

植物学报 ›› 2024, Vol. 59 ›› Issue (1): 89-98.DOI: 10.11983/CBB23021 cstr: 32102.14.CBB23021
陈佳欣, 梅浩, 黄彩翔, 梁宗原, 全依桐, 李东鹏, 布威麦尔耶姆·赛麦提, 李欣欣*( ), 廖红
), 廖红
收稿日期:2023-02-20
接受日期:2023-05-31
出版日期:2024-01-10
发布日期:2024-01-10
通讯作者:
*E-mail: 基金资助:
Jiaxin Chen, Hao Mei, Caixiang Huang, Zongyuan Liang, Yitong Quan, Dongpeng Li, Buweimaieryemu·Saimaiti , Xinxin Li*( ), Hong Liao
), Hong Liao
Received:2023-02-20
Accepted:2023-05-31
Online:2024-01-10
Published:2024-01-10
Contact:
*E-mail: 摘要: 建立高效的大豆(Glycine max)转基因毛状根嵌合植株体系对于推动大豆功能基因组学研究具有重要意义。该研究利用3种大豆基因型材料比较了不同共培养条件下毛状根诱导率及成活率。结果显示, 用发根农杆菌(Agrobacterium rhizogenes)侵染外植体并在黑暗条件下共培养1天是诱导毛状根形成的有效策略。研究发现清除下胚轴处不定根可显著增加毛状根的数目并促进根系生长, 进而提高转基因毛状根的阳性率。毛状根诱导14天接种根瘤菌, 可增强生长初期转基因毛状根与根瘤菌的接触, 从而提高大豆的结瘤效率。该研究成功建立了一种高效培育大豆转基因毛状根嵌合植株的方法, 可广泛应用于大豆基因功能研究。
陈佳欣, 梅浩, 黄彩翔, 梁宗原, 全依桐, 李东鹏, 布威麦尔耶姆·赛麦提, 李欣欣, 廖红. 利用转基因毛状根高效培育大豆嵌合植株的方法. 植物学报, 2024, 59(1): 89-98.
Jiaxin Chen, Hao Mei, Caixiang Huang, Zongyuan Liang, Yitong Quan, Dongpeng Li, Buweimaieryemu·Saimaiti , Xinxin Li, Hong Liao. A Highly Efficient Method to Generate Chimeric Soybean Plant with Transgenic Hairy Roots. Chinese Bulletin of Botany, 2024, 59(1): 89-98.
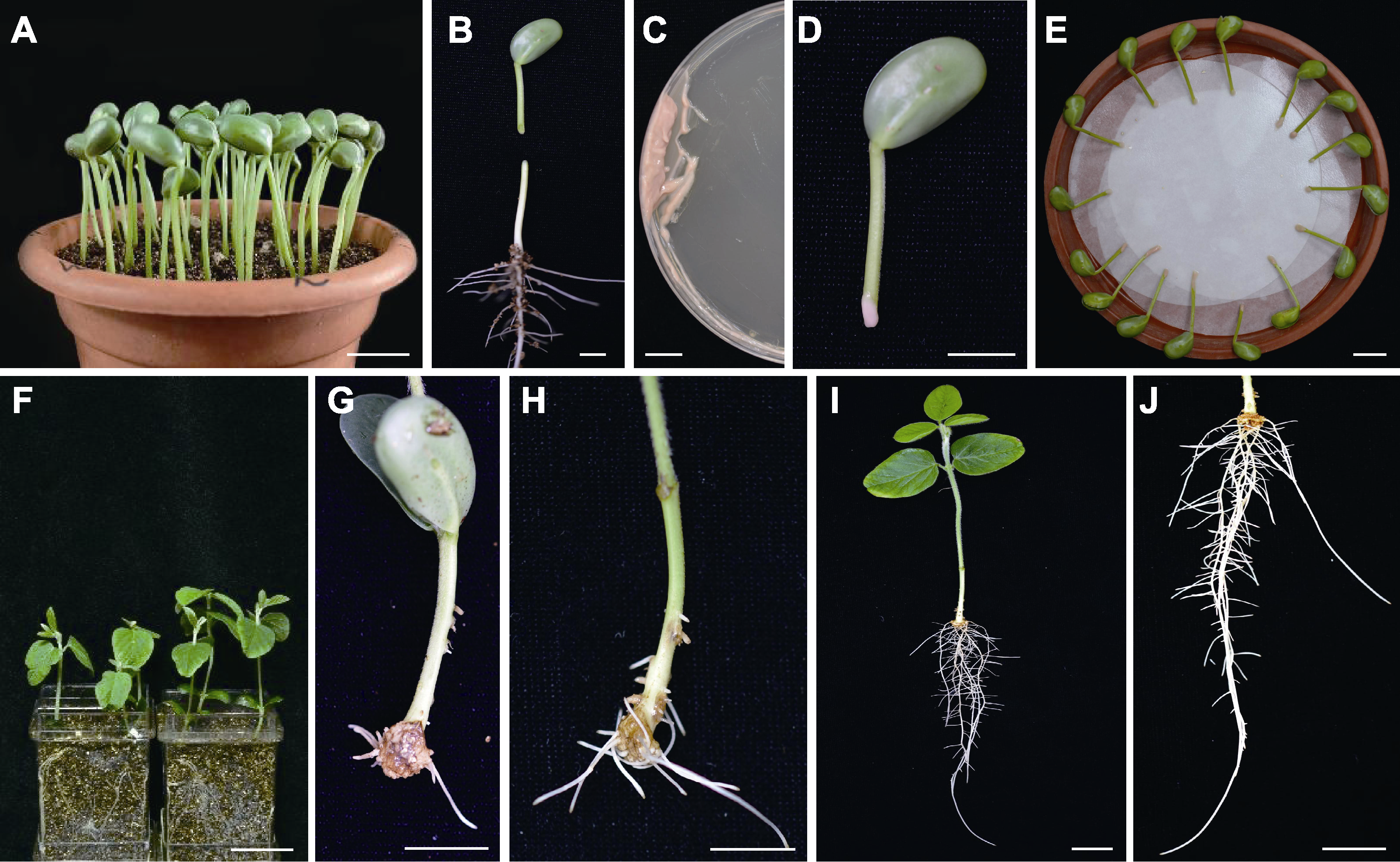
图1 发根农杆菌诱导大豆下胚轴转基因毛状根产生嵌合植株流程 (A) 萌发4天后的大豆幼苗(bar=2 cm); (B) 在下胚轴处以45°角切开形成伤口(bar=1 cm); (C) 从培养皿中收集发根农杆菌(bar=1 cm); (D) 在伤口处涂菌(bar=1 cm); (E) 共培养(bar=1 cm); (F) 将外植体移入蛭石中(bar=5 cm); (G) 培养10天后诱导出愈伤组织(bar=1 cm); (H) 诱导14天后毛状根形成(bar=1 cm); (I) 毛状根生长20天后的状态(bar=2 cm); (J) I图的局部放大(bar=2 cm)
