

植物学报 ›› 2025, Vol. 60 ›› Issue (3): 425-434.DOI: 10.11983/CBB24092 cstr: 32102.14.CBB24092
曾文丹1, 严华兵1,*( ), 吴正丹2, 尚小红1, 曹升1, 陆柳英1, 肖亮1, 施平丽1, 程冬1, 龙紫媛1, 李婕宇1
), 吴正丹2, 尚小红1, 曹升1, 陆柳英1, 肖亮1, 施平丽1, 程冬1, 龙紫媛1, 李婕宇1
收稿日期:2024-06-11
接受日期:2024-12-26
出版日期:2025-05-10
发布日期:2024-12-27
通讯作者:
*严华兵, 男, 1979年5月生, 博士。现任广西农业科学院经济作物研究所所长, 兼任广西薯类创新团队首席专家、国家食药同源产业科技创新联盟葛根专委会主任委员, 荣获第八批广西“十百千”知识产权领军人才。其科研团队主要从事木薯、葛根等特色薯类植物资源收集、生物技术育种、良种繁育、高效栽培以及资源开发利用等研究工作。先后主持IAEA国际合作项目、国家重点研发计划子课题、国家自然科学基金、自治区主席科技资金等农业科研项目20余项。科研成果获得广西科技进步二等奖1项、广西科技进步三等奖2项。带领团队育成“桂木薯6号”等木薯新品种5个。以第一完成人获得国家发明专利10余项。以第一作者或通讯作者在Horticulture Research、DNA Research和植物生理学报等专业刊物上发表论文90余篇, 其中国际刊物20余篇。主编专著2部, 作为副主编合编著作2部。E-mail: h.b.yan@hotmail.com
基金资助:
Zeng Wendan1, Yan Huabing1,*( ), Wu Zhengdan2, Shang Xiaohong1, Cao Sheng1, Lu Liuying1, Xiao Liang1, Shi Pingli1, Cheng Dong1, Long Ziyuan1, Li Jieyu1
), Wu Zhengdan2, Shang Xiaohong1, Cao Sheng1, Lu Liuying1, Xiao Liang1, Shi Pingli1, Cheng Dong1, Long Ziyuan1, Li Jieyu1
Received:2024-06-11
Accepted:2024-12-26
Online:2025-05-10
Published:2024-12-27
Contact:
*E-mail: h.b.yan@hotmail.com
摘要: 为建立高效的野葛(Pueraria lobata)毛状根遗传转化体系, 以野葛组培苗为外植体, 探讨不同因素对野葛毛状根遗传转化效率的影响。结果表明, 基因型是建立野葛毛状根高效遗传转化体系的主要限制因子; 发根农杆菌K599为最适宜的菌株; 以培养5-13代组培苗继代培养8天、第1-2节位刚展开的幼嫩叶片为最佳外植体材料, 预培养3天, 菌液侵染15分钟, 毛状根诱导率最高, 可达22.4%。野葛毛状根继代增殖的最佳培养基类型为固体培养基, 其毛状根鲜重是液体培养基中毛状根鲜重的75倍; PCR检测和荧光显微观察结果显示, GFP及rolB基因在野葛毛状根基因组中稳定表达, 共转化率为80%。研究初步建立了发根农杆菌介导的野葛毛状根遗传转化体系, 旨在为野葛基因功能研究奠定基础。
曾文丹, 严华兵, 吴正丹, 尚小红, 曹升, 陆柳英, 肖亮, 施平丽, 程冬, 龙紫媛, 李婕宇. 发根农杆菌介导的野葛毛状根遗传转化体系. 植物学报, 2025, 60(3): 425-434.
Zeng Wendan, Yan Huabing, Wu Zhengdan, Shang Xiaohong, Cao Sheng, Lu Liuying, Xiao Liang, Shi Pingli, Cheng Dong, Long Ziyuan, Li Jieyu. Agrobacterium rhizogenes-mediated Transformation System of Pueraria lobata Hairy Roots. Chinese Bulletin of Botany, 2025, 60(3): 425-434.
