

植物学报 ›› 2021, Vol. 56 ›› Issue (4): 404-413.DOI: 10.11983/CBB20207 cstr: 32102.14.CBB20207
车永梅†, 孙艳君†, 卢松冲, 侯丽霞, 范欣欣, 刘新*( )
)
收稿日期:2020-12-22
接受日期:2021-04-19
出版日期:2021-07-01
发布日期:2021-06-30
通讯作者:
刘新
作者简介:*E-mail: liuxin6080@126.com†共同第一作者
基金资助:
Yongmei Che†, Yanjun Sun†, Songchong Lu, Lixia Hou, Xinxin Fan, Xin Liu*( )
)
Received:2020-12-22
Accepted:2021-04-19
Online:2021-07-01
Published:2021-06-30
Contact:
Xin Liu
About author:First author contact:†These authors contributed equally to this paper
摘要: 转录因子MYB77与信号分子一氧化碳(NO)是侧根发育的重要调节因子, 但MYB77和NO在干旱胁迫下侧根发生中的作用及机制尚不明确。该文以拟南芥(Arabidopsis thaliana)野生型、AtMYB77缺失突变体Atmyb77-1及过表达株系AtOE77-1和AtOE77-3为材料, 研究了MYB77和NO在干旱胁迫下侧根发生中的作用。结果表明, AtMYB77受干旱胁迫诱导, AtMYB77缺失导致干旱胁迫下侧根发育相关基因CYCA2;1和CDKA;1表达下调, 同时Atmyb77-1的侧根数目和长度显著低于野生型, AtMYB77过表达则作用相反, 表明AtMYB77参与干旱胁迫下侧根发育的调控过程。干旱胁迫下, 拟南芥根系NO含量显著升高, NO合成关键酶NO合酶(NOS)和硝酸还原酶(NR)活性及基因表达上调, Atmyb77-1中NO含量、NOS和NR活性及基因表达量显著低于野生型, 而AtOE77-1和AtOE77-3根系NO含量及合成酶活性和基因表达量显著高于野生型。外施NO供体硝普钠(SNP)能缓解AtMYB77缺失对CYCA2;1和CDKA;1表达及侧根生长的抑制, NO清除剂或合成抑制剂则削弱AtMYB77过表达对侧根生长的促进作用。上述结果表明, AtMYB77通过促进NO合成参与干旱诱导的拟南芥侧根生长过程, 研究结果为深入解析干旱诱导侧根生长的信号转导机制和培育耐旱植物奠定了理论基础。
车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育. 植物学报, 2021, 56(4): 404-413.
Yongmei Che, Yanjun Sun, Songchong Lu, Lixia Hou, Xinxin Fan, Xin Liu. AtMYB77 Involves in Lateral Root Development via Regulating Nitric Oxide Biosynthesis under Drought Stress in Arabidopsis thaliana. Chinese Bulletin of Botany, 2021, 56(4): 404-413.
