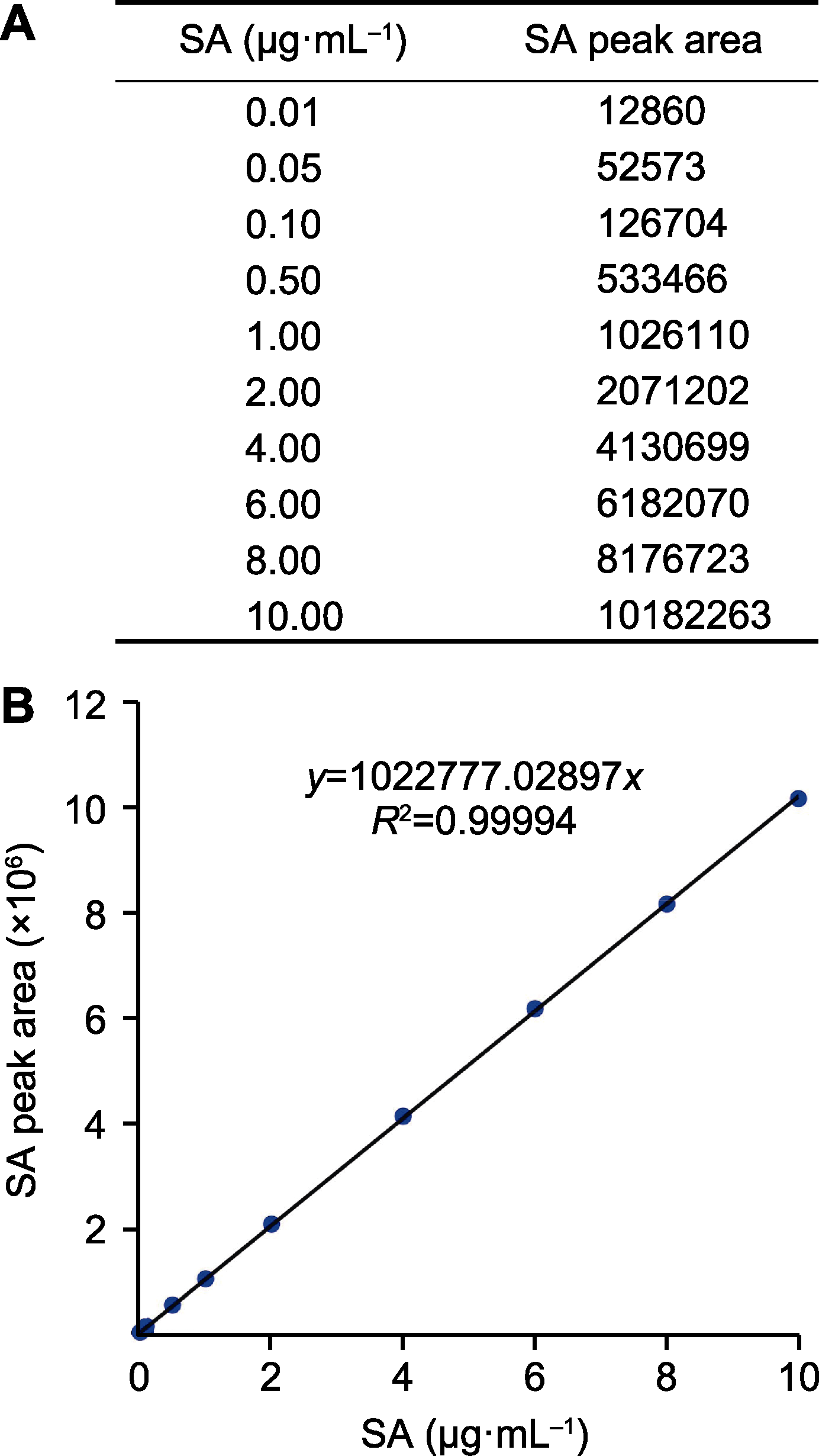
图6 水杨酸(SA)标准曲线 (A) 不同浓度SA标准样品对应的峰面积; (B) 标准曲线(截距为0, R2表示决定系数)
Figure 6 Salicylic acid (SA) standard curve (A) Peak areas corresponding to different concentrations of SA standards; (B) The standard curve (with an intercept of 0, R2 indicates the coefficient of determination)
| [1] | Aboul-Soud MAM, Cook K, Loake GJ (2004). Measurement of salicylic acid by a high-performance liquid chromatography procedure based on ion-exchange. Chromatographia 59, 129-133. |
| [2] |
Balcke GU, Handrick V, Bergau N, Fichtner M, Henning A, Stellmach H, Tissier A, Hause B, Frolov A (2012). An UPLC-MS/MS method for highly sensitive high-throughput analysis of phytohormones in plant tissues. Plant Methods 8, 47.