| Primer name | Primer sequence (5′−3′) |
|---|---|
| GFP | F: CAGTGCTTCAGCCGCTAC R: TTCTCGTTGGGGTCTTTG |
| rolB | F: AAGTGCTGAGGAACAATC R: CAAGTGAATGAACAAGGAAC |
| virG | F: CCTTGGGCGTCGTCATAC R: TCGTCCTCGGTCGTTTCC |
表1 引物名称及序列
Table 1 Primer names and sequences
| Primer name | Primer sequence (5′−3′) |
|---|---|
| GFP | F: CAGTGCTTCAGCCGCTAC R: TTCTCGTTGGGGTCTTTG |
| rolB | F: AAGTGCTGAGGAACAATC R: CAAGTGAATGAACAAGGAAC |
| virG | F: CCTTGGGCGTCGTCATAC R: TCGTCCTCGGTCGTTTCC |
| Genotype | Hairy root frequency of different explants (%) | ||||
|---|---|---|---|---|---|
| Unfolding im- mature leaves | Mature leaves | Stems | Petioles | Shoot me- ristems | |
| YG-18 | 0 | 0 | 0 | 0 | 0 |
| YG-19 | 10.2±0.9 | 6.1±0.1 | 0 | 5.2±1.2 | 0 |
| YG-50 | 0 | 0 | 0 | 0 | 0 |
| YG-51 | 1.9±0.6 | 0 | 0 | 3.5±0.4 | 0 |
| YG-81 | 2.1±0.3 | 1.1±0.4 | 0 | 1.9±0.1 | 0 |
表2 不同基因型及不同外植体对野葛毛状根诱导效率的影响
Table 2 Effects of different genotypes and different explants on the induction efficiency of Pueraria lobata hairy roots
| Genotype | Hairy root frequency of different explants (%) | ||||
|---|---|---|---|---|---|
| Unfolding im- mature leaves | Mature leaves | Stems | Petioles | Shoot me- ristems | |
| YG-18 | 0 | 0 | 0 | 0 | 0 |
| YG-19 | 10.2±0.9 | 6.1±0.1 | 0 | 5.2±1.2 | 0 |
| YG-50 | 0 | 0 | 0 | 0 | 0 |
| YG-51 | 1.9±0.6 | 0 | 0 | 3.5±0.4 | 0 |
| YG-81 | 2.1±0.3 | 1.1±0.4 | 0 | 1.9±0.1 | 0 |
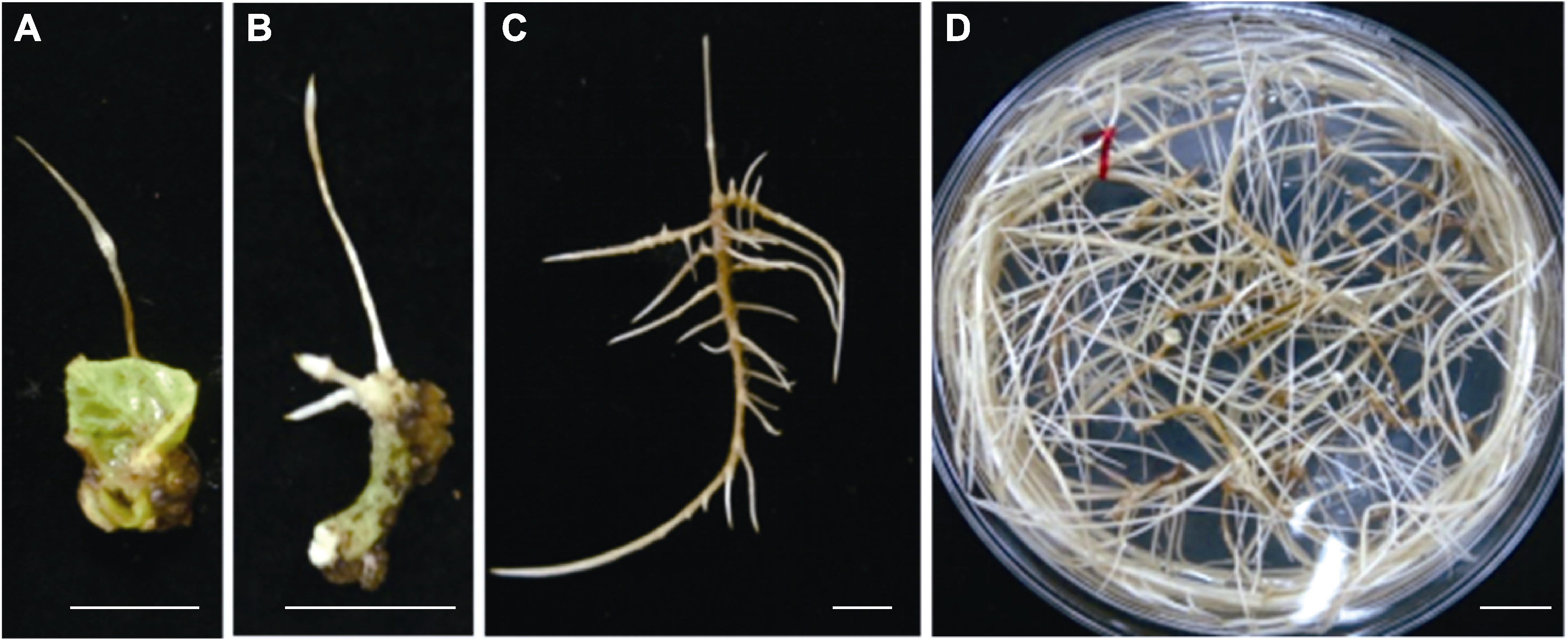
图2 野葛毛状根诱导及继代培养 (A) 幼嫩叶片诱导产生的毛状根; (B) 叶柄诱导产生的毛状根; (C), (D) 继代培养的毛状根。Bars=1 cm
Figure 2 Induction and subculture of Pueraria lobata hairy roots (A) Hairy roots from immature leaf explants; (B) Hairy roots from petiole explants; (C), (D) Subcultured hairy roots. Bars=1 cm
| Agrobacterium rhizogenes | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| CK | - | 0 c | 0 c |
| K599 | 14 | 12.1±0.6 a | 2.2±0.1 a |
