

植物学报 ›› 2019, Vol. 54 ›› Issue (1): 72-80.DOI: 10.11983/CBB18118 cstr: 32102.14.CBB18118
李俊华1,2,3,†,刘世宇1,†,李成龙1,韩林林1,董亚辉1,张晓丽1,2,3,赵喜亭1,2,3,李明军1,2,3,*( )
)
收稿日期:2018-05-15
接受日期:2018-09-13
出版日期:2019-01-01
发布日期:2019-07-31
通讯作者:
李俊华,刘世宇,李明军
基金资助:
Junhua Li1,2,3,†,Shiyu Liu1,†,Chenglong Li1,Linlin Han1,Yahui Dong1,Xiaoli Zhang1,2,3,Xiting Zhao1,2,3,Mingjun Li1,2,3,*( )
)
Received:2018-05-15
Accepted:2018-09-13
Online:2019-01-01
Published:2019-07-31
Contact:
Junhua Li,Shiyu Liu,Mingjun Li
摘要: 怀山药(Dioscorea opposita)遗传转化是对其进行基因功能分析和遗传改良的基础, 但目前国内外尚未见相关报道。以怀山药优良品种铁棍山药(D. opposita cv. ‘Tiegun’)的微型块茎为受体材料, 对影响遗传转化的因素进行优化, 建立了由根癌农杆菌介导的山药遗传转化体系。过表达质粒载体pCAMBIA1301-DoSERK2含GUS标记基因和潮霉素(Hyg)抗性筛选基因, 沉默质粒载体pART27-DoSERK2含卡那霉素(Kan)抗性筛选基因。根癌农杆菌抑制剂特美汀(Tim)的最佳浓度为500 mg·L -1; 再生芽和生根时, Hyg的最佳浓度分别为15和20 mg·L -1, Kan的最佳浓度分别为120和160 mg·L -1。对转化植株进行PCR和GUS组织化学检测, 结果显示外源基因已整合到铁棍山药转基因株系的基因组中并在细胞中表达。该研究建立了一套取材便利的铁棍山药遗传转化方法, 对其它品种山药的转化也具有参考价值。
李俊华,刘世宇,李成龙,韩林林,董亚辉,张晓丽,赵喜亭,李明军. 铁棍山药微型块茎遗传转化体系的建立. 植物学报, 2019, 54(1): 72-80.
Junhua Li,Shiyu Liu,Chenglong Li,Linlin Han,Yahui Dong,Xiaoli Zhang,Xiting Zhao,Mingjun Li. Establishment of a Genetic Transformation System for Dioscorea opposita Using Microtuber. Chinese Bulletin of Botany, 2019, 54(1): 72-80.
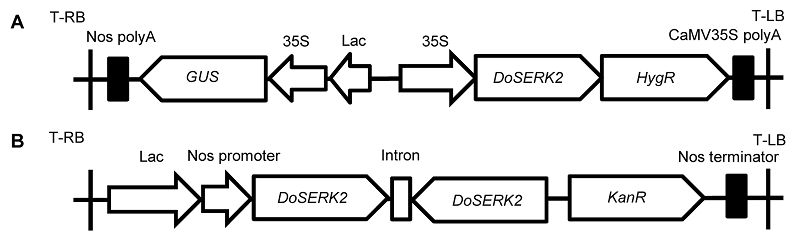
图1 过表达载体和沉默载体结构示意图 (A) 过表达载体pCAMBIA1301-DoSERK2; (B) 沉默载体pART27-DoSERK2
Figure 1 Schematic maps of the overexpression vector and silencing vector used in this study (A) The overexpression vector pCAMBIA1301-DoSERK2; (B) The silencing vector pART27-DoSERK2
| Media | Composition |
|---|---|
| MS0 | MS major salts, MS minor salts and MS vitamins +30 g·L-1 sucrose+6 g·L-1 fungible agar, pH5.8- 6.2 |
| MS0 1 | MS0+1 mg·L-1 6-BA+1 mg?L-1 IAA |
| MS0 2 | MS0+2 mg?L-1 PP333+0.05 mg?L-1 NAA |
| MS0 1-A | MS0 1+100 μmol?L-1 Acetosyringone (AS) |
| MS0 1-T | MS0 1+500 mg?L-1 Timentin (Tim) |
| MS0 1-TH | MS0 1+500 mg?L-1 Tim+15 mg?L-1 Hyg |
| MS0 1-TK | MS0 1+500 mg?L-1 Tim+120 mg?L-1 Kan |
| MS0 2-H | MS0 2+20 mg?L-1 Hyg |
| MS0 2-K | MS0 2+160 mg?L-1 Kan |
表1 铁棍山药遗传转化和植株再生培养基
Table 1 Medium used for genetic transformation and plant regeneration of Dioscorea opposita cv. ‘Tiegun’
