

植物学报 ›› 2024, Vol. 59 ›› Issue (6): 878-882.DOI: 10.11983/CBB24171 cstr: 32102.14.CBB24171
所属专题: 玉米生物学与分子设计(2024年59卷6期)
收稿日期:2024-11-09
接受日期:2024-11-15
出版日期:2024-11-10
发布日期:2024-11-15
通讯作者:
*林荣呈, 湘湖实验室(农业浙江省实验室)研究员, 博士生导师, 国家“杰出青年基金”获得者。曾在中国科学院植物研究所工作, 入选中国科学院人才计划、国家百千万人才工程和国家级人才计划领军人才。主要从事植物光信号与光合作用研究, 论文发表于Science、Nature Communications、Proceedings of the National Academy of Sciences of the USA、Molecular Plant和Plant Cell等学术期刊。兼任中国植生学会常务理事及光合作用专业委员会主任、中国植物学会理事及智能植物工厂分会会长等职。担任《植物学报》副主编。E-mail: linrongcheng@xhlab.ac.cn
基金资助:Received:2024-11-09
Accepted:2024-11-15
Online:2024-11-10
Published:2024-11-15
Contact:
*E-mail: linrongcheng@xhlab.ac.cn
摘要: 隐花色素(CRY)是调节植物光反应的蓝光受体。CRY在黑暗中以无活性的单体形式存在, 吸收光子后构象变化并发生寡聚化, 同时改变了其与互作蛋白间的亲和力, 进而调控光反应蛋白的转录或稳定性以调节植物的生长发育。最近的一项研究发现了CRY2的一个精巧作用机制, CRY不仅可被蓝光“激活”, 还可被黑暗信号“激活”, 从而构建起光信号和暗信号依赖的光受体信号转导更节能的模式。他们发现CRY2即便在黑暗中也能抑制根尖分生组织中的细胞分裂, 调控根的伸长, 并控制大量基因的表达。FL1和FL3与细胞分裂基因的染色质结合以促进其转录。需要说明的是, 只有黑暗中的CRY2单体可与FL1/FL3相互作用, 从而抑制后者促进根伸长的功能, 蓝光则解除该抑制作用。这一发现重塑了人们对光受体的认识, 为理解植物感知和响应不同信号以调节生长和适应性提供了全新的视角, 对深入理解基因的功能极具启发意义。
景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”. 植物学报, 2024, 59(6): 878-882.
Yanjun Jing, Rongcheng Lin. Blue Light Receptor CRY2 Transforms into a ‘dark dancer’. Chinese Bulletin of Botany, 2024, 59(6): 878-882.
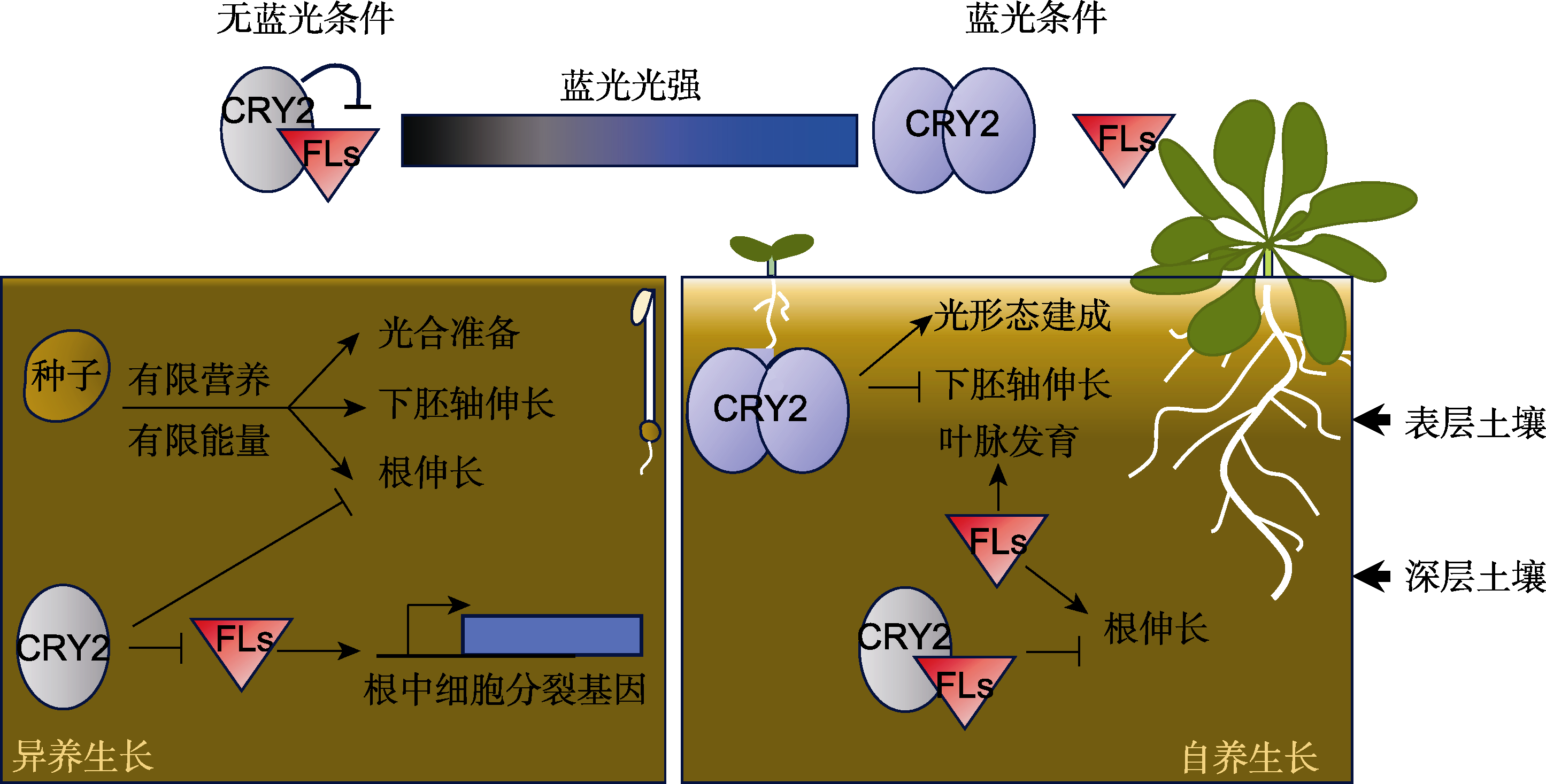
图1 不同蓝光条件下CRY2的作用模型 箭头表示促进作用; T形线表示抑制作用。在深层土壤(无蓝光)中, CRY2与FL1/FL3互作, 抑制FLs促进根伸长的能力。种子中有限的营养和能量被用于下胚轴伸长和异养生长过程中光合作用的准备(左)。在表层土壤中, 蓝光激活的CRY2抑制下胚轴伸长, CRY2与FLs间的互作被抑制, 释放了FLs促进根伸长的能力(右)。在成熟植物中, CRY2和FLs协同调控植物地上部的光形态建成和叶脉发育, 同时抑制深层土壤中的根伸长。