| R1601 | 16 | 8.3±0.5 b | 1.1±0.1 b |
| ATCC15834 | - | 0 c | 0 c |
| Ar4 | - | 0 c | 0 c |
表3 不同发根农杆菌对野葛毛状根诱导效率的影响
Table 3 Effects of different Agrobacterium rhizogenes strains on the induction efficiency in Pueraria lobata hairy roots
| Agrobacterium rhizogenes | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| CK | - | 0 c | 0 c |
| K599 | 14 | 12.1±0.6 a | 2.2±0.1 a |
| R1601 | 16 | 8.3±0.5 b | 1.1±0.1 b |
| ATCC15834 | - | 0 c | 0 c |
| Ar4 | - | 0 c | 0 c |
| Different nodes | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| Unfully expanded immature leaves | 16 | 2.3±0.2 bc | 1.0±0 b |
| Immature leaves from 1st to 2nd nodes | 13 | 18.1±1.2 a | 1.9±0.4 a |
| Immature leaves from 3rd to 4th nodes | 14 | 10.7±0.3 b | 1.7±0.2 a |
| Immature leaves from 5th to 6th nodes | 16 | 5.5±0.5 c | 1.2±0.2 b |
表4 不同部位幼嫩叶片对野葛毛状根诱导效率的影响
Table 4 Effects of immature leaves from different nodes on the induction efficiency of Pueraria lobata hairy roots
| Different nodes | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| Unfully expanded immature leaves | 16 | 2.3±0.2 bc | 1.0±0 b |
| Immature leaves from 1st to 2nd nodes | 13 | 18.1±1.2 a | 1.9±0.4 a |
| Immature leaves from 3rd to 4th nodes | 14 | 10.7±0.3 b | 1.7±0.2 a |
| Immature leaves from 5th to 6th nodes | 16 | 5.5±0.5 c | 1.2±0.2 b |
| Precultivation time (d) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 0 | 16 | 2.4±0.1 c | 1.1±0.4 b |
| 1 | 13 | 8.1±1.5 b | 1.2±0.1 b |
| 2 | 13 | 18.9±2.1 a | 2.0±0.0 a |
| 3 | 10 | 21.3±1.7 a | 1.8±0.4 a |
| 4 | 14 | 11.1±0.9 b | 1.0±0.2 b |
表5 不同预培养时间对野葛毛状根诱导效率的影响
Table 5 Effects of preculture time on the induction efficiency of Pueraria lobata hairy roots
| Precultivation time (d) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 0 | 16 | 2.4±0.1 c | 1.1±0.4 b |
| 1 | 13 | 8.1±1.5 b | 1.2±0.1 b |
| 2 | 13 | 18.9±2.1 a | 2.0±0.0 a |