| Primer name | Primer sequence (5°-3°) |
|---|---|
| AtMYB77-FP | GGAGAAGGACGTAGAGGTGAG |
| AtMYB77-RP | GGTGTTATTACTCCACAATCCCTA |
| AtCDKA;1-FP | GAGGATACATGGCGTGGGGTA |
| AtCDKA;1-RP | GCGTTGATTCTTTTGGTCGGA |
| AtCYCA2;1-FP | GCCCCTGAAATCCACTACAAT |
| AtCYCA2;1-RP | AGAGACCTCCACAAGCCAATC |
| AtNia1-FP | AGGTCCACTAGGGCACATCG |
| AtNia1-RP | TTCGTCCTCTGGATCACTCAATAT |
| AtNia2-FP | TTCTTACAAACCTCCCGTTCCAG |
| AtNia2-RP | GATTTTCTTATCATCTCCTTGTAGT |
| AtNOS1-FP | GATTCTCCGGGATTTGTCGA |
| AtNOS1-RP | CCTCCATTACCACCAACTGCT |
表1 定量PCR引物序列
Table 1 The primers used for quantitative PCR analysis
| Primer name | Primer sequence (5°-3°) |
|---|---|
| AtMYB77-FP | GGAGAAGGACGTAGAGGTGAG |
| AtMYB77-RP | GGTGTTATTACTCCACAATCCCTA |
| AtCDKA;1-FP | GAGGATACATGGCGTGGGGTA |
| AtCDKA;1-RP | GCGTTGATTCTTTTGGTCGGA |
| AtCYCA2;1-FP | GCCCCTGAAATCCACTACAAT |
| AtCYCA2;1-RP | AGAGACCTCCACAAGCCAATC |
| AtNia1-FP | AGGTCCACTAGGGCACATCG |
| AtNia1-RP | TTCGTCCTCTGGATCACTCAATAT |
| AtNia2-FP | TTCTTACAAACCTCCCGTTCCAG |
| AtNia2-RP | GATTTTCTTATCATCTCCTTGTAGT |
| AtNOS1-FP | GATTCTCCGGGATTTGTCGA |
| AtNOS1-RP | CCTCCATTACCACCAACTGCT |
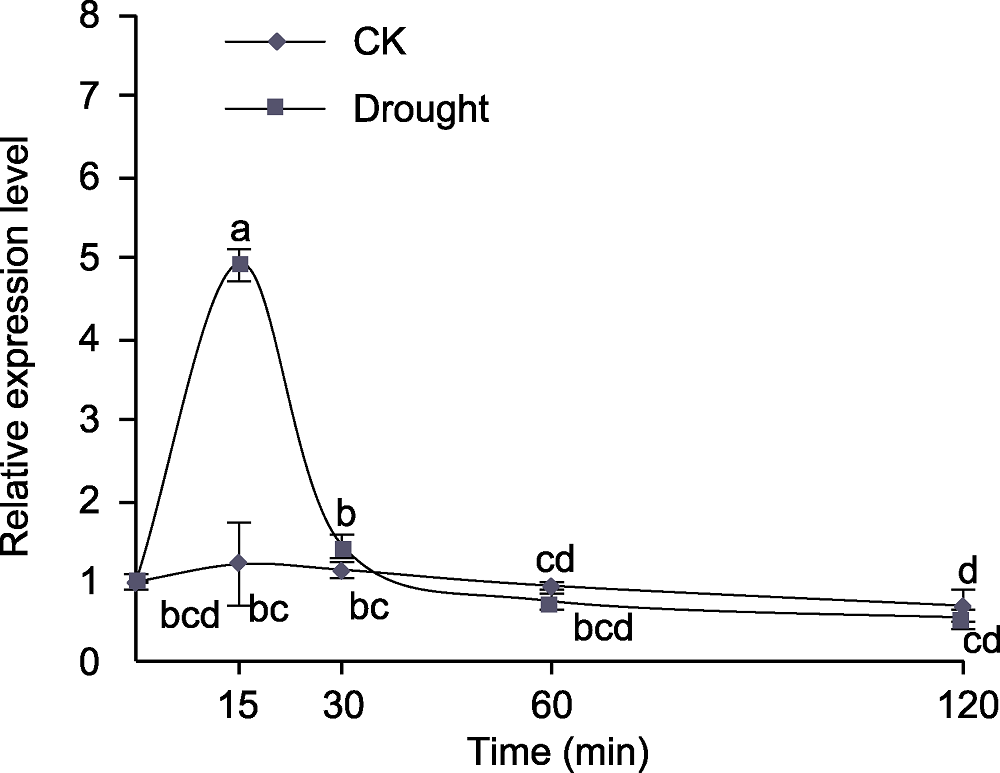
图1 干旱胁迫对拟南芥根部AtMYB77表达的影响 CK: 对照。不同小写字母表示不同处理间差异显著(P<0.05)。
Figure 1 The effect of drought stress on AtMYB77 expression in Arabidopsis roots CK: Control. Different lowercase letters indicate significant differences among different treatments at P<0.05.
