

植物学报 ›› 2021, Vol. 56 ›› Issue (2): 175-182.DOI: 10.11983/CBB20133 cstr: 32102.14.CBB20133
收稿日期:2020-07-26
接受日期:2020-12-07
出版日期:2021-03-01
发布日期:2021-03-17
通讯作者:
张可伟
作者简介:*E-mail: kwzhang@zjnu.edu.cn基金资助:
Qilu Yu, Jiangzhe Zhao, Xiaoxian Zhu, Kewei Zhang( )
)
Received:2020-07-26
Accepted:2020-12-07
Online:2021-03-01
Published:2021-03-17
Contact:
Kewei Zhang
摘要: 植物激素是植物体内合成的一类重要小分子物质, 其含量可因外界条件变化而改变, 并作为信号物质调控植物生长发育和适应环境。水培所用介质体积过小会造成植物生长受限、植株矮小, 通常认为是小体积生长介质中营养成分不足所致。研究表明, 在不同体积且不含任何营养物质的纯水中培养的水稻(Oryza sativa)亦表现出不同的生长速度, 幼苗在小体积水中生长缓慢而在大体积水中则生长快速且健壮。用液相色谱-质谱(LC-MS)测定培养液和水稻幼苗的激素含量, 发现相比于大体积培养条件, 小体积培养液中的植物体内积累了较多的ABA、SA和JA-Ile等胁迫相关激素, 最终导致幼苗生长缓慢和生物量积累减少。结果表明植物可能通过感知根际激素浓度来预测外界水量限制, 并据此调节生长速度, 以最大限度地适应外界环境。研究结果对揭示根分泌激素的生理功能以及优化植物工厂的水培条件具有借鉴意义。
俞启璐, 赵江哲, 朱晓仙, 张可伟. 水稻根分泌激素调节生长速度. 植物学报, 2021, 56(2): 175-182.
Qilu Yu, Jiangzhe Zhao, Xiaoxian Zhu, Kewei Zhang. Regulation of Rice Growth by Root-secreted Phytohormones. Chinese Bulletin of Botany, 2021, 56(2): 175-182.
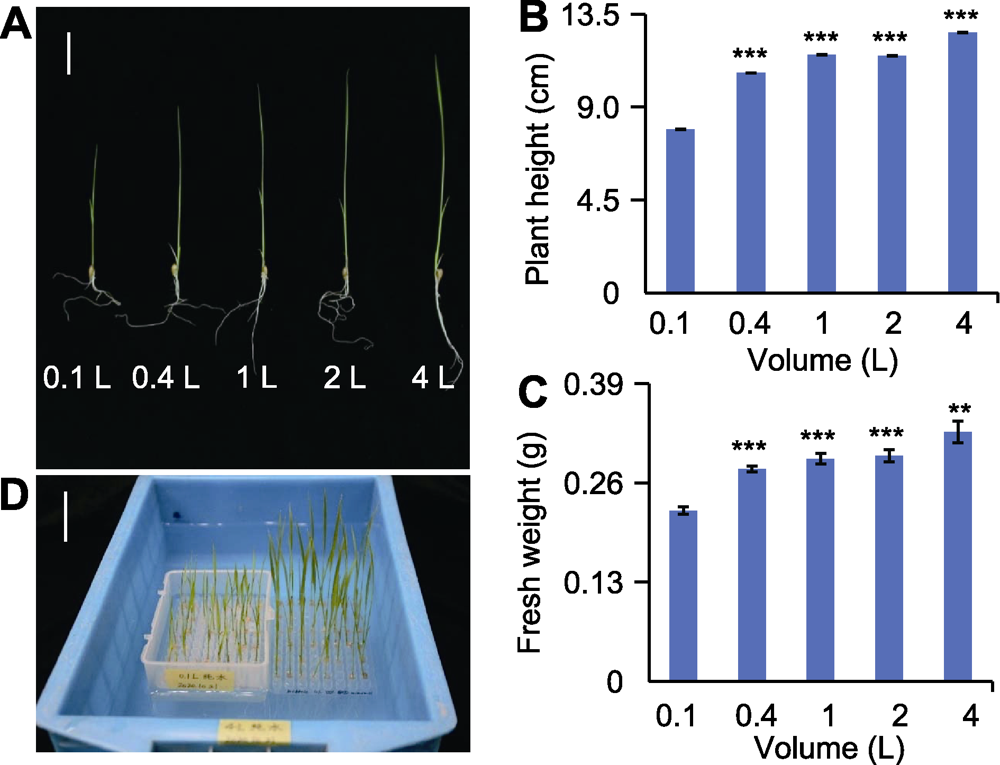
图1 不同体积水培养12天的水稻幼苗表型 (A)不同体积纯水培养12天后水稻幼苗表型(bar=3 cm); (B) 不同体积纯水培养12天后水稻幼苗株高(n=30); (C)不同体积纯水培养12天后水稻幼苗鲜重(n=30); (D) 0.1 L与4 L培养容器及幼苗表型(bar=3 cm)。** 差异显著(P<0.01), *** 差异极显著(P<0.001)。
Figure 1 The phenotype of 12-day rice seedlings grown in different water culture systems (A) Phenotypes of rice seedlings grown in different water culture systems for 12 days (bar=3 cm); (B) Plant height of rice seedlings grown in different water culture systems for 12 days (n=30); (C) Fresh weight of rice seedlings grown in different water culture systems for 12 days (n=30); (D) 0.1 and 4 L containers used in this experiment and the phenotypes of rice seedlings grown in them, respectively (bar=3 cm). ** significant difference at P<0.01; *** significant differences at P<0.001.