| 3 | 10 | 21.3±1.7 a | 1.8±0.4 a |
| 4 | 14 | 11.1±0.9 b | 1.0±0.2 b |
| Infection time (min) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 15 | 10 | 20.9±2.7 a | 1.7±0.6 a |
| 20 | 11 | 19.7±3.3 a | 1.8±0.2 a |
| 25 | 13 | 14.3±2.1 a | 1.0±0 b |
| 30 | 16 | 9.1±1.4 b | 1.0±0 b |
表6 不同侵染时间对野葛毛状根诱导效率的影响
Table 6 Effects of infection time on the induction efficiency of Pueraria lobata hairy roots
| Infection time (min) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 15 | 10 | 20.9±2.7 a | 1.7±0.6 a |
| 20 | 11 | 19.7±3.3 a | 1.8±0.2 a |
| 25 | 13 | 14.3±2.1 a | 1.0±0 b |
| 30 | 16 | 9.1±1.4 b | 1.0±0 b |
| Culture days (d) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 5 | 11 | 14.7±4.5 bc | 2.0±0 a |
| 8 | 10 | 22.4±3.7 a | 1.9±0.5 a |
| 11 | 11 | 16.4±3.5 b | 1.6±0.3 ab |
| 14 | 13 | 12.0±2.5 c | 1.0±0 b |
| 17 | 14 | 6.1±3.1 d | 1.0±0 b |
| 20 | - | 0 e | - |
| 23 | - | 0 e | - |
| 26 | - | 0 e | - |
| 29 | - | 0 e | - |
表7 不同培养天数对野葛毛状根诱导效率的影响
Table 7 Effects of culture days on the induction efficiency of Pueraria lobata hairy roots
| Culture days (d) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 5 | 11 | 14.7±4.5 bc | 2.0±0 a |
| 8 | 10 | 22.4±3.7 a | 1.9±0.5 a |
| 11 | 11 | 16.4±3.5 b | 1.6±0.3 ab |
| 14 | 13 | 12.0±2.5 c | 1.0±0 b |
| 17 | 14 | 6.1±3.1 d | 1.0±0 b |
| 20 | - | 0 e | - |
| 23 | - | 0 e | - |
| 26 | - | 0 e | - |
| 29 | - | 0 e | - |
| Subculture times (t) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 1 | 9 | 2.2±0.8 d | 1.0±0 b |
| 3 | 10 | 11.1±2.3 b | 1.0±0 b |
| 5 | 9 | 18.9±1.7 a | 1.9±0.2 a |
| 7 | 10 | 16.7±2.6 a | 2.1±0.1 a |
| 9 | 10 | 17.0±1.1 a | 2.0±0 a |
| 11 | 11 | 15.6±3.7 a | 1.8±0.2 a |
| 13 | 11 | 14.1±2.1 ab | 1.8±0.4 a |
| 15 | 12 | 11.2±0.4 b | 1.4±0.2 ab |
| 17 | 13 | 6.7±2.2 c | 1.1±0.3 b |
| 19 | 13 | 4.3±1.9 cd | 1.0±0 b |
| 21 | - | 0 e | - |
| 23 | - | 0 e | - |