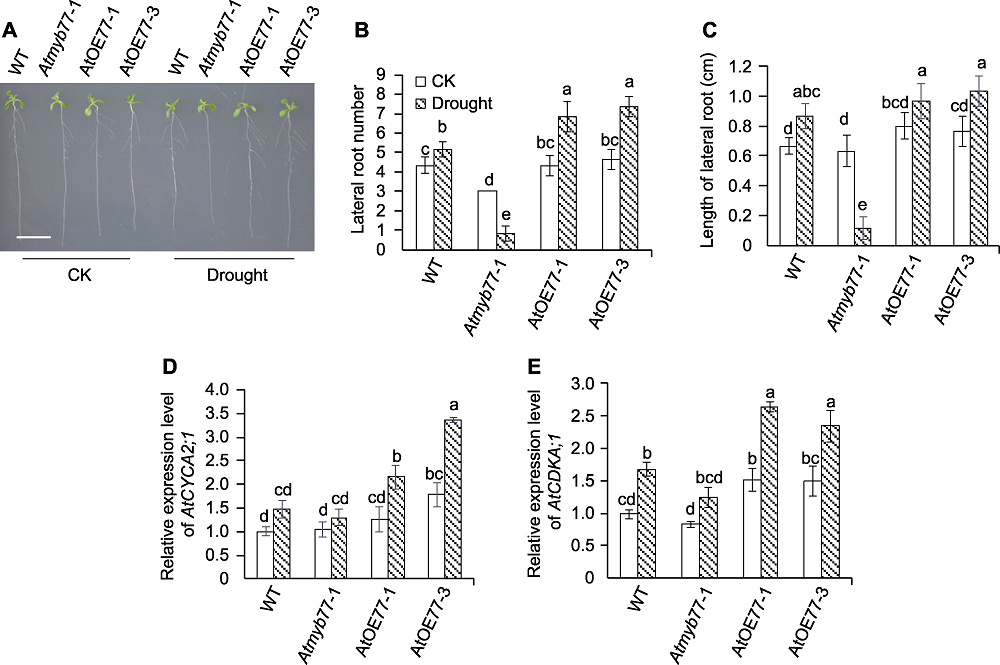
图2 干旱对拟南芥Atmyb77-1突变体和AtMYB77过表达株系侧根生长和发育相关基因表达的影响 (A) 干旱胁迫下Atmyb77-1突变体根系生长表型(Bar=1 cm); (B) 干旱对拟南芥Atmyb77-1突变体和AtMYB77过表达株系侧根数目的影响; (C) 干旱对拟南芥Atmyb77-1突变体和AtMYB77过表达株系侧根长度的影响; (D) 干旱对拟南芥Atmyb77-1突变体和AtMYB77过表达株系根部AtCYCA2;1表达的影响; (E) 干旱对拟南芥Atmyb77-1突变体和AtMYB77过表达株系根部AtCDKA;1表达的影响。CK: 对照; WT: 野生型。不同小写字母表示不同株系的不同处理间差异显著(P<0.05)。
Figure 2 Effects of drought stress on lateral root growth and expression of lateral root development related genes in Arabidopsis Atmyb77-1 mutant and AtMYB77 overexpression lines (A) Root phenotypes of Atmyb77-1 mutant under drought stress (Bar=1 cm); (B) Effects of drought stress on lateral root number of Atmyb77-1 mutant and AtMYB77 overexpression lines; (C) Effects of drought stress on lateral root length of Atmyb77-1 mutant and AtMYB77 overexpression lines; (D) Effects of drought stress on relative expression level of AtCYCA2;1 in roots of Arabidopsis Atmyb77-1 mutant and AtMYB77 overexpression lines; (E) Effects of drought stress on relative expression level of AtCDKA;1 in roots of Arabidopsis Atmyb77-1 mutant and AtMYB77 overexpression lines. CK: Control; WT: Wild type. Different lowercase letters indicate significant differences among different treatments of different lines at P<0.05.