| Media | Composition |
|---|---|
| MS0 | MS major salts, MS minor salts and MS vitamins +30 g·L-1 sucrose+6 g·L-1 fungible agar, pH5.8- 6.2 |
| MS0 1 | MS0+1 mg·L-1 6-BA+1 mg?L-1 IAA |
| MS0 2 | MS0+2 mg?L-1 PP333+0.05 mg?L-1 NAA |
| MS0 1-A | MS0 1+100 μmol?L-1 Acetosyringone (AS) |
| MS0 1-T | MS0 1+500 mg?L-1 Timentin (Tim) |
| MS0 1-TH | MS0 1+500 mg?L-1 Tim+15 mg?L-1 Hyg |
| MS0 1-TK | MS0 1+500 mg?L-1 Tim+120 mg?L-1 Kan |
| MS0 2-H | MS0 2+20 mg?L-1 Hyg |
| MS0 2-K | MS0 2+160 mg?L-1 Kan |
| Primers | Sequences (5'-3') |
|---|---|
| DoSERK2-OE-F | ATGACGGCTTGGGTTTTC |
| DoSERK2-OE-R | TCACCTCGGACCAGATAGC |
| DoSERK2-RNAi-F | CAGATGATACAGAAAAGCACCG |
| DoSERK2-RNAi-R | TAACTTTCGGTAGAGCGGAC |
| DoActin-F | CTCATTGATCGGCATGGAAGC |
| DoActin-R | GGGGAACATAGTTGAACCACCAC |
| DoSERK2-qRT-F | TATCTGGACCAGTTCCATCC |
| DoSERK2-qRT-R | CTTCAGCAGGCACATCATAG |
表2 引物序列
Table 2 Sequences of primers
| Primers | Sequences (5'-3') |
|---|---|
| DoSERK2-OE-F | ATGACGGCTTGGGTTTTC |
| DoSERK2-OE-R | TCACCTCGGACCAGATAGC |
| DoSERK2-RNAi-F | CAGATGATACAGAAAAGCACCG |
| DoSERK2-RNAi-R | TAACTTTCGGTAGAGCGGAC |
| DoActin-F | CTCATTGATCGGCATGGAAGC |
| DoActin-R | GGGGAACATAGTTGAACCACCAC |
| DoSERK2-qRT-F | TATCTGGACCAGTTCCATCC |
| DoSERK2-qRT-R | CTTCAGCAGGCACATCATAG |
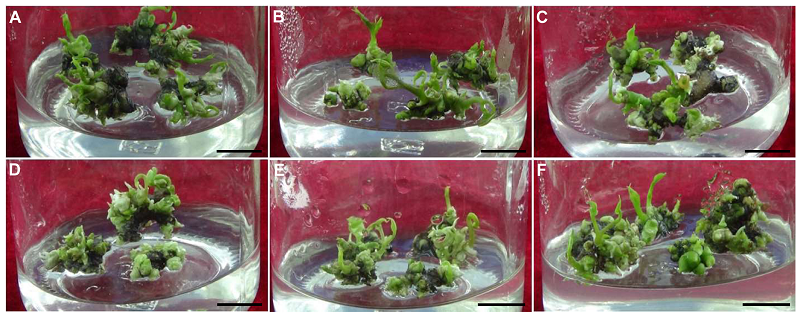
图2 不同浓度Tim对铁棍山药类原球茎(PLBs)增殖分化的影响 cv. ‘Tiegun’(A) 0 mg·L-1; (B) 100 mg·L-1; (C) 200 mg·L-1; (D) 300 mg·L-1; (E) 400 mg·L-1; (F) 500 mg·L-1。Bars=1 cm
Figure 2 Effects of different concentrations of Tim on the proliferation and differentiation of protocorm-like bodies (PLBs) in Dioscorea opposita (A) 0 mg·L-1; (B) 100 mg·L-1; (C) 200 mg·L-1; (D) 300 mg·L-1; (E) 400 mg·L-1; (F) 500 mg·L-1. Bars=1 cm

图3 筛选剂对铁棍山药微型块茎苗再生及再生苗生根的影响 (A)-(F) 不同浓度Hyg对微型块茎苗再生的影响, Hyg浓度分别为0、5、10、15、20和25 mg·L-1; (G)-(L) 不同浓度Kan对微型块茎苗再生的影响, Kan浓度分别为0、80、100、120、140和160 mg·L-1; (M) 不同浓度Hyg对再生苗生根的影响; (N) 不同浓度Kan对再生苗生根的影响。(A)-(L) Bars=0.5 cm; (M), (N) Bars=2 cm