| 25 | - | 0 e | - |
表8 不同继代次数对野葛毛状根诱导效率的影响
Table 8 Effects of subculture time on the induction efficien- cy of Pueraria lobata hairy roots
| Subculture times (t) | Rooting time (d) | Hairy root frequency (%) | Hairy root density |
|---|---|---|---|
| 1 | 9 | 2.2±0.8 d | 1.0±0 b |
| 3 | 10 | 11.1±2.3 b | 1.0±0 b |
| 5 | 9 | 18.9±1.7 a | 1.9±0.2 a |
| 7 | 10 | 16.7±2.6 a | 2.1±0.1 a |
| 9 | 10 | 17.0±1.1 a | 2.0±0 a |
| 11 | 11 | 15.6±3.7 a | 1.8±0.2 a |
| 13 | 11 | 14.1±2.1 ab | 1.8±0.4 a |
| 15 | 12 | 11.2±0.4 b | 1.4±0.2 ab |
| 17 | 13 | 6.7±2.2 c | 1.1±0.3 b |
| 19 | 13 | 4.3±1.9 cd | 1.0±0 b |
| 21 | - | 0 e | - |
| 23 | - | 0 e | - |
| 25 | - | 0 e | - |
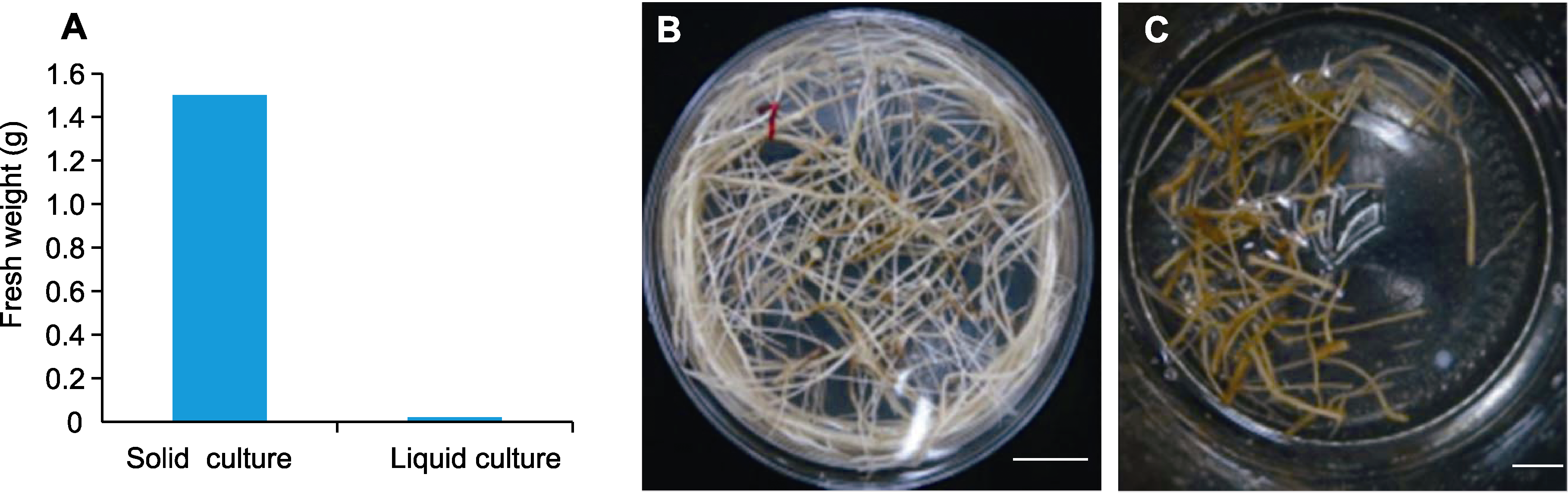
图3 野葛毛状根的继代增殖 (A) 不同培养方式下野葛毛状根的鲜重; (B) 接种于固体培养基20天的毛状根; (C) 接种于液体培养基20天的毛状根。Bars=1 cm
Figure 3 Subculture proliferation of Pueraria lobata hairy roots (A) Fresh weight of P. lobata hairy roots under different cultivation methods; (B) Morphological characterization of P. lobata hairy roots grown on solid media 20 d after inoculation; (C) Morphological characterization of P. lobata hairy roots grown in liquid media 20 d after inoculation. Bars=1 cm
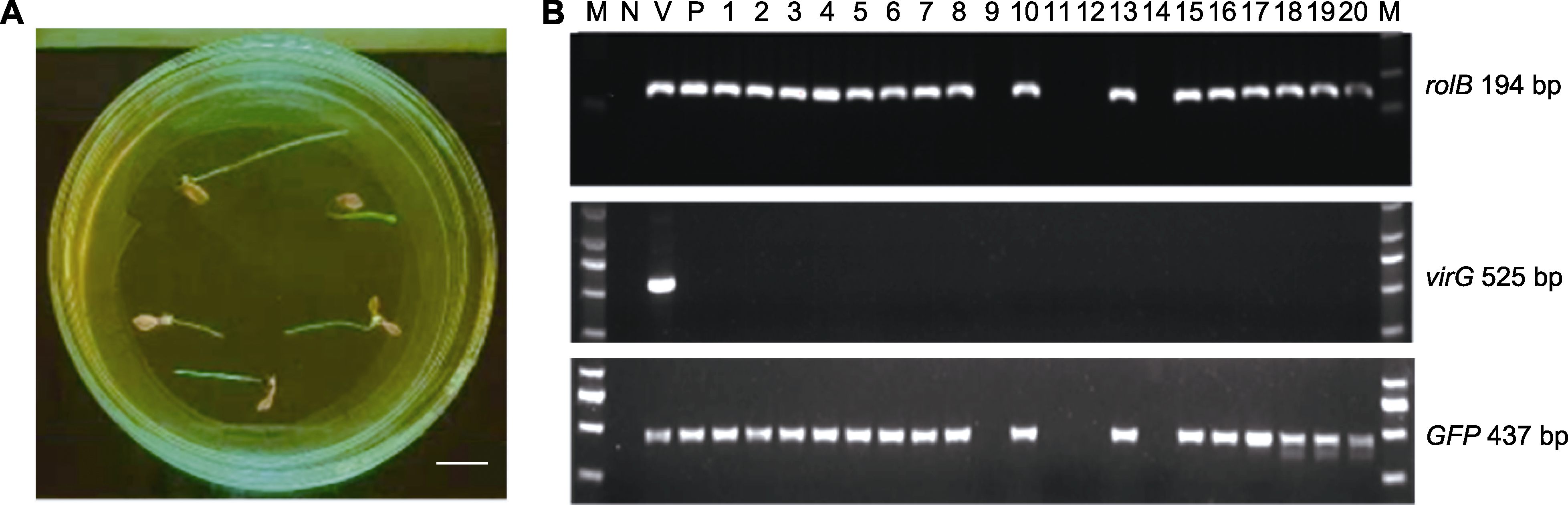
图4 野葛转基因毛状根GFP荧光观察及PCR检测 (A) 毛状根GFP荧光观察(bar=1 cm); (B) PCR检测(M: DL2000 DNA marker; N: 超纯水; V: K599; P: 阳性对照; 1-20: 不同转基因毛状根株系)
Figure 4 Green fluorescence assay and PCR analysis of the GFP in Pueraria lobata transgenic hairy roots (A) GFP green fluorescence assay of hairy roots (bar=1 cm); (B) PCR detection (M: DL2000 DNA marker; N: ddH2O; V: K599; P: Positive control; 1-20: Different transgenic hairy root lines)
| [1] |
Aggarwal PR, Nag P, Choudhary P, Chakraborty N, Chakraborty S (2018). Genotype-independent Agrobacterium rhizogenes-mediated root transformation of chickpea: a rapid and efficient method for reverse genetics studies. Plant Methods 14, 55.