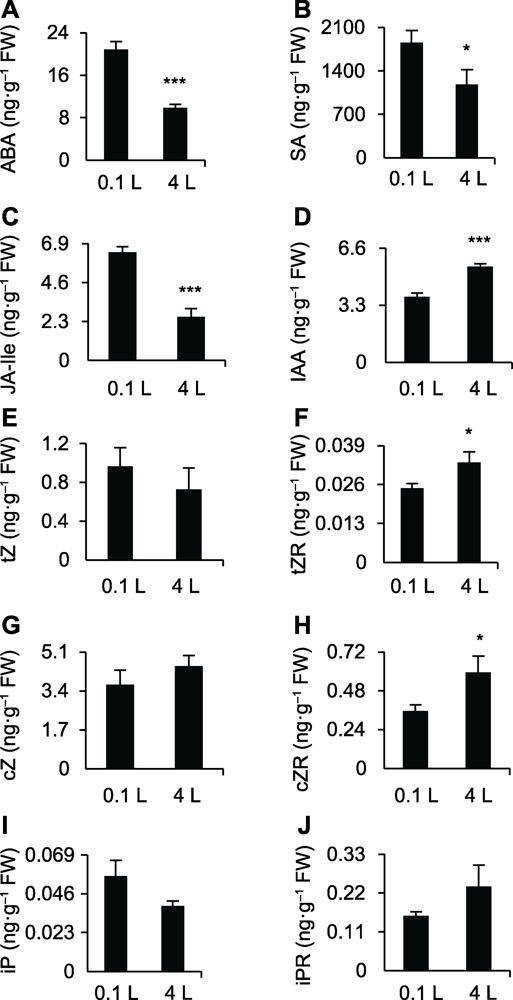
图2 不同体积水培条件下水稻幼苗地上部组织激素含量 (A)-(J)0.1和4 L纯水培养12天后水稻幼苗地上部组织ABA、SA、JA-Ile、IAA、tZ、tZR、cZ、cZR、iP和iPR的含量。* 差异显著(P<0.05); *** 差异极显著(P<0.001)。
Figure 2 Hormone profiling of shoot of rice seedlings grown in different water culture sytems (A)-(J) The ABA, SA, JA-Ile, IAA, tZ, tZR, cZ, cZR, iP, and iPR contents in shoot of rice seedlings after planting in 0.1 and 4 L water culture systems for 12 days, respectively. * significant differences at P<0.05; *** significant differences at P<0.001.