Figure 1 Procedure of generating chimeric soybean plants with Agrobacterium rhizogenes induced hypocotyl transgenic hairy roots (A) Soybean seedlings at 4 d post germination (bar=2 cm); (B) Hypocotyl was cut to form wound site with an angle of 45° (bar=1 cm); (C) A. rhizogenes were collected in petri dishes (bar=1 cm); (D) Apply bacteria to the wound (bar=1 cm); (E) Co-cultivation (bar=1 cm); (F) Inoculated explants were planted into vermiculite (bar=5 cm); (G) Callus came out after 10 d of cultivation (bar=1 cm); (H) Hairy roots emerged at 14 d post induction (bar=1 cm); (I) Growth performance of hairy roots after 20 d of growth (bar=2 cm); (J) Partial enlarged view of image I (bar=2 cm)
| Treatments | Genotype | Co-cultivation time (d) | Efficiency of induction (%) | Number of chimeric plant with HR | Surviving rate (%) | Number of surviving plants | Total number of plants |
|---|---|---|---|---|---|---|---|
| Dark | Ws82 | 0 | 87.0 | 20 | 76.7 | 23 | 30 |
| 1 | 96.2 | 25 | 86.7 | 26 | 30 | ||
| 3 | 89.7 | 26 | 96.7 | 29 | 30 | ||
| HN66 | 0 | 87.5 | 21 | 80.0 | 24 | 30 | |
| 1 | 95.7 | 22 | 76.7 | 23 | 30 | ||
| 3 | 83.3 | 15 | 60.0 | 18 | 30 | ||
| BX10 | 0 | 93.3 | 14 | 75.0 | 15 | 20 | |
| 1 | 94.7 | 18 | 95.0 | 19 | 20 | ||
| 3 | 82.4 | 14 | 85.0 | 17 | 20 | ||
| Light (100 µmol∙m-2∙s-1) | Ws82 | 0 | 82.4 | 21 | 76.7 | 23 | 30 |
| 1 | 91.3 | 21 | 76.7 | 23 | 30 | ||
| 3 | 68.2 | 15 | 73.3 | 22 | 30 | ||
| HN66 | 0 | 90.0 | 18 | 66.7 | 20 | 30 | |
| 1 | 83.3 | 20 | 80.0 | 24 | 30 | ||
| 3 | 90.5 | 19 | 70.0 | 21 | 30 | ||
| BX10 | 0 | 86.7 | 13 | 75.0 | 15 | 20 | |
| 1 | 83.3 | 15 | 90.0 | 18 | 20 | ||
| 3 | 80.0 | 12 | 75.0 | 15 | 20 |
表1 不同基因型大豆在光/暗条件下毛状根(HR)形成的对比