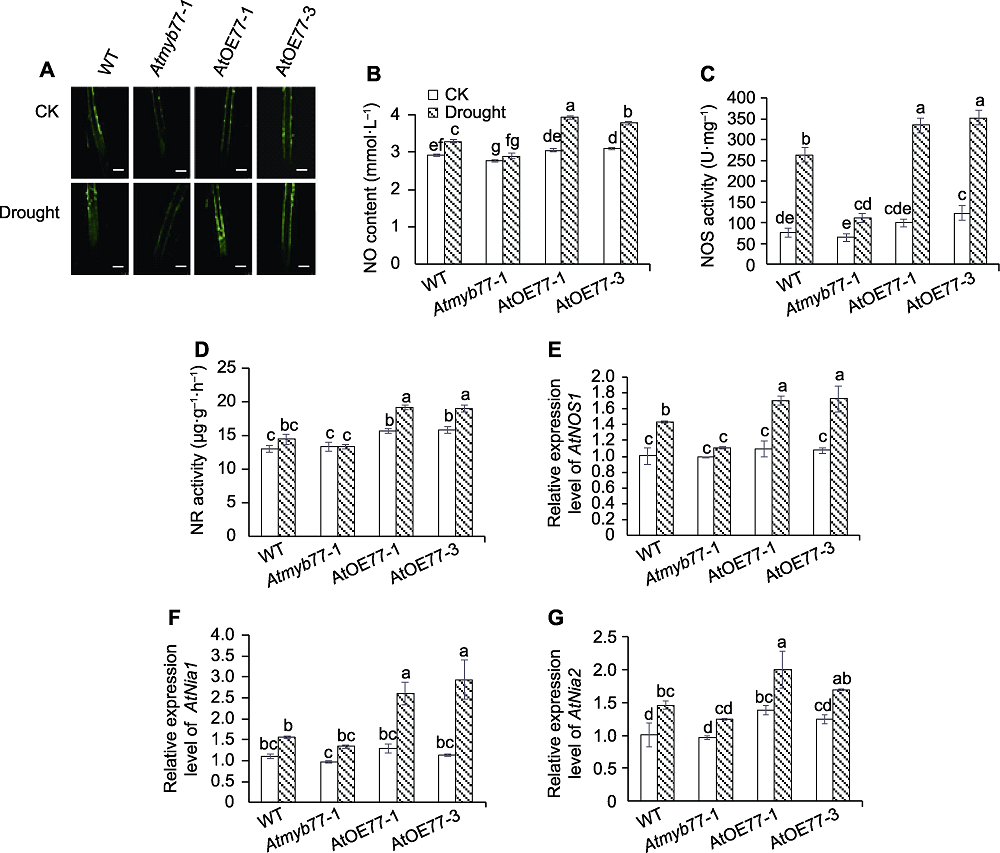
图3 干旱对拟南芥根部NO含量及NO合成酶活性和相关基因表达的影响 (A) 干旱胁迫下拟南芥根部NO荧光成像(Bars=100 μm); (B) 干旱对拟南芥根部NO含量的影响; (C) 干旱对拟南芥根部NOS活性的影响; (D) 干旱对拟南芥根部NR活性的影响; (E) 干旱对拟南芥根部AtNOS1表达量的影响; (F) 干旱对拟南芥根部AtNia1表达量的影响; (G) 干旱对拟南芥根部AtNia2表达量的影响。CK: 对照; WT: 野生型; NOS: 一氧化氮合酶; NR: 硝酸还原酶。不同小写字母表示不同株系的不同处理间差异显著(P<0.05)。
Figure 3 Effects of drought stress on NO content, activities and gene expression of NO synthesis enzymes in Arabidopsis roots (A) NO fluorescence imaging of Arabidopsis roots under drought stress (Bars=100 μm); (B) Effects of drought stress on NO content in Arabidopsis roots; (C) Effects of drought stress on NOS activity in Arabidopsis roots; (D) Effects of drought stress on NR activity in Arabidopsis roots; (E) Effects of drought stress on relative expression level of AtNOS1 in Arabidopsis roots; (F) Effects of drought stress on relative expression level of AtNia1 in Arabidopsis roots; (G) Effects of drought stress on relative expression level of AtNia2 in Arabidopsis roots. CK: Control; WT: Wild type; NOS: Nitric oxide synthase; NR: Nitrate reductase. Different lowercase letters indicate significant differences among different treatments of different lines at P<0.05.