Figure 1 Proposed model of CRY2 function under different blue-light regimes Arrows indicate positive regulation and bars indicate negative regulation. Without blue light, such as in deep soil, CRY2 physically interacts with FL1 and FL3 to inhibit the function of FLs in promoting root elongation by accelerating cell division in the root. Limited nutrients and energy in seeds are directed toward hypocotyl elongation and preparation for photosynthesis during hete-rotrophic growth (left). In topsoil, blue light not only enhances CRY2’s photoresponse to inhibit hypocotyl elongation but also hinders the interaction between CRY2 and FLs. This liberates the capacity of FLs to then stimulate root elongation (right). In mature plants, CRY2 and FLs coordinate photomorphogenesis and leaf-vein development above ground while inhibiting root elongation in deep soil.
| [1] |
Canamero RC, Bakrim N, Bouly JP, Garay A, Dudkin EE, Habricot Y, Ahmad M (2006). Cryptochrome photoreceptors cry1 and cry2 antagonistically regulate primary root elongation in Arabidopsis thaliana. Planta 224, 995-1003.
DOI PMID |
| [2] | Guo TT, Liu MQ, Chen L, Liu Y, Li L, Li YP, Cao XL, Mao ZL, Wang WX, Yang HQ (2023). Photoexcited cryptochromes interact with ADA2b and SMC5 to promote the repair of DNA double-strand breaks in Arabidopsis. Nat Plants 9, 1280-1290. |
| [3] | Jiang BC, Zhong ZH, Gu LF, Zhang XF, Wei JB, Ye C, Lin GF, Qu GP, Xiang X, Wen CJ, Hummel M, Bailey-Seres J, Wang Q, He C, Wang X, Lin CT (2023). Light-induced LLPS of the CRY2/SPA1/FIO1 complex regulating m-RNA methylation and chlorophyll homeostasis in Arabidopsis. Nat Plants 9, 2042-2058. |
| [4] | Li C, Qi LJ, Gu XF, Li JG (2022). Research progress on TZP, a novel key regulator of light signal transduction in plants. Chin Bull Bot 57, 579-587. (in Chinese) |
|
李聪, 齐立娟, 谷晓峰, 李继刚 (2022). 植物光信号途径重要新调控因子TZP的研究进展. 植物学报 57, 579-587.