Table 1 Comparison of hairy roots (HR) generated from different soybean genotypes under light/dark conditions
| Treatments | Genotype | Co-cultivation time (d) | Efficiency of induction (%) | Number of chimeric plant with HR | Surviving rate (%) | Number of surviving plants | Total number of plants |
|---|---|---|---|---|---|---|---|
| Dark | Ws82 | 0 | 87.0 | 20 | 76.7 | 23 | 30 |
| 1 | 96.2 | 25 | 86.7 | 26 | 30 | ||
| 3 | 89.7 | 26 | 96.7 | 29 | 30 | ||
| HN66 | 0 | 87.5 | 21 | 80.0 | 24 | 30 | |
| 1 | 95.7 | 22 | 76.7 | 23 | 30 | ||
| 3 | 83.3 | 15 | 60.0 | 18 | 30 | ||
| BX10 | 0 | 93.3 | 14 | 75.0 | 15 | 20 | |
| 1 | 94.7 | 18 | 95.0 | 19 | 20 | ||
| 3 | 82.4 | 14 | 85.0 | 17 | 20 | ||
| Light (100 µmol∙m-2∙s-1) | Ws82 | 0 | 82.4 | 21 | 76.7 | 23 | 30 |
| 1 | 91.3 | 21 | 76.7 | 23 | 30 | ||
| 3 | 68.2 | 15 | 73.3 | 22 | 30 | ||
| HN66 | 0 | 90.0 | 18 | 66.7 | 20 | 30 | |
| 1 | 83.3 | 20 | 80.0 | 24 | 30 | ||
| 3 | 90.5 | 19 | 70.0 | 21 | 30 | ||
| BX10 | 0 | 86.7 | 13 | 75.0 | 15 | 20 | |
| 1 | 83.3 | 15 | 90.0 | 18 | 20 | ||
| 3 | 80.0 | 12 | 75.0 | 15 | 20 |
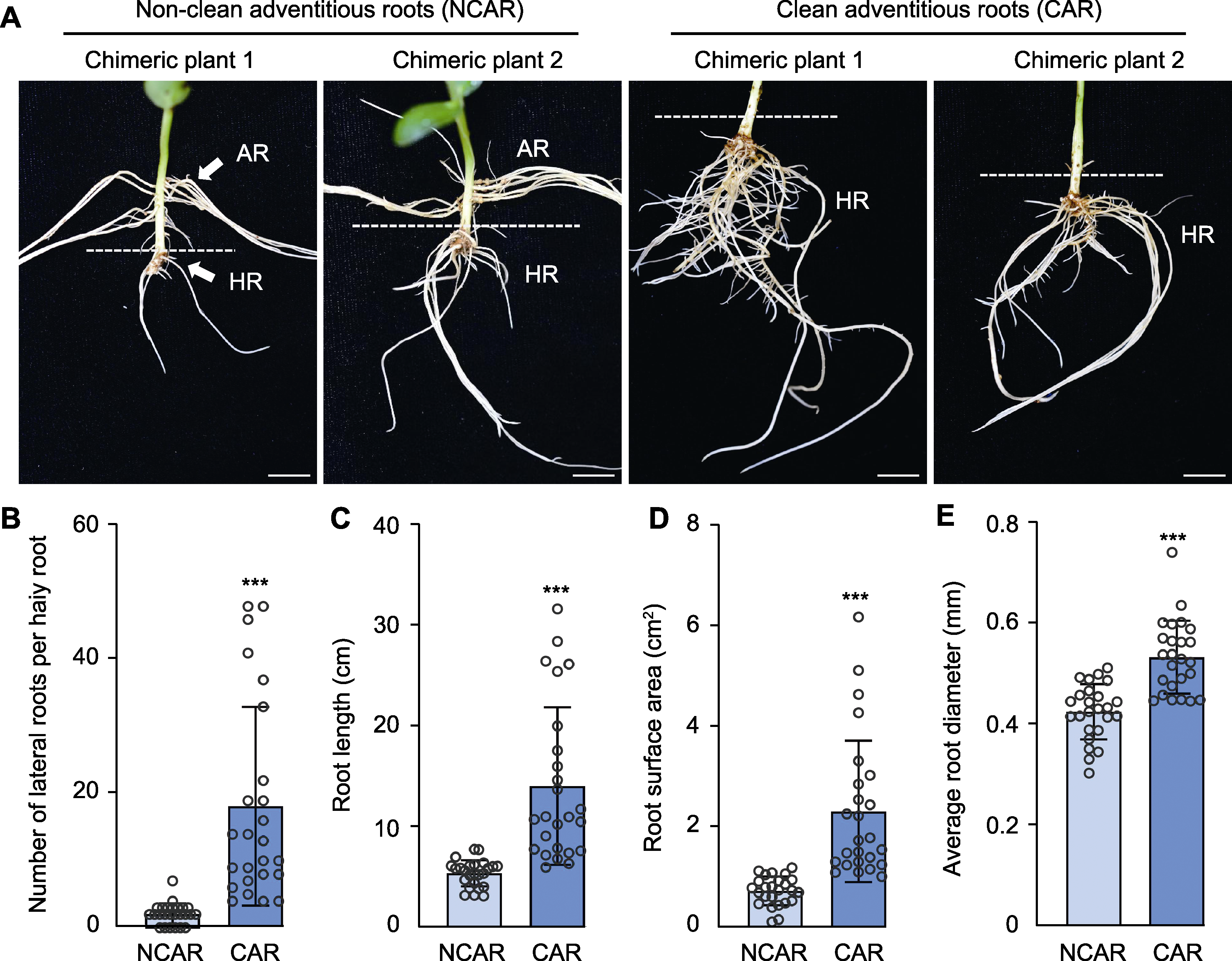
图2 清理下胚轴处的不定根对毛状根诱导的影响 (A) 不清理与清理不定根条件下毛状根生长对比(bars=1 cm); (B) 每条毛状根生长的侧根数目; (C) 根长; (D) 根表面积; (E) 根平均直径。数据为25个毛状根的平均值±标准差。***表示在0.001水平差异显著。AR: 不定根; HR: 毛状根
Figure 2 Effect of cleaning adventitious root at hypocotyl on induction of hairy roots (A) Comparison of hairy roots between non-clean and clean adventitious root condition (bars=1 cm); (B) Number of lateral roots per hairy root; (C) Root length; (D) Root surface area; (E) Average root diameter. Data are means±SD from 25 hairy roots. *** indicate significant differences at 0.001 level. AR: Adventitious root; HR: Hairy root