Figure 3 Effects of antibiotic on microtuber regeneration and rooting of regenerated seedlings in Dioscorea opposita cv. ‘Tiegun’ (A)-(F) Effects of different concentrations of Hyg on microtuber regeneration, the concentrations of Hyg are 0, 5, 10, 15, 20, 25 mg·L-1, respectively; (G)-(L) Effects of different concentrations of Kan on microtuber regeneration, the concentrations of Kan are 0, 80, 100, 120, 140, 160 mg·L-1, respectively; (M) Effects of different concentrations of Hyg on rooting of regenerated plantlets; (N) Effects of different concentrations of Kan on rooting of regenerated plantlets. (A)-(L) Bars=0.5 cm; (M), (N) Bars=2 cm
| Concentration of Hyg (mg·L-1) | Regeneration (%) | Concentration of Kan (mg·L-1) | Regeneration (%) | Growth of seedlings |
|---|---|---|---|---|
| 0 | 60.00±2.00 | 0 | 65.56±2.52 | Strong growth vigor |
| 5 | 55.56±1.15 | 80 | 48.89±1.53 | Well |
| 10 | 33.33±2.00 | 100 | 34.44±1.53 | Slow, albefaction in some seedlings |
| 15 | 25.56±0.58 | 120 | 8.89±3.06 | Slow, albefaction in many seedlings |
| 20 | 2.22±1.15 | 140 | 1.11±0.58 | Growth stopped, albefaction seriously |
| 25 | 0 | 160 | 0 | All dead |
表3 不同浓度筛选剂对铁棍山药微型块茎切片再生的影响
Table 3 Effects of antibiotic concentrations on regeneration of microtuber slices in Dioscorea opposita cv. ‘Tiegun’
| Concentration of Hyg (mg·L-1) | Regeneration (%) | Concentration of Kan (mg·L-1) | Regeneration (%) | Growth of seedlings |
|---|---|---|---|---|
| 0 | 60.00±2.00 | 0 | 65.56±2.52 | Strong growth vigor |
| 5 | 55.56±1.15 | 80 | 48.89±1.53 | Well |
| 10 | 33.33±2.00 | 100 | 34.44±1.53 | Slow, albefaction in some seedlings |
| 15 | 25.56±0.58 | 120 | 8.89±3.06 | Slow, albefaction in many seedlings |
| 20 | 2.22±1.15 | 140 | 1.11±0.58 | Growth stopped, albefaction seriously |
| 25 | 0 | 160 | 0 | All dead |
| Concentration of Hyg (mg·L-1) | Rooting (%) | Concentration of Kan (mg·L-1) | Rooting (%) | Growth of roots |
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | Strong and flourishing |
| 5 | 100 | 120 | 100 | Well |
| 15 | 3±2.00 | 140 | 1±1.20 | Seldom rooting |
| 20 | 0 | 160 | 0 | Hardly rooting |
| 25 | 0 | 180 | 0 | Hardly rooting, albefaction |
表4 不同浓度筛选剂对铁棍山药再生苗生根的影响
Table 4 Effects of antibiotic concentrations on rooting of regenerated seedlings in Dioscorea opposita cv. ‘Tiegun’
| Concentration of Hyg (mg·L-1) | Rooting (%) | Concentration of Kan (mg·L-1) | Rooting (%) | Growth of roots |