DOI PMID |
| [3] | Cao H, Bowling SA, Gordon AS, Dong XN (1994). Characterization of an Arabidopsis mutant that is nonresponsive to inducers of systemic acquired resistance. Plant Cell 6, 1583-1592. |
| [4] | Defraia CT, Schmelz EA, Mou ZL (2008). A rapid biosensor-based method for quantification of free and glucose- conjugated salicylic acid. Plant Methods 4, 28. |
| [5] |
Delaney TP, Uknes S, Vernooij B, Friedrich L, Weymann K, Negrotto D, Gaffney T, Gut-Rella M, Kessmann H, Ward E, Ryals J (1994). A central role of salicylic acid in plant disease resistance. Science 266, 1247-1250.
DOI PMID |
| [6] |
Ding YL, Sun TJ, Ao K, Peng YJ, Zhang YX, Li X, Zhang YL (2018). Opposite roles of salicylic acid receptors NPR1 and NPR3/NPR4 in transcriptional regulation of plant immunity. Cell 173, 1454-1467.
DOI PMID |
| [7] |
Fu ZQ, Dong XN (2013). Systemic acquired resistance: turning local infection into global defense. Annu Rev Plant Biol 64, 839-863.
DOI PMID |
| [8] | Fu ZQ, Yan SP, Saleh A, Wang W, Ruble J, Oka N, Mohan R, Spoel SH, Tada Y, Zheng N, Dong XN (2012). NPR3 and NPR4 are receptors for the immune signal salicylic acid in plants. Nature 486, 228-232. |
| [9] |
Gaffney T, Friedrich L, Vernooij B, Negrotto D, Nye G, Uknes S, Ward E, Kessmann H, Ryals J (1993). Requirement of salicylic acid for the induction of systemic acquired resistance. Science 261, 754-756.
DOI PMID |
| [10] |
Huang WE, Wang H, Zheng HJ, Huang LF, Singer AC, Thompson I, Whiteley AS (2005). Chromosomally located gene fusions constructed in Acinetobacter sp. ADP1 for the detection of salicylate. Environ Microbiol 7, 1339-1348.
PMID |
| [11] | Kumar S, Zavaliev R, Wu QL, Zhou Y, Cheng J, Dillard L, Powers J, Withers J, Zhao JS, Guan ZQ, Borgnia MJ, Bartesaghi A, Dong XN, Zhou P (2022). Structural basis of NPR1 in activating plant immunity. Nature 605, 561-566. |
| [12] | Lim EK, Doucet CJ, Li Y, Elias L, Worrall D, Spencer SP, Ross J, Bowles DJ (2002). The activity of Arabidopsis glycosyltransferases toward salicylic acid, 4-hydroxybenzoic acid, and other benzoates. J Biol Chem 277, 586-592. |
| [13] |
Marek G, Carver R, Ding YZ, Sathyanarayan D, Zhang XD, Mou ZZ (2010). A high-throughput method for isolation of salicylic acid metabolic mutants. Plant Methods 6, 21.
DOI PMID |
| [14] | Mishra S, Roychowdhury R, Ray S, Hada A, Kumar A, Sarker U, Aftab T, Das R (2024). Salicylic acid (SA)-mediated plant immunity against biotic stresses: an insight on molecular components and signaling mechanism. Plant Stress 11, 100427. |
| [15] |
Nawrath C, Métraux JP (1999). Salicylic acid induction- deficient mutants of Arabidopsis express PR-2 and PR-5 and accumulate high levels of camalexin after pathogen inoculation. Plant Cell 11, 1393-1404.
DOI PMID |
| [16] |
Raskin I, Turner IM, Melander WR (1989). Regulation of heat production in the inflorescences of an Arum lily by endogenous salicylic acid. Proc Natl Acad Sci USA 86, 2214-2218.
PMID |
| [17] |
Schmelz EA, Engelberth J, Alborn HT, O’Donnell P, Sammons M, Toshima H, Tumlinson JH (2003). Simultaneous analysis of phytohormones, phytotoxins, and volatile organic compounds in plants. Proc Natl Acad Sci USA 100, 10552-10557.