图3 清理下胚轴处的不定根对毛状根阳性率的影响 (A) 不清理与清理不定根条件下毛状根GUS染色, 椭圆形及箭头示非转基因毛状根(bars=1 cm); (B) 每个外植体产生的毛状根总数; (C) 每个外植体阳性毛状根数目; (D) 阳性率。数据为14个外植体的平均值±标准差。*和***分别表示在0.05和0.001水平差异显著。
Figure 3 Effect of cleaning adventitious root at hypocotyl on positive rate of transgenic hairy roots (A) GUS staining of hairy roots under non-clean and clean adventitious roots conditions, the oval and arrows indicate non-transgenic hairy roots (bars=1 cm); (B) Total number of hairy roots generated from each explant; (C) Number of transgenic hairy roots per explant; (D) Efficiency of transgenic hairy roots. Data are means±SD from 14 explants. * and *** indicate significant differences at 0.05 and 0.001 levels, respectively.

图4 清理不定根对毛状根结瘤的影响 (A), (D) 不清理(A)与清理(D)不定根的大豆嵌合植株(bars= 2 cm); (B), (E) 分别为图A和D方框中的局部放大(bars=1 cm); (C), (F) 毛状根GUS染色(bars=1 cm)。红色箭头指示毛状根上的根瘤。AR同图2。
Figure 4 Effect of cleaning adventitious root on nodulation of hairy roots (A), (D) Soybean chimeric plants without (A) or with (D) cleaning adventitious root (bars=2 cm); (B), (E) Partial enlarged view of image A and D in box (bars=1 cm); (C), (F) GUS staining of hairy roots (bars=1 cm). The red arrows indicate nodules on hairy roots. AR is the same as shown in Figure 2.
| Treatments | Chimeric plants | Hairy roots | Nodule number |
|---|---|---|---|
| NCAR | 1st | 1st | 3 |
| 2nd | 4 | ||
| 3rd | 2 | ||
| 2nd | 1st | 2 | |
| 2nd | 1 | ||
| 3rd | 3 | ||
| 3rd | 1st | 4 | |
| 2nd | 2 | ||
| 3rd | 1 | ||