DOI |
|
| [5] | Liu HT, Yu XH, Li KW, Klejnot J, Yang HY, Lisiero D, Lin CT (2008). Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322, 1535-1539. |
| [6] | Liu Q, Wang Q, Deng WX, Wang X, Piao M, Cai DW, Li YX, Barshop WD, Yu XL, Zhou TT, Liu B, Oka Y, Wohlschlegel J, Zuo ZC, Lin CT (2017). Molecular basis for blue light-dependent phosphorylation of Arabidopsis cryptochrome 2. Nat Commun 8, 15234. |
| [7] |
Prabhakaran Mariyamma N, Clarke KJ, Yu HL, Wilton EE, Van Dyk J, Hou HW, Schultz EA (2018). Members of the Arabidopsis FORKED1-LIKE gene family act to localize PIN1 in developing veins. J Exp Bot 69, 4773-4790.
DOI PMID |
| [8] | Shalitin D, Yang HY, Mockler TC, Maymon M, Guo HW, Whitelam GC, Lin CT (2002). Regulation of Arabidopsis cryptochrome 2 by blue-light-dependent phosphorylation. Nature 417, 763-767. |
| [9] |
Shao K, Zhang X, Li X, Hao YH, Huang XW, Ma ML, Zhang MH, Yu F, Liu HT, Zhang P (2020). The oligomeric structures of plant cryptochromes. Nat Struct Mol Biol 27, 480-488.
DOI PMID |
| [10] |
Wang H, Ma LG, Li JM, Zhao HY, Deng XW (2001). Direct interaction of Arabidopsis cryptochromes with COP1 in light control development. Science 294, 154-158.
DOI PMID |
| [11] |
Wang Q, Lin CT (2020). Mechanisms of cryptochrome- mediated photoresponses in plants. Annu Rev Plant Biol 71, 103-129.
DOI PMID |
| [12] |
Wang Q, Zuo ZC, Wang X, Gu LF, Yoshizumi T, Yang ZH, Yang L, Liu Q, Liu W, Han YJ, Kim JI, Liu B, Wohlschlegel JA, Matsui M, Oka Y, Lin CT (2016). Photoactivation and inactivation of Arabidopsis cryptochrome 2. Science 354, 343-347.
PMID |
| [13] | Yang LW, Liu SR, Lin RC (2019) . Advances in light and hormones in regulating seed dormancy and germination. Chin Bull Bot 54, 569-581. (in Chinese) |
|
杨立文, 刘双荣, 林荣呈 (2019). 光信号与激素调控种子休眠和萌发研究进展. 植物学报 54, 569-581.
DOI |
|
| [14] | Yang YJ, Zuo ZC, Zhao XY, Li X, Klejnot J, Li Y, Chen P, Liang SP, Yu XH, Liu XM, Lin CT (2008). Blue-light-independent activity of Arabidopsis cryptochromes in the regulation of steady-state levels of protein and mRNA expression. Mol Plant 1, 167-177. |
| [15] | Zeng D, Lv J, Li X, Liu H (2024). The Arabidopsis blue-light photoreceptor CRY2 is active in darkness to inhibit root growth. Cell doi: 10.1016/j.cell.2024.10.031. |
| [16] | Zuo ZC, Liu HT, Liu B, Liu XM, Lin CT (2011). Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21, 841-847. |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制[J]. 植物学报, 2025, 60(4): 551-561. |
| [2] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [3] | 孙苗苗, 张蔚, 张林霞, 霍竣涛, 李志能, 刘国锋. 矮牵牛花朵大小遗传规律及相关基因的表达分析[J]. 植物学报, 2024, 59(3): 422-432. |
| [4] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [5] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [6] | 朱宝, 赵江哲, 张可伟, 黄鹏. 水稻细胞分裂素氧化酶9参与调控水稻叶夹角发育[J]. 植物学报, 2024, 59(1): 10-21. |
| [7] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [8] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [9] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [10] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [11] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [12] | 李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展[J]. 植物学报, 2021, 56(4): 462-469. |
| [13] | 王婷, 羊欢欢, 赵弘巍, JosefVoglmeir, 刘丽. 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021, 56(3): 262-274. |
| [14] | 宋松泉, 刘军, 杨华, 张文虎, 张琪, 高家东. 细胞分裂素调控种子发育、休眠与萌发的研究进展[J]. 植物学报, 2021, 56(2): 218-231. |
| [15] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