DOI PMID |
| [2] | Cao XS, Xie HT, Song ML, Lu JH, Ma P, Huang BY, Wang MG, Tian YF, Chen F, Peng J, Lang ZB, Li GF, Zhu JK (2023). Cut-dip-budding delivery system enables genetic modifications in plants without tissue culture. Innovation (Camb) 4, 100345. |
| [3] | Chen YM, Liu JY, Zhang T, Lu QY, Yan Q (2017). Induction of hairy roots in heavy metal hyperaccumulator Sedum alfredii. J Trop Subtrop Bot 25, 136-140. (in Chinese) |
| 陈友明, 刘静轶, 张腾, 卢倩云, 晏琼 (2017). 重金属超富集植物东南景天毛状根的诱导. 热带亚热带植物学报 25, 136-140. | |
| [4] | Cheng Y, Ma PT, Wu J (2022). Study on comparison of regeneration capacity of different varieties of common bean. J Sichuan Agric Univ 40, 465-471, 535. (in Chinese) |
| 程媛, 马朋涛, 武晶 (2022). 不同基因型普通菜豆再生能力研究. 四川农业大学学报 40, 465-471, 535. | |
| [5] | Crane C, Wright E, Dixon RA, Wang ZY (2016). Transgenic Medicago truncatula plants obtained from Agrobacterium tumefaciens-transformed roots and Agrobacterium rhizogenes-transformed hairy roots. Planta 223, 1344-1354. |
| [6] | Cui ML, Liu C, Piao CL, Liu CL (2020). A stable Agrobacterium rhizogenes-mediated transformation of cotton (Gos-sypium hirsutum L.) and plant regeneration from transformed hairy root via embryogenesis. Front Plant Sci 11, 604255. |
| [7] |
Du JY, Chen KY, Pu J, Zhou HY, Zhu GT, Zhang CZ, Du H (2023). The modification of gene editing vector for efficient GFPuv fluorescence screening and its application in potato genetic transformation. Sci Agric Sin 56, 2223-2236. (in Chinese)
DOI |
| 杜静雅, 陈凯园, 普金, 周会英, 祝光涛, 张春芝, 杜慧 (2023). 高效GFPuv荧光筛选基因编辑载体的改造及其在马铃薯遗传转化中的应用. 中国农业科学 56, 2223-2236. | |
| [8] | Fu CX, Jin ZP, Yang R, Wu FY, Zhao DX (2004). Establishment of Saussurea involucrata hairy roots culture and plantlet regeneration. Chin J Biotechnol 20, 366-371. (in Chinese) |
| 付春祥, 金治平, 杨睿, 吴风燕, 赵德修 (2004). 新疆雪莲毛状根的诱导及其植株再生体系的建立. 生物工程学报 20, 366-371. | |
| [9] | Geng LL, Niu LH, Gresshoff PM, Shu CL, Song FP, Huang DF, Zhang J (2012). Efficient production of Agrobacterium rhizogenes-transformed roots and composite plants in peanut (Arachis hypogaea L.). Plant Cell Tissue Organ Cult 109, 491-500. |
| [10] | Hu JQ (2021). Study on Genetic Transformation System of Mulberry Mediated by Agrobacterium. Master's thesis. Chongqing: Southwest University. pp. 1-62. (in Chinese) |
| 胡建琼 (2021). 农杆菌介导的桑树遗传转化体系研究. 硕士论文. 重庆: 西南大学. pp. 1-62. | |
| [11] | Kiryushkin AS, Ilina EL, Guseva ED, Pawlowski K, Demchenko KN (2022). Hairy CRISPR: genome editing in plants using hairy root transformation. Plants (Basel) 11, 51. |
| [12] | Liang P, Shi HP, Qi Y (2004). Effect of sucrose concentration on the growth and production of secondary metabolites in Pueraria phaseoloides hairy roots. Acta Biol Exp Sin 37, 384-390. (in Chinese) |
| 梁朋, 施和平, 齐莹 (2004). 蔗糖浓度对三裂叶野葛毛状根生长及其次生物质产生的影响. 实验生物学报 37, 384-390. | |
| [13] | Liu CF, Yu SH, Li L, Shi HP, Pan RZ (2000). The genetic transformation of Pueraria as medicinal plant by Agrobacterium rhizogenes. J Integr Plant Biol 42, 936-939. (in Chinese) |
| 刘传飞, 于树宏, 李玲, 施和平, 潘瑞炽 (2000). 发根土壤杆菌对葛属药用植物的遗传转化. 植物学报 42, 936-939. | |
| [14] | Mei GG, Chen A, Wang YR, Li SQ, Wu MY, Hu YL, Liu X, Hou XL (2024). A simple and efficient in planta transformation method based on the active regeneration capacity of plants. Plant Commun 5, 100822. |
| [15] | Park SU, Facchini PJ (2000). Agrobacterium rhizogenes-mediated transformation of opium poppy, Papaver somniferum L., and California poppy, Eschscholzia californica Cham., root cultures. J Exp Bot 347, 1005-1016. |
| [16] | Ren RY, Xue JK, Guo HY, Wei JC (2017). Induction of hairy roots of Scrophularia buergeriana and its plant regeneration. Chin Bull Bot 52, 783-787. (in Chinese) |
|
任如意, 薛巨坤, 国会艳, 魏继承 (2017). 北玄参毛状根诱导及其植株再生. 植物学报 52, 783-787.
DOI |
|
| [17] |
Shi HP, Kintzios S (2003). Genetic transformation of Pueraria phaseoloides with Agrobacterium rhizogenes and puerarin production in hairy roots. Plant Cell Rep 21, 1103-1107.
PMID |
| [18] | Shi HP, Quan H, Kintzios S (2003). Induction of hairy roots of Pueraria phaseoloides and its culture in liquid and solid medium. Chin J Biotechnol 19, 307-311. (in Chinese) |
| 施和平, 权宏 Kintzios S (2003). 三裂叶野葛毛状根的诱导及其固体培养和液体培养. 生物工程学报 19, 307-311. | |
| [19] | Shi HP, Wang P, Yang SN, Guo YP (2016). Induction of hairy roots of Dianthus chinensis and its plant regeneration. Chin Bull Bot 51, 363-368. (in Chinese) |
|
施和平, 王蓓, 杨树楠, 郭亚鹏 (2016). 五寸石竹毛状根诱导及其植株再生. 植物学报 51, 363-368.