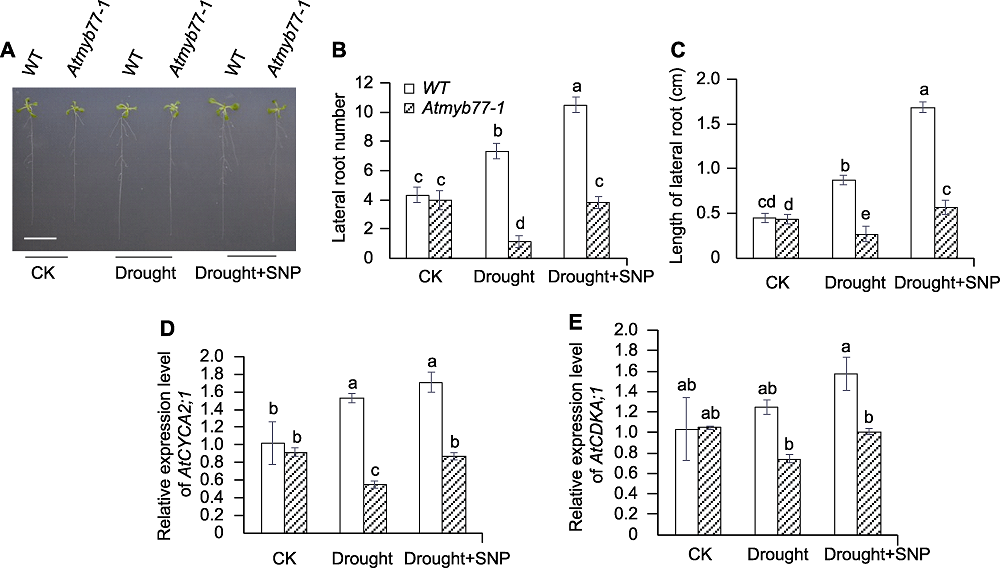
图4 NO供体硝普钠(SNP)对干旱条件下拟南芥Atmyb77-1缺失突变体侧根生长和发育关键基因表达的影响 (A) SNP对干旱胁迫下Atmyb77-1突变体根系生长的影响(Bar=1 cm); (B) SNP对干旱条件下Atmyb77-1缺失突变体侧根数目的影响; (C) SNP对干旱条件下Atmyb77-1缺失突变体侧根长度的影响; (D) SNP对干旱条件下Atmyb77-1缺失突变体AtCYCA2;1表达的影响; (E) SNP对干旱条件下Atmyb77-1缺失突变体AtCDKA;1表达的影响。CK: 对照; WT: 野生型。不同小写字母表示不同株系的不同处理间差异显著(P<0.05)。
Figure 4 Effects of NO donor sodium nitroprusside (SNP) on lateral root growth and expression of lateral root development related genes in Arabidopsis Atmyb77-1 mutant under drought condition (A) The effect of SNP on root growth of Atmyb77-1 mutant under drought stress (Bar=1 cm); (B) The effect of SNP on lateral root number in Atmyb77-1 mutant under drought condition; (C) The effect of SNP on lateral root length in Atmyb77-1 mutant under drought condition; (D) The effect of SNP on relative expression level of AtCYCA2;1 in Atmyb77-1 mutant root under drought condition; (E) The effect of SNP on relative expression level of AtCDKA;1 in Atmyb77-1 mutant root under drought condition. CK: Control; WT: Wild type. Different lowercase letters indicate significant differences among different treatments of different lines at P<0.05.
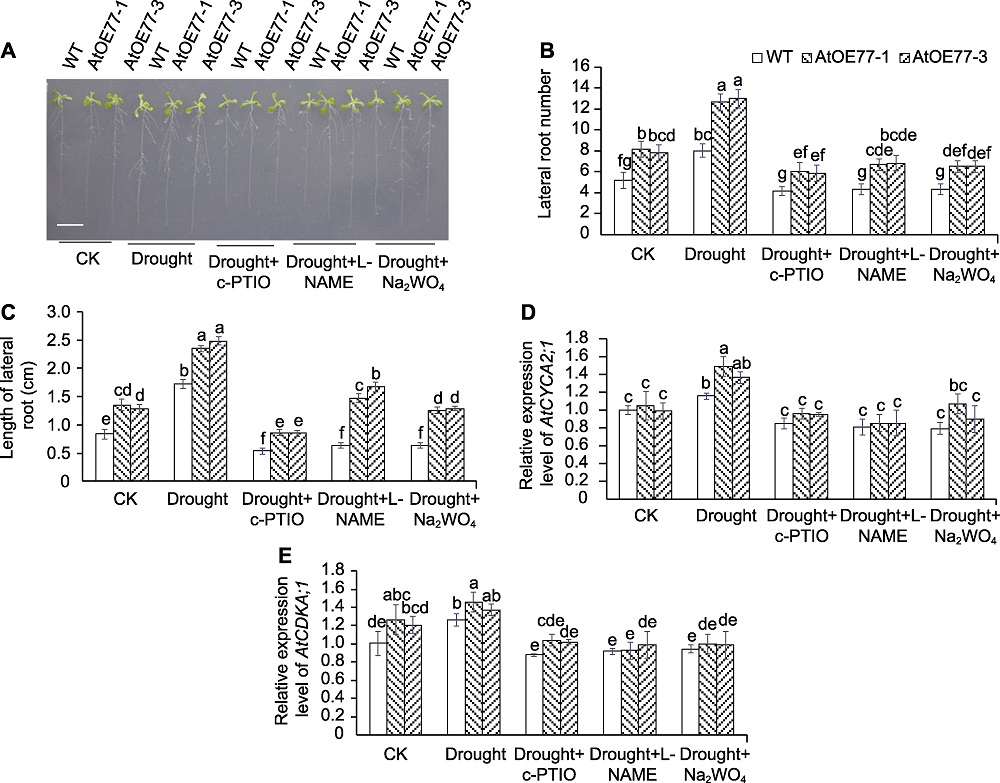