| Average | 2.4 | ||
| CAR | 1st | 1st | 7 |
| 2nd | 6 | ||
| 3rd | 8 | ||
| 2nd | 1st | 10 | |
| 2nd | 8 | ||
| 3rd | 6 | ||
| 3rd | 1st | 5 | |
| 2nd | 7 | ||
| 3rd | 9 | ||
| Average | 7.3*** | ||
表2 不清理与清理不定根对转基因毛状根根瘤数目的影响
Table 2 Effect of non-clean (NCAR) and clean (CAR) adventitious root on nodule number in transgenic hairy roots
| Treatments | Chimeric plants | Hairy roots | Nodule number |
|---|---|---|---|
| NCAR | 1st | 1st | 3 |
| 2nd | 4 | ||
| 3rd | 2 | ||
| 2nd | 1st | 2 | |
| 2nd | 1 | ||
| 3rd | 3 | ||
| 3rd | 1st | 4 | |
| 2nd | 2 | ||
| 3rd | 1 | ||
| Average | 2.4 | ||
| CAR | 1st | 1st | 7 |
| 2nd | 6 | ||
| 3rd | 8 | ||
| 2nd | 1st | 10 | |
| 2nd | 8 | ||
| 3rd | 6 | ||
| 3rd | 1st | 5 | |
| 2nd | 7 | ||
| 3rd | 9 | ||
| Average | 7.3*** | ||
| [1] | 程凤娴, 曹桂芹, 王秀荣, 赵静, 严小龙, 廖红 (2008). 华南酸性低磷土壤中大豆根瘤菌高效株系的发现及应用. 科学通报 53, 2903-2910. |
| [2] | 杜梦柯, 连文婷, 张晓, 李欣欣 (2021). 氮处理对大豆根瘤固氮能力及GmLbs基因表达的影响. 植物学报 56, 391-403. |
| [3] | 李锦锦, 王昉, 张万科, 文自翔, 李海朝, 袁道华, 李金英, 张辉, 杨青华, 卢为国 (2012). 发根农杆菌介导不同基因型大豆转化效率的筛选. 河南农业科学 41(5), 37-41. |
| [4] | 李欣欣, 赵静, 廖红 (2011). 大豆毛状根-VA菌根真菌双重培养体系的建立. 植物生理学报 47, 475-480. |
| [5] | 栾健, 张斌, 胡钰 (2022). 中国大豆产业的发展态势、政策演进与趋势展望. 农业展望 18(8), 35-41. |
| [6] | 邱丽娟, 王昌陵, 周国安, 陈受宜, 常汝镇 (2007). 大豆分子育种研究进展. 中国农业科学 40, 2418-2436. |
| [7] | 田志喜, 刘宝辉, 杨艳萍, 李明, 姚远, 任小波, 薛勇彪 (2018). 我国大豆分子设计育种成果与展望. 中国科学院院刊 33, 915-922. |
| [8] |
Bahramnejad B, Naji M, Bose R, Jha S (2019). A critical review on use of Agrobacterium rhizogenes and their associated binary vectors for plant transformation. Biotechnol Adv 37, 107405.
DOI URL |
| [9] |