|---|---|---|---|---|
| 0 | 100 | 0 | 100 | Strong and flourishing |
| 5 | 100 | 120 | 100 | Well |
| 15 | 3±2.00 | 140 | 1±1.20 | Seldom rooting |
| 20 | 0 | 160 | 0 | Hardly rooting |
| 25 | 0 | 180 | 0 | Hardly rooting, albefaction |
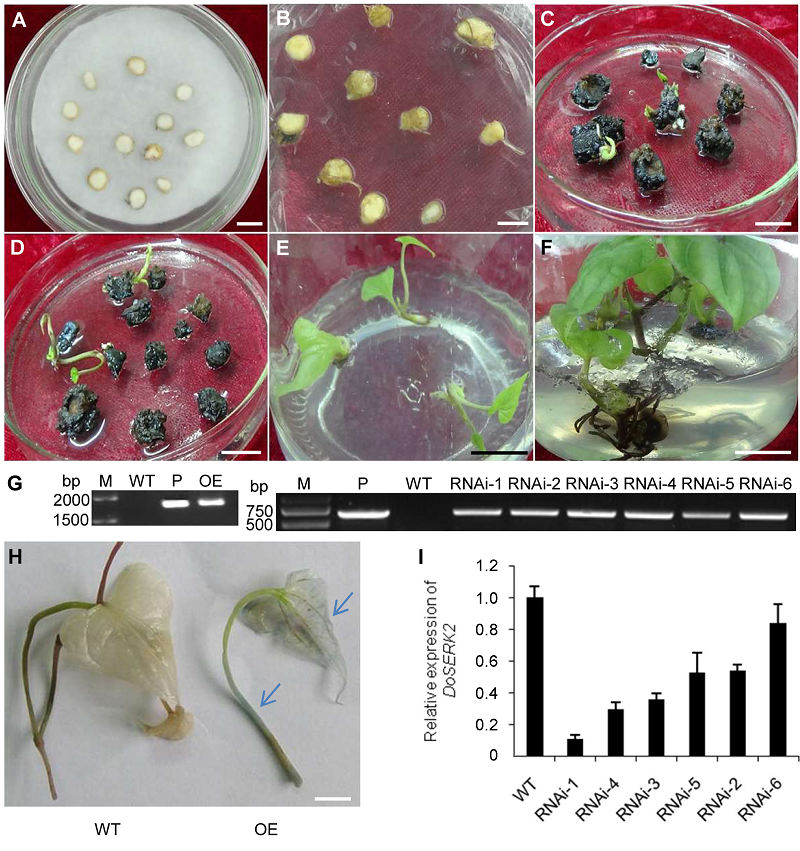
图4 铁棍山药微型块茎切片的遗传转化、植株再生及转基因植株的分子鉴定 (A) 农杆菌侵染后的切片; (B) 共培养3天后的切片; (C) 再生苗培养基上培养40天的愈伤组织; (D) 再生苗培养基上培养70天的愈伤组织; (E) 抗性再生苗转入生根培养基; (F) 生根培养基上生长30天的再生苗; (G) 转基因植株基因组DNA的PCR鉴定(M: DNA分子量标记; P: 分别以质粒pCAMBIA1301-DoSERK2 (左)和pART27-DoSERK2 (右)为模板; WT: 以野生型植株的基因组DNA为模板; OE: 以过表达转基因株系基因组DNA为模板; RNAi-1-6: 以沉默载体转基因株系1-6的基因组DNA为模板); (H) 过表达转化叶片及叶柄中的GUS表达情况, 野生型植株(WT)和转基因植株(OE)分别经GUS染色液染色; (I) 内源DoSERK2表达水平的RT-qPCR检测(WT: 以野生型植株的cDNA为模板; RNAi-1-6: 以沉默载体转基因株系1-6 cDNA为模板)。(A)-(F), (H) Bars=1 cm
Figure 4 Genetic transformation and regeneration of Dioscorea opposita cv. ‘Tiegun’ microtuber slices and molecular verification of trans- genic plants (A) Microtuber slices infected by Agrobacterium; (B) Microtuber slices after co-cultivation with Agrobacterium for 3 days; (C) Tissues cultured on regeneration medium for 40 days; (D) Tissues cultured on regeneration medium for 70 days; (E) Antibiotic-resistant regenerated seedlings that had been transferred to rooting medium; (F) Regenerated seedlings that had been cultured on rooting medium for 30 days; (G) PCR assay of genome DNA from transgenic plants (M: DNA marker; P: Plasmid pCAMBIA1301-DoSERK2 (left panel) and pART27-DoSERK2 (right panel) as template; WT: Genome DNA of wild-type plants as template; OE: Genome DNA from overexpression transgenic plants as template; RNAi-1-6: Genome DNA from line 1-6 of transgenic plants of the silencing vector as template); (H) GUS activity assay in the leaf of an overexpression transformant, nodal or leaf segments of a wild-type plant (WT) or transgenic plant (OE) were stained with GUS staining solution; (I) RT-qPCR assay of the endogenous expression levels of DoSERK2 (WT: cDNA of wild-type plants as template; RNAi-1-6: cDNA from line 1-6 of transgenic plants of the silencing vector as template). (A)-(F), (H) Bars=1 cm
| 1 |
范俊强 ( 2008). 根癌农杆菌介导的盾叶薯蓣转化体系的建立. 硕士论文. 武汉: 华中农业大学. pp. 1-5.