图5 NO清除剂(c-PTIO)或合成抑制剂(L-NAME)对干旱条件下AtMYB77过表达株系侧根生长和发育关键基因表达的影响 (A) NO清除剂或合成抑制剂对干旱胁迫下AtMYB77过表达株系根系生长的影响(Bar=1 cm); (B) NO清除剂或合成抑制剂对干旱条件下AtMYB77过表达株系侧根数目的影响; (C) NO清除剂或合成抑制剂对干旱条件下AtMYB77过表达株系侧根长度的影响; (D) NO清除剂或合成抑制剂对干旱条件下AtMYB77过表达株系根部AtCYCA2;1表达量的影响; (E) NO清除剂或合成抑制剂对干旱条件下AtMYB77过表达株系根部AtCDKA;1表达量的影响。CK: 对照; WT: 野生型。不同小写字母表示不同株系的不同处理间差异显著(P<0.05)。
Figure 5 Effects of NO scavenger (c-PTIO) or biosynthesis inhibitor (L-NAME) on lateral root growth and expression of lateral root development related genes in AtMYB77 overexpression lines under drought condition (A) The effects of NO scavenger or biosynthesis inhibitor on root growth of AtMYB77 overexpression lines subjected to drought stress (Bar=1 cm); (B) The effects of NO scavenger or biosynthesis inhibitor on lateral root number of AtMYB77 overexpression lines under drought condition; (C) The effects of NO scavenger or biosynthesis inhibitor on lateral root length of AtMYB77 overexpression lines under drought condition; (D) The effects of NO scavenger or biosynthesis inhibitor on relative expression level of AtCYCA2;1 in AtMYB77 overexpression lines under drought condition; (E) The effects of NO scavenger or biosynthesis inhibitor on relative expression level of AtCDKA;1 in AtMYB77 