DOI PMID |
| [18] | Snyder LR, Kirkland JJ, Dolan JW (陈小明, 唐雅妍译) (2012). 现代液相色谱技术导论(第3版). 北京: 人民卫生出版社. pp. 126-131. |
| [19] | Song JT (2006). Induction of a salicylic acid glucosyltransferase, AtSGT1, is an early disease response in Arabidopsis thaliana. Mol Cells 22, 233-238. |
| [20] |
Yan SP, Dong XN (2014). Perception of the plant immune signal salicylic acid. Curr Opin Plant Biol 20, 64-68.
DOI PMID |
| [21] | Ye C, Yao LB, Jin Y, Gao R, Tan Q, Li XY, Zhang YJ, Chen XF, Ma BJ, Zhang W, Zhang KW (2025). Establishment and application of a high-throughput screening method for salicylic acid metabolic mutants in rice. Chin Bull Bot 60, 586-596. (in Chinese) |
|
叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟 (2025). 水稻水杨酸代谢突变体高通量筛选方法的建立与应用. 植物学报 60, 586-596.
DOI |
|
| [22] | Yu XD, Cui XY, Wu C, Shi SX, Yan SP (2022). Salicylic acid inhibits gibberellin signaling through receptor interactions. Mol Plant 15, 1759-1771. |
| [23] |
Yu XD, Xu YR, Yan SP (2021). Salicylic acid and ethylene coordinately promote leaf senescence. J Integr Plant Biol 63, 823-827.
DOI |
| [24] | Zhang YJ, Zhao L, Zhao JZ, Li YJ, Wang JB, Guo R, Gan SS, Liu CJ, Zhang KW (2017). S5H/DMR6 encodes a salicylic acid 5-hydroxylase that fine-tunes salicylic acid homeostasis. Plant Physiol 175, 1082-1093. |
| [1] | 江亚楠, 徐雨青, 魏毅铤, 陈钧, 张容菀, 赵蓓蓓, 林宇翔, 饶玉春. 水稻抗病调控机制研究进展[J]. 植物学报, 2025, 60(5): 734-748. |
| [2] | 刘德水, 岳宁, 刘玉乐. 植物免疫机制新突破[J]. 植物学报, 2025, 60(5): 669-678. |
| [3] | 粟思琳, 唐先宇, 陈祎, 王婷, 夏石头. 植物系统获得性抗性的转录调控[J]. 植物学报, 2025, 60(5): 722-733. |
| [4] | 吴玉俊, 李英菊, 罗巧玉, 马永贵. 光控植物免疫: 从光信号通路到免疫应答的调控网络[J]. 植物学报, 2025, 60(5): 786-803. |
| [5] | 肖银燕, 于华, 万里. 植物免疫研究: 机制突破和应用创新[J]. 植物学报, 2025, 60(5): 693-703. |
| [6] | 朱孝波, 王立印, 陈学伟. 水杨酸介导的植物免疫反应: 从代谢、感知到免疫激活[J]. 植物学报, 2025, 60(5): 679-692. |
| [7] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 586-596. |
| [8] | 杨莉, 曲茜彤, 陈子航, 邹婷婷, 王全华, 王小丽. 菠菜AT-hook基因家族鉴定与表达谱分析[J]. 植物学报, 2025, 60(3): 377-392. |
| [9] | 李园, 范开建, 安泰, 李聪, 蒋俊霞, 牛皓, 曾伟伟, 衡燕芳, 李虎, 付俊杰, 李慧慧, 黎亮. 玉米自然群体自交系农艺性状的多环境全基因组预测初探[J]. 植物学报, 2024, 59(6): 1041-1053. |
| [10] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [11] | 陈佳欣, 梅浩, 黄彩翔, 梁宗原, 全依桐, 李东鹏, 布威麦尔耶姆·赛麦提, 李欣欣, 廖红. 利用转基因毛状根高效培育大豆嵌合植株的方法[J]. 植物学报, 2024, 59(1): 89-98. |
| [12] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| [13] | 田传玉, 方妍力, 沈晴, 王宏杰, 陈析丰, 郭威, 赵开军, 王春连, 纪志远. 2019-2021年我国南方稻区白叶枯病菌的毒力与遗传多样性调查研究[J]. 植物学报, 2023, 58(5): 743-749. |
| [14] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [15] | 袁民航, 辛秀芳. 烽火狼烟: 水杨酸甲酯介导的植物间通讯和气传性免疫的机制解析[J]. 植物学报, 2023, 58(5): 682-686. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