DOI URL |
| 2 |
付洪冰, 崔崇士, 赵曦, 刘琦 ( 2010). 农杆菌介导南瓜遗传转化体系的建立. 植物学报 45, 472-478.
DOI URL |
| 3 |
郭利军, 曾炳山, 刘英 ( 2013). 农杆菌介导巨桉Eg5高效遗传转化. 植物学报 48, 87-93.
DOI URL |
| 4 |
韩林林, 李俊华, 赵喜亭, 张晓丽, 宋志辉, 刘世宇, 李明军 ( 2016). 农杆菌介导的怀山药叶片瞬时表达方法的建立. 河南师范大学学报(自然科学版) 44, 135-139.
DOI URL |
| 5 | 李明军 (2013).提高山药商品性栽培技术问答. 北京: 金盾出版社. pp. 36-50. |
| 6 |
李明军, 陈明霞, 洪森荣, 徐鑫, 张晓丽 ( 2004). NAA、IBA和PP333对怀山药试管苗生长发育的影响. 广西植物 24, 376-379.
DOI URL |
| 7 | 李明军, 刘世宇, 刘雯, 李俊华, 张晓丽, 赵喜亭 ( 2017). 怀山药微型块茎形成过程中的生理生化变化. 植物生理学报 53, 807-814. |
| 8 |
李明军, 张峰, 陈明霞, 于相丽 ( 2003). 怀山药病毒病的研究. 中草药 34, U003-U005.
DOI URL |
| 9 | 李瑞雪, 李纪强, 蒲腾飞, 张晓丽, 赵喜亭, 李俊华, 李明军 ( 2018). 怀山药类原球茎的诱导形成与植株再生. 植物学报 53, 334-340. |
| 10 |
李卫, 郭光沁, 郑国锠 ( 2000). 根癌农杆菌介导遗传转化研究的若干新进展. 科学通报 45, 798-807.
DOI URL |
| 11 |
廖华兰, 黎秀琼, 李可, 李伯凌, 熊茜, 苏童, 李春霞, 陈银华, 罗丽娟 ( 2016). 农杆菌介导的木薯遗传转化体系的优化. 热带生物学报 7, 427-434.
DOI URL |
| 12 | 王善平, 许智宏, 卫志明 ( 1990). 毛白杨叶外植体的遗传转化. 植物学报 32, 172-177. |
| 13 | 王运英 ( 2016). 怀山药脱毒微型块茎管内管外萌发条件及生理生化机制研究. 硕士论文. 新乡: 河南师范大学. pp. 9-19. |
| 14 | 许云 ( 2014). 大薯遗传多样性的AFLP分析和类原球茎遗传转化体系的研究. 硕士论文. 海口: 海南大学. pp. 61-66. |
| 15 | 杨亚萍, 李永兰, 梁月荣, 陆建良, 郑新强 ( 2015). 发根农杆菌抑菌剂的抑菌效果及对茶组培苗丛生芽的影响. 茶叶科学 35, 437-442. |
| 16 |
张宁, 司怀军, 李学才, 王蒂 ( 2004). 根癌农杆菌介导的马铃薯高效遗传转化体系的研究. 中国马铃薯 18, 132-135.
DOI URL |
| 17 |
赵喜亭, 蒋丽薇, 王苗, 朱玉婷, 张文芳, 李明军 ( 2016). 怀黄菊间接体胚受体再生体系的建立及CmTGA1的遗传转化. 植物学报 51, 525-532.
DOI URL |
| 18 |
Edwards K, Johnstone C, Thompson C ( 1991). A simple and rapid method for the preparation of plant genomic DNA for PCR analysis. Nucleic Acids Res 19, 1349.