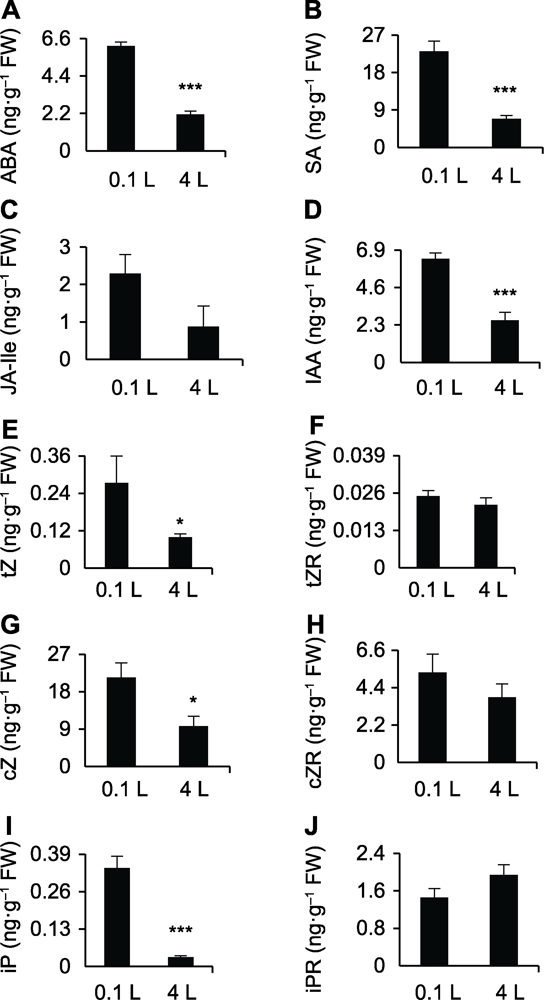
图3 不同体积水培条件下水稻幼苗根部激素含量 (A)-(J)0.1和4 L纯水培养12天后水稻幼苗根部ABA、SA、JA-Ile、IAA、tZ、tZR、cZ、cZR、iP和iPR的含量。* 差异显著(P<0.05); *** 差异极显著(P<0.001)。
Figure 3 Profiling of phytohormones in the root of rice after planting in different water culture systems (A)-(J)The ABA, SA, JA-Ile, IAA, tZ, tZR, cZ, cZR, iP, and iPR contents in the root of rice after planting in 0.1 and 4 L water culture systems, respectively. * significant differences at P<0.05; *** significant differences at P<0.001.
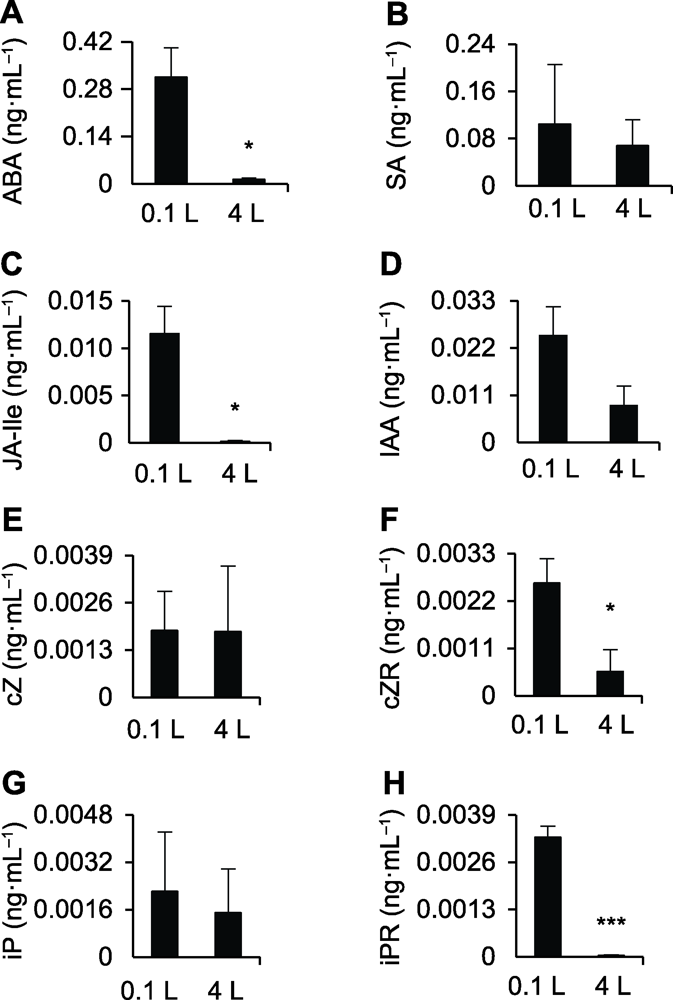
图4 不同体积水培后水中激素含量 (A)-(H)0.1和4 L纯水培养水稻幼苗12天后水中ABA、SA、JA-Ile、IAA、cZ、cZR、iP和iPR的含量。* 差异显著(P<0.05); *** 差异极显著(P<0.001)。
Figure 4 Phytohormone profiling of the different hydroculture systems (A)-(H) The ABA, SA, JA-Ile, IAA, cZ, cZR, iP, and iPR contents in 0.1 and 4 L water culture systems after being planted with rice seedlings for 12 days, respectively. * significant differences at P<0.05; *** significant differences at P<0.001.
| [1] | 程立超, 曾令鑫 (2020). 不同水培条件绿萝生长状况研究. 中国林副特产 (1),24-26, 30. |
| [2] |
代宇佳, 罗晓峰, 周文冠, 陈锋, 帅海威, 杨文钰, 舒凯 (2019). 生物和非生物逆境胁迫下的植物系统信号. 植物学报 54,255-264.