Cheng YY, Wang XL, Cao L, Ji J, Liu TF, Duan KX (2021). Highly efficient Agrobacterium rhizogenes-mediated hairy root transformation for gene functional and gene editing analysis in soybean. Plant Methods 17, 73.
DOI |
| [10] |
Cho HJ, Farrand SK, Noel GR, Widholm JM (2000). High-efficiency induction of soybean hairy roots and propagation of the soybean cyst nematode. Planta 210, 195-204.
DOI PMID |
| [11] |
Fan YL, Zhang XH, Zhong LJ, Wang XY, Jin LS, Lyu SH (2020). One-step generation of composite soybean plants with transgenic roots by Agrobacterium rhizogenes-mediated transformation. BMC Plant Biol 20, 208.
DOI |
| [12] |
Gantait S, Mukherjee E (2021). Hairy root culture technology: applications, constraints and prospect. Appl Microbiol Bio- technol 105, 35-53.
DOI |
| [13] |
Gomes C, Dupas A, Pagano A, Grima-Pettenati J, Paiva JAP (2019). Hairy root transformation: a useful tool to explore gene function and expression in Salix spp. recalcitrant to transformation. Front Plant Sci 10, 1427.
DOI URL |
| [14] |
Guo WB, Zhao J, Li XX, Qin L, Yan XL, Liao H (2011). A soybean β-expansin gene GmEXPB2 intrinsically involved in root system architecture responses to abiotic stresses. Plant J 66, 541-552.
DOI URL |
| [15] |
Herridge DF, Peoples MB, Boddey RM (2008). Global inputs of biological nitrogen fixation in agricultural systems. Plant Soil 311, 1-18.
DOI URL |
| [16] |
Huang PH, Lu MY, Li XB, Sun HY, Cheng ZY, Miao YC, Fu YF, Zhang XM (2022). An efficient Agrobacterium rhizogenes-mediated hairy root transformation method in a soybean root biology study. Int J Mol Sci 23, 12261.