DOI URL PMID |
| 19 | Li MJ, Li JH, Wang YP, Liu W, Guo XB, Li SJ, Han LL, Song ZH, Zhao XT, Yang QX ( 2015). A simple method for microtuber production in Dioscorea opposita using single nodal segments . Pakistan J Bot 47, 665-668. |
| 20 | Mignouna HD, Abang MM, Asiedu R (2008). Genomics of yams, a common source of food and medicine in the tropics. In: Moore PH, Ming R, eds. Genomics of Tropical Crop Plants. Plant Genetics and Genomics: Crops and Models, Vol. 1. Berlin: Springer. pp. 549-570. |
| 21 | Nyaboga E, Tripathi JN, Manoharan R, Tripathi L ( 2014). Agrobacterium-mediated genetic transformation of yam ( Dioscorea rotundata): an important tool for functional study of genes and crop improvement.Front Plant Sci 5, 463. |
| 22 | Tör M, Ainsworth C, Mantell SH ( 1993). Stable transfor-mation of the food yam Dioscorea alata L.by particle bombardment. Plant Cell Rep 12, 468-473. |
| 23 |
Tör M, Twyford CT, Funes I, Boccon-Gibod J, Ainsworth CC, Mantell SH ( 1998). Isolation and culture of protoplasts from immature leaves and embryogenic cell suspensions of Dioscorea yams: tools for transient gene expression studies.Plant Cell Tissue Organ Cult 53, 113-126.
DOI URL |
| 24 |
Zhao XT, Zhang XL, Guo XB, Li SJ, Han LL, Song ZH, Wang YY, Li JH, Li MJ ( 2016). Identification and validation of reference genes for qRT-PCR studies of gene expression in Dioscorea opposita.Biomed Res Int 2016, 3089584.
DOI URL PMID |
| [1] | 李晶晶, 李艳飞, 王安琪, 王佳颖, 邓成燕, 卢敏, 马剑英, 戴思兰. 菊花品种‘万代风光’再生及遗传转化体系的建立[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 曾文丹, 严华兵, 吴正丹, 尚小红, 曹升, 陆柳英, 肖亮, 施平丽, 程冬, 龙紫媛, 李婕宇. 发根农杆菌介导的野葛毛状根遗传转化体系[J]. 植物学报, 2025, 60(3): 425-434. |
| [3] | 李宇琛, 赵海霞, 姜希萍, 黄馨田, 刘亚玲, 吴振映, 赵彦, 付春祥. 根癌农杆菌介导的蒙古冰草稳定遗传转化体系建立[J]. 植物学报, 2024, 59(4): 600-612. |
| [4] | 余晓敏, 王亚琴, 刘雨菡, 易庆平, 程文翰, 朱钰, 段枫, 张莉雪, 何燕红. 根癌农杆菌介导万寿菊遗传转化体系的建立[J]. 植物学报, 2023, 58(5): 760-769. |
| [5] | 杨澜, 刘雅, 项阳, 孙秀娟, 颜景畏, 张阿英. 谷子茎尖体外遗传转化体系的建立与优化[J]. 植物学报, 2021, 56(1): 71-79. |
| [6] | 李瑞雪, 李纪强, 蒲腾飞, 张晓丽, 赵喜亭, 李俊华, 李明军. 怀山药类原球茎的诱导形成与植株再生[J]. 植物学报, 2018, 53(3): 334-340. |
| [7] | 吴国栋, 修宇, 王华芳. 优化子叶节转化法培育大豆MtDREB2A转基因植株[J]. 植物学报, 2018, 53(1): 59-71. |
| [8] | 赵喜亭, 蒋丽微, 王苗, 朱玉婷, 张文芳, 李明军. 怀黄菊间接体胚受体再生体系的建立及CmTGA1的遗传转化[J]. 植物学报, 2016, 51(4): 525-532. |
| [9] | 郭利军, 曾炳山, 刘英. 农杆菌介导巨桉Eg5高效遗传转化[J]. 植物学报, 2013, 48(1): 87-93. |
| [10] | 江莺, 刘秀明, 马吉胜, 李巍, 朱海林, 杜美丽, 李海燕, 李校堃. 转基因红花中角质细胞生长因子KGF-1的表达[J]. 植物学报, 2011, 46(3): 311-318. |
| [11] | 刘宣雨, 王青云, 刘树君, 宋松泉. 高粱遗传转化研究进展[J]. 植物学报, 2011, 46(2): 216-223. |
| [12] | 付洪冰;崔崇士;赵曦;刘琦. 农杆菌介导南瓜遗传转化体系的建立[J]. 植物学报, 2010, 45(04): 472-478. |
| [13] | 范源伟;刘挨枝;王华芳 . 胡杨转基因体系的建立[J]. 植物学报, 2009, 44(06): 728-734. |
| [14] | 王道杰, 杨翠玲, 陆鸣. 真空渗透法转化油菜及转化种子的筛选[J]. 植物学报, 2009, 44(02): 216-222. |
| [15] | 邹智;卢长明*. 整株转化法及其在油菜上的应用与展望[J]. 植物学报, 2009, 44(02): 236-244. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||