DOI URL |
| [3] | 甘林, 代玉立, 杨秀娟, 杜宜新, 石妞妞, 阮宏椿, 陈福如 (2020). 香蕉抗(感)病品种根系分泌物对枯萎病菌和枯草芽孢杆菌的生物效应. 应用生态学报 31,2279-2286. |
| [4] | 洪常青, 聂艳丽 (2003). 根系分泌物及其在植物营养中的作用. 生态环境 12,508-511. |
| [5] | 梁银丽, 康绍忠, 张成娥 (1999). 不同水分条件下小麦生长特性及氮磷营养的调节作用. 干旱地区农业研究 17(4),58-64. |
| [6] | 罗晓蔓, 周书宇, 杨雪 (2019). 植物根系分泌物的分类和作用. 安徽农业科学 47(4),37-39, 45. |
| [7] | 任伟, 高慧娟, 王润娟, 吕昕培, 何傲蕾, 邵坤仲, 汪永平, 张金林 (2020). 高等植物适应干旱生境研究进展. 草学 (3),4-15. |
| [8] | 孙珂, 周亚峰, 黄雅敏, 李会松, 孔倩倩 (2020). 果菜类蔬菜水培研究进展. 农业科技通讯 (3),25-27. |
| [9] | 岳杨, 曹世文, 王颖 (2010). 辽河下游平原区淹灌条件下水稻的需水规律. 东北水利水电 (2),58-59. |
| [10] | 张奇, 张清旭, 庞晓敏, 叶江华, 王海斌, 贾小丽, 何海斌 (2020). 稗草根系分泌物对水稻种子萌发和苗期生长的影响. 亚热带农业研究 16,8-15. |
| [11] | 张瑜, 刘玉红, 扎西顿珠, 杨亚辉, 代安国 (2020). 不同营养液浓度对水培生菜生长的影响. 西藏农业科技 42,54-56. |
| [12] | Albrecht T, Argueso CT (2017). Should I fight or should I grow now? The role of cytokinins in plant growth and immunity and in the growth-defence trade-off. Ann Bot 119,725-735. |
| [13] |
Bandurska H, Niedziela J, Pietrowska-Borek M, Nuc K, Chadzinikolau T, Radzikowska D (2017). Regulation of proline biosynthesis and resistance to drought stress in two barley ( Hordeum vulgare L.) genotypes of different origin. Plant Physiol Biochem 118,427-437.
DOI URL |
| [14] | Bhaskara GB, Nguyen TT, Verslues PE (2012). Unique drought resistance functions of the highly ABA-induced clade A protein phosphatase 2Cs. Plant Physiol 160,379-395. |
| [15] |
Bielach A, Hrtyan M, Tognetti VB (2017). Plants under stress: involvement of auxin and cytokinin. Int J Mol Sci 18,1427.
DOI URL |
| [16] |
Cortleven A, Leuendorf JE, Frank M, Pezzetta D, Bolt S, Schmülling T (2019). Cytokinin action in response to abiotic and biotic stresses in plants. Plant Cell Environ 42,998-1018.
DOI PMID |
| [17] |
Fang YJ, Xiong LZ (2015). General mechanisms of drought response and their application in drought resistance improvement in plants. Cell Mol Life Sci 72,673-689.
DOI URL |
| [18] |
Gargallo-Garriga A, Preece C, Sardans J, Oravec M, Urban O, Peñuelas J (2018). Root exudate metabolomes change under drought and show limited capacity for recovery. Sci Rep 8,12696.
DOI URL |
| [19] |
Hu HH, Xiong LZ (2014). Genetic engineering and breeding of drought-resistant crops. Annu Rev Plant Biol 65,715- 741.
DOI URL |
| [20] |
Huang JL, Zhai JQ, Jiang T, Wang YJ, Li XC, Wang R, Xiong M, Su BD, Thomas F (2018). Analysis of future drought characteristics in China using the regional climate model CCLM. Climate Dyn 50,507-525.
DOI URL |
| [21] | Kramer PJ (1983). Water Relation of Plant. New York: Academic Press. pp.168-191. |
| [22] |
Luo J, Zhou JJ, Zhang JZ (2018). Aux/ IAA gene family in plants: molecular structure, regulation, and function. Int J Mol Sci 19,259.