DOI URL |
| [17] | Hungria M, Mendes IC (2015). Nitrogen fixation with soybean:the perfect symbiosis? In: de Bruijn FJ, ed. Biological Nitrogen Fixation. New Jersey: Wiley. pp. 1005-1019. |
| [18] |
Ke XL, Xiao H, Peng YQ, Wang J, Lv Q, Wang XL (2022). Phosphoenolpyruvate reallocation links nitrogen fixation rates to root nodule energy state. Science 378, 971-977.
DOI PMID |
| [19] |
Kereszt A, Li DX, Indrasumunar A, Nguyen CDT, Nontachaiyapoom S, Kinkema M, Gresshoff PM (2007). Agrobacterium rhizogenes-mediated transformation of soybean to study root biology. Nat Protoc 2, 948-952.
DOI PMID |
| [20] |
Kiryushkin AS, Ilina EL, Guseva ED, Pawlowski K, Demchenko KN (2021). Hairy CRISPR: genome editing in plants using hairy root transformation. Plants (Basel) 11, 51.
DOI URL |
| [21] |
Li R, Chen HF, Yang ZL, Yuan SL, Zhou XA (2020). Research status of soybean symbiosis nitrogen fixation. Oil Crop Sci 5, 6-10.
DOI URL |
| [22] | Li XX, Zhao J, Tan ZY, Zeng RS, Liao H (2015). GmEXPB2, a cell wall β-expansin, affects soybean nodulation through modifying root architecture and promoting nodule formation and development. Plant Physiol 169, 2640-2653. |
| [23] |
Li XX, Zheng JK, Yang YQ, Liao H (2018). INCREASING NODULE SIZE1 expression is required for normal rhizobial symbiosis and nodule development. Plant Physiol 178, 1233-1248.
DOI URL |
| [24] |
Liu SL, Zhang M, Feng F, Tian ZX (2020). Toward a ‘‘green revolution’’ for soybean. Mol Plant 13, 688-697.
DOI URL |
| [25] |
Morey KJ, Peebles CAM (2022). Hairy roots: an untapped potential for production of plant products. Front Plant Sci 13, 937095.
DOI URL |
| [26] |
Olhoft PM, Flagel LE, Donovan CM, Somers DA (2003). Efficient soybean transformation using hygromycin B selection in the cotyledonary-node method. Planta 216, 723-735.
DOI PMID |
| [27] |
Pistelli L, Giovannini A, Ruffoni B, Bertoli A, Pistelli L (2010). Hairy root cultures for secondary metabolites production. Adv Exp Med Biol 698, 167-184.
PMID |
| [28] |
Qin L, Zhao J, Tian J, Chen LY, Sun ZA, Guo YX, Lu X, Gu M, Xu GH, Liao H (2012). The high-affinity phosphate transporter GmPT5 regulates phosphate transport to nodules and nodulation in soybean. Plant Physiol 159, 1634-1643.
DOI PMID |
| [29] |
Wang T, Guo J, Peng YQ, Lyu X, Liu B, Sun SY, Wang XL (2021). Light-induced mobile factors from shoots regulate rhizobium-triggered soybean root nodulation. Science 374, 65-71.
DOI PMID |
| [30] |
Wang XR, Wang YX, Tian J, Lim BL, Yan XL, Liao H (2009). Overexpressing AtPAP15enhances phosphorus efficiency in soybean. Plant Physiol 151, 233-240.
DOI URL |
| [31] |
Xu HY, Li YJ, Zhang KF, Li MJ, Fu SY, Tian YZ, Qin TF, Li XX, Zhong YJ, Liao H (2021). miR169c-NFYA-C-ENOD40 modulates nitrogen inhibitory effects in soybean nodulation. New Phytol 229, 3377-3392.
DOI URL |
| [32] |
Yang ZJ, Gao Z, Zhou HW, He Y, Liu YX, Lai YL, Zheng JK, Li XX, Liao H (2021). GmPTF1 modifies root architecture responses to phosphate starvation primarily through regulating GmEXPB2 expression in soybean. Plant J 107, 525-543.