overexpression lines under drought condition. CK: Control; WT: Wild type. Different lowercase letters indicate significant differences among different treatments of different lines at P<0.05.
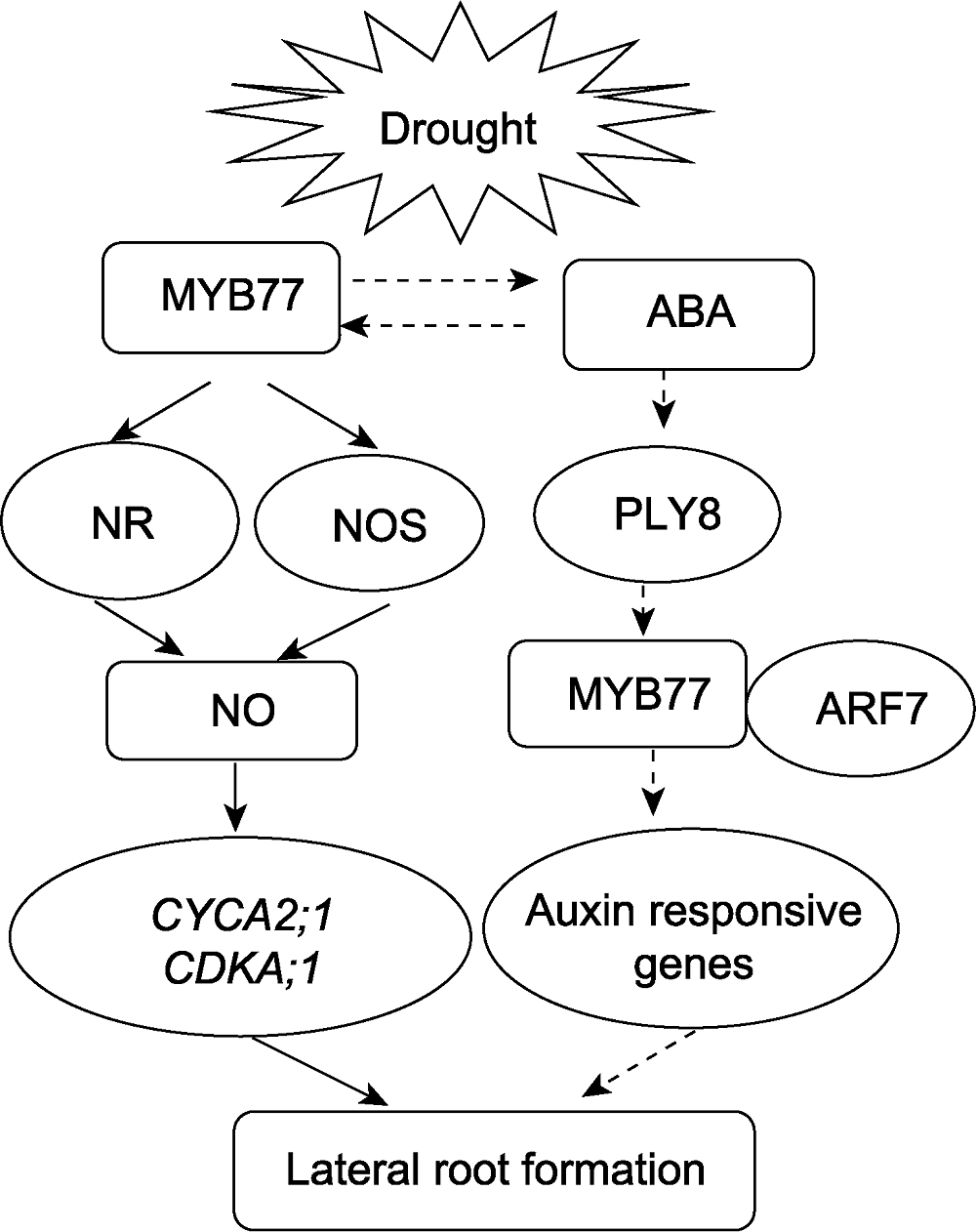
图6 AtMYB77参与干旱胁迫下拟南芥侧根发育的工作模型 实线部分为本文结果, 虚线部分为根据已有报道及推测可能存在的作用。ABA: 脱落酸; NR: 硝酸还原酶; NOS: 一氧化氮合酶
Figure 6 Working model of AtMYB77 function in regulating lateral roots development under drought stress in Arabidopsis The solid lines indicate the results of this study, and the dotted lines indicate the possible roles based on reports and speculation. ABA: Abscisic acid; NR; Nitrate reductase; NOS: Nitric oxide synthase
| [1] | 车永梅, 孙艳君, 卢松冲, 赵方贵, 侯丽霞, 刘新 (2018). AtWRKY40参与拟南芥干旱胁迫响应过程. 植物生理学报 54, 456-464. |
| [2] | 刘国华, 刘菁, 侯丽霞, 唐静, 刘新 (2009). NO可能作为Ca2+的下游信号介导乙烯诱导的蚕豆气孔关闭. 分子细胞生物学报 42, 145-155. |
| [3] |
张玲玲, 吴丹, 赵子捷, 赵立群 (2017). 植物一氧化氮信号分子的研究进展. 植物学报 52, 337-345.
DOI |
| [4] |
张雨, 赵明洁, 张蔚 (2020). 植物次生细胞壁生物合成的转录调控网络. 植物学报 55, 351-368.
DOI |
| [5] |
An JP, Wang XF, Zhang XW, Xu HF, Bi SQ, You CX, Hao YJ (2020). An apple MYB transcription factor regulates cold tolerance and anthocyanin accumulation and undergoes MIEL1-mediated degradation. Plant Biotechnol J 18, 337-353.
DOI URL |
| [6] |