DOI URL |
| [23] | Mohammadian MA, Watling JR, Hill RS (2007). The impact of epicuticular wax on gas-exchange and photoinhibition in Leucadendron lanigerum (Proteaceae). Acta Oecol 31,93-101. |
| [24] | North GB, Nobel PS (1992). Drought-induced changes in hydraulic conductivity and structure in roots of Ferocactus acanthodes and Opuntia ficus-indica. New Phytol 120,9-19. |
| [25] | Ruan JJ, Zhou YX, Zhou ML, Yan J, Khurshid M, Weng WF, Cheng JP, Zhang KX (2019). Jasmonic acid signaling pathway in plants. Int J Mol Sci 20,2479. |
| [26] | Seki M, Umezawa T, Urano K, Shinozaki K (2007). Regulatory metabolic networks in drought stress responses. Curr Opin Plant Biol 10,296-302. |
| [27] | Shinozaki K, Yamaguchi-Shinozaki K (1997). Gene expression and signal transduction in water-stress response. Plant Physiol 115,327-334. |
| [28] | Takahashi F, Kuromori T, Sato H, Shinozaki K (2018). Regulatory gene networks in drought stress responses and resistance in plants. Adv Exp Med Biol 1081,189-214. |
| [29] | Tardieu F (2013). Plant response to environmental conditions: assessing potential production, water demand, and negative effects of water deficit. Front Physiol 4,17. |
| [30] | Valenzuela CE, Acevedo-Acevedo O, Miranda GS, Vergara-Barros P, Holuigue L, Figueroa CR, Figueroa PM (2016). Salt stress response triggers activation of the jasmonate signaling pathway leading to inhibition of cell elongation in Arabidopsis primary root. J Exp Bot 67,4209- 4220. |
| [31] |
Verma V, Ravindran P, Kumar PP (2016). Plant hormone-mediated regulation of stress responses. BMC Plant Biol 16,86.
DOI URL |
| [32] |
Vives-Peris V, Gómez-Cadenas A, Pérez-Clemente RM (2017). Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep 36, 1971- 1984.
DOI URL |
| [33] |
Vlot AC, Dempsey DMA, Klessig DF (2009). Salicylic acid, a multifaceted hormone to combat disease. Annu Rev Phytopathol 47,177-206.
DOI URL |
| [34] |
Wybouw B, De Rybel B (2019). Cytokinin—a developing story. Trends Plant Sci 24,177-185.
DOI URL |
| [35] |
Xiong LM, Schumaker KS, Zhu JK (2002). Cell signaling during cold, drought, and salt stress. Plant Cell 14,S165- S183.
DOI URL |
| [36] |
Zhao JZ, Yu NN, Ju M, Fan B, Zhang YJ, Zhu EG, Zhang MY, Zhang KW (2019). ABC transporter OsABCG18 controls the shootward transport of cytokinins and grain yield in rice. J Exp Bot 70,6277-6291.
DOI URL |
| [37] |
Zou JJ, Wei FJ, Wang C, Wu JJ, Ratnasekera D, Liu WX, Wu WH (2010). Arabidopsis calcium-dependent protein kinase CPK10 functions in abscisic acid- and Ca 2+-mediated stomatal regulation in response to drought stress. Plant Physiol 154,1232-1243.
DOI URL |
| [1] | 王娟, 张登山, 肖元明, 裴全帮, 王博, 樊博, 周国英. 长期围封后高寒草原植物根系分泌物特征与环境因子关系[J]. 植物生态学报, 2025, 49(4): 596-609. |
| [2] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [3] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [4] | 刘旭鹏, 王敏, 韩守安, 朱学慧, 王艳蒙, 潘明启, 张雯. 植物器官脱落调控因素及分子机理研究进展[J]. 植物学报, 2025, 60(3): 472-482. |
| [5] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [6] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [7] | 李青洋, 刘翠, 何李, 彭姗, 马嘉吟, 胡子祎, 刘宏波. 甘蓝型油菜BnaA02.CPSF6基因的克隆及功能分析(长英文摘要)[J]. 植物学报, 2025, 60(1): 62-73. |
| [8] | 王亚萍, 包文泉, 白玉娥. 单细胞转录组学在植物生长发育及胁迫响应中的应用进展[J]. 植物学报, 2025, 60(1): 101-113. |
| [9] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [10] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [11] | 闫恒宇, 李朝霞, 李玉斌. 高温对玉米生长的影响及中国耐高温玉米筛选研究进展[J]. 植物学报, 2024, 59(6): 1007-1023. |
| [12] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| [13] | 杜庆国, 李文学. lncRNA调控玉米生长发育和非生物胁迫研究进展[J]. 植物学报, 2024, 59(6): 950-962. |
| [14] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [15] | 路笃贤, 张严妍, 刘艳, 李岩竣, 左新秀, 林金星, 崔亚宁. 非编码RNA在植物生长发育及逆境响应中的研究进展[J]. 植物学报, 2024, 59(5): 709-725. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||