DOI URL |
| [33] |
Zhang FL, Chen C, Ge HL, Liu JM, Luo YL, Liu K, Chen L, Xu KD, Zhang Y, Tan GX, Li CW (2014). Efficient soybean regeneration and Agrobacterium-mediated transformation using a whole cotyledonary node as an explant. Biotechnol Appl Biochem 61, 620-625.
DOI PMID |
| [1] | 郑立媛, 徐茜竹, 尹嘉淇, 孙小雯, 王艳. 沈阳城郊近河农田退耕地野大豆群落生态位和种间联结研究[J]. 植物生态学报, 2025, 49(濒危植物的保护与恢复): 1-. |
| [2] | 干靓 刘巷序 鲁雪茗 岳星. 全球生物多样性热点地区大城市的保护政策与优化方向[J]. 生物多样性, 2025, 33(5): 24529-. |
| [3] | 曾文丹, 严华兵, 吴正丹, 尚小红, 曹升, 陆柳英, 肖亮, 施平丽, 程冬, 龙紫媛, 李婕宇. 发根农杆菌介导的野葛毛状根遗传转化体系[J]. 植物学报, 2025, 60(3): 425-434. |
| [4] | 曹婕, 卢秋连, 翟健平, 刘宝辉, 方超, 李世晨, 苏彤. 大豆TPS基因家族在盐胁迫下的表达变化及单倍型选择规律分析(长英文摘要)[J]. 植物学报, 2025, 60(2): 172-185. |
| [5] | 王林, 尹梓杨, 黄慧芳, 王静. 基于Carter-Morley Jones蛋形模型的参数估计新方法[J]. 生物多样性, 2025, 33(1): 24203-. |
| [6] | 李园, 范开建, 安泰, 李聪, 蒋俊霞, 牛皓, 曾伟伟, 衡燕芳, 李虎, 付俊杰, 李慧慧, 黎亮. 玉米自然群体自交系农艺性状的多环境全基因组预测初探[J]. 植物学报, 2024, 59(6): 1041-1053. |
| [7] | 秦涛, 崔荣赫, 宋蕊, 富丽莎. 我国野生动物肇事公众责任保险: 发展模式、现实困境与优化策略[J]. 生物多样性, 2024, 32(5): 23431-. |
| [8] | 崔国发. 关于自然保护地整合优化工作中几个关键问题的讨论与建议[J]. 生物多样性, 2023, 31(9): 22447-. |
| [9] | 陈天傲, 李想. 我国国家公园管理体系优化路径: 以中央层面为例[J]. 生物多样性, 2023, 31(3): 22485-. |
| [10] | 宋蕊, 邓晶, 秦涛. 野生动物肇事公众责任保险发展困境与优化路径[J]. 生物多样性, 2022, 30(7): 22291-. |
| [11] | 王韫慧, 王一帆, 蔺佳雨, 李金红, 姚士恩, 冯湘池, 曹振林, 王俊, 李美娜. 植物驱动蛋白: 从微管阵列到生理活动调控[J]. 植物学报, 2022, 57(3): 358-374. |
| [12] | 胡惠玲, 姚致远, 高世斌, 朱波. 紫色土线虫对长期不同施肥措施的响应[J]. 生物多样性, 2022, 30(12): 22189-. |
| [13] | 杜梦柯, 连文婷, 张晓, 李欣欣. 氮处理对大豆根瘤固氮能力及GmLbs基因表达的影响[J]. 植物学报, 2021, 56(4): 391-403. |
| [14] | 夏正俊, 李玉卓, 朱金龙, 吴红艳, 徐坤, 翟红. 快速、无损大豆种子连续取样技术及其DNA制备[J]. 植物学报, 2021, 56(1): 56-61. |
| [15] | 王研, 贾博为, 孙明哲, 孙晓丽. 野生大豆耐逆分子调控机制研究进展[J]. 植物学报, 2021, 56(1): 104-115. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||