DOI |
|
| [20] | Shi HP, Zhu YF, Zeng BQ, Zhou ZH, Yu ZA, Huang SQ (2017). Factors influencing induction and in vitro culture of hairy roots in Phytolacca americana L. Chin J Biotechnol 33, 272-283. (in Chinese) |
| 施和平, 朱远锋, 曾宝强, 周卓辉, 余震傲, 黄胜琴 (2017). 美洲商陆毛状根诱导及其离体培养的影响因素. 生物工程学报 33, 272-283. | |
| [21] | Sun H, Zhang JY, Luo LJ, Liu PD (2024). Development of transgenic hairy root induction and protoplast preparation systems for Stylosanthes leiocarpa. Acta Agrestia Sin 32, 1583-1591. (in Chinese) |
|
孙昊, 张建禹, 罗丽娟, 刘攀道 (2024). 光果柱花草转基因毛状根及其原生质体制备体系的建立. 草地学报 32, 1583-1591.
DOI |
|
| [22] | Wan YM, Xiao SH, Bai YC, Fan JY, Wang Y, Wu CA (2023). Establishment and optimization of a high-efficient hairy-root system in foxtail millet (Setaria italica L.). Acta Agron Sin 49, 1758-1768. (in Chinese) |
|
万夷曼, 肖圣慧, 白依超, 范佳音, 王琰, 吴长艾 (2023). 谷子毛状根诱导方法的建立与优化. 作物学报 49, 1758-1768.
DOI |
|
| [23] | Wang Y, Huang LJ, Li YW, Feng ZS, Mu ZH, Wang J, Wu XY, Wang BG, Lu ZF, Li GJ, Wu XH (2022). Checking transformation efficiency for different Lagenaria siceraria genotypes by using seed germination pouches as a growth carrier. Plant Cell Tissue Organ Cult 151, 199-206. |
| [24] | Wang YH (2006). Study on establishment of system with high frequency for genetic transformation of Phellodendron chinese and plant regeneration. J Chin Med Mater 29, 641-644. (in Chinese) |
| 王跃华 (2006). 川黄柏高效遗传转化系统建立和植株再生研究. 中药材 29, 641-644. | |
| [25] | Xie XT, Huang QY, Wen GC, Yuan HW, He Y, Yan DL, Huang JQ, Wang XF, Zheng BS (2022). Construction of Agrobacterium rhizogenes-mediated transformation system of Carya illinoinensis without dependence on tissue culture. J Fruit Sci 39, 131-140. (in Chinese) |
| 谢晓婷, 黄巧宇, 温广超, 袁虎威, 何漪, 闫道良, 黄坚钦, 王晓飞, 郑炳松 (2022). 非组培依赖的发根农杆菌介导的薄壳山核桃转化体系构建. 果树学报 39, 131-140. | |
| [26] | Xu Y, Cao YP, Wang Y, Fu CX, Dai SJ (2019). Agrobacterium rhizogenes-mediated transformation system of Spinacia oleracea. Chin Bull Bot 54, 515-521. (in Chinese) |
|
徐悦, 曹英萍, 王玉, 付春祥, 戴绍军 (2019). 发根农杆菌介导的菠菜毛状根遗传转化体系的建立. 植物学报 54, 515-521.
DOI |
|
| [27] | Yu SH, Liu CF, Li L, Pan RC (2001). Factors affecting genetic transformation of Pueraria lobata by Agrobacterium rhizogene. Chin J Appl Environ Biol 7, 474-477. (in Chinese) |
| 于树宏, 刘传飞, 李玲, 潘瑞炽 (2001). 影响发根农杆菌对结野葛遗传转化效率的因素. 应用与环境生物学报 7, 474-477. | |
| [28] | Yu SH, Liu CF, Li L, Pan RC (2002). Pueraria lobata hairy root culture in vitro and isoflavone production. J Plant Physiol Mol Biol 28, 281-286. (in Chinese) |
| 于树宏, 刘传飞, 李玲, 潘瑞炽 (2002). 野葛毛状根离体培养与异黄酮生产. 植物生理与分子生物学学报 28, 281-286. | |
| [29] | Zhang CC (2011). Research on Agrobacterium rhizogenes-mediated Genetic Transformation in Cotton. Master's thesis. Beijing: Chinese Academy of Agricultural Sciences. pp. 1-73. (in Chinese) |
| 张程程 (2011). 发根农杆菌介导的棉花遗传转化的研究. 硕士论文. 北京: 中国农业科学院. pp. 1-73. | |
| [30] | Zhang LL, Zhang J, Lü HL, Tan B, Wang W, Cheng J, Feng JC (2022). Establishment of leaf regeneration system for European plum. J Fruit Sci 39, 1945-1953. (in Chinese) |
| 张郎郎, 张洁, 吕虹霖, 谭彬, 王伟, 程钧, 冯建灿 (2022). 欧洲李叶片再生体系的建立. 果树学报 39, 1945-1953. | |
| [31] | Zhao XK, Sun H, Yang LY, Luo LJ, Liu PD (2023). Establishment of the genetic transformation system of Stylosanthes gracilis mediated with Agrobacterium rhizogenes. Acta Agrestia Sin 31, 581-586. (in Chinese) |
|
赵兴坤, 孙昊, 杨丽云, 罗丽娟, 刘攀道 (2023). 发根农杆菌介导的细茎柱花草毛状根转化体系的建立. 草地学报 31, 581-586.
DOI |
|
| [32] | Zhu HY (2005). Study on Establishment of Regeneration System In Vitro and Transgenic Receptor System in Gerbera (Gerbera jamesonii Bolus). Master's thesis. Yangling: Northwest A&F University. pp. 1-64. (in Chinese) |
| 祝红艺 (2005). 非洲菊(Gerbera jamesonii Bolus)再生体系及遗传转化受体系统建立的研究. 硕士论文. 杨凌: 西北农林科技大学. pp. 1-64. | |
| [33] | Zhu WF, Li JL, Meng XW, Zhang PZ, Wu WT, Liu RH (2021). Research advances in chemical constituents and pharmacological activities of Pueraria genus. China J Chin Mater Med 46, 1311-1325. (in Chinese) |
| 朱卫丰, 李佳莉, 孟晓伟, 张普照, 吴文婷, 刘荣华 (2021). 葛属植物的化学成分及药理活性研究进展. 中国中药杂志 46, 1311-1325. |
| [1] | 李晶晶, 李艳飞, 王安琪, 王佳颖, 邓成燕, 卢敏, 马剑英, 戴思兰. 菊花品种‘万代风光’再生及遗传转化体系的建立[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 李宇琛, 赵海霞, 姜希萍, 黄馨田, 刘亚玲, 吴振映, 赵彦, 付春祥. 根癌农杆菌介导的蒙古冰草稳定遗传转化体系建立[J]. 植物学报, 2024, 59(4): 600-612. |
| [3] | 陈佳欣, 梅浩, 黄彩翔, 梁宗原, 全依桐, 李东鹏, 布威麦尔耶姆·赛麦提, 李欣欣, 廖红. 利用转基因毛状根高效培育大豆嵌合植株的方法[J]. 植物学报, 2024, 59(1): 89-98. |
| [4] | 余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红. 根癌农杆菌介导万寿菊遗传转化体系的建立[J]. 植物学报, 2023, 58(5): 760-769. |
| [5] | 杨澜, 刘雅, 项阳, 孙秀娟, 颜景畏, 张阿英. 谷子茎尖体外遗传转化体系的建立与优化[J]. 植物学报, 2021, 56(1): 71-79. |
| [6] | 徐悦,曹英萍,王玉,付春祥,戴绍军. 发根农杆菌介导的菠菜毛状根遗传转化体系的建立[J]. 植物学报, 2019, 54(4): 515-521. |
| [7] | 李俊华,刘世宇,李成龙,韩林林,董亚辉,张晓丽,赵喜亭,李明军. 铁棍山药微型块茎遗传转化体系的建立[J]. 植物学报, 2019, 54(1): 72-80. |
| [8] | 吴国栋, 修宇, 王华芳. 优化子叶节转化法培育大豆MtDREB2A转基因植株[J]. 植物学报, 2018, 53(1): 59-71. |
| [9] | 任如意, 薛巨坤, 国会艳, 魏继承. 北玄参毛状根诱导及其植株再生[J]. 植物学报, 2017, 52(6): 783-787. |
| [10] | 王大鹏, 唐嘉泽, 邵明成, 张文彪, 王华芳. 胡杨组织培养叶片及插穗毛状根发生[J]. 植物学报, 2017, 52(2): 210-217. |
| [11] | 施西子, 郭亚鹏, 施和平. 多效唑(PP333)对美洲商陆毛状根生长和商陆皂苷甲产生的影响[J]. 植物学报, 2016, 51(6): 801-806. |
| [12] | 赵喜亭, 蒋丽微, 王苗, 朱玉婷, 张文芳, 李明军. 怀黄菊间接体胚受体再生体系的建立及CmTGA1的遗传转化[J]. 植物学报, 2016, 51(4): 525-532. |
| [13] | 施和平, 王蓓, 杨树楠, 郭亚鹏. 五寸石竹毛状根诱导及其植株再生[J]. 植物学报, 2016, 51(3): 363-368. |
| [14] | 郭利军, 曾炳山, 刘英. 农杆菌介导巨桉Eg5高效遗传转化[J]. 植物学报, 2013, 48(1): 87-93. |
| [15] | 刘宣雨, 王青云, 刘树君, 宋松泉. 高粱遗传转化研究进展[J]. 植物学报, 2011, 46(2): 216-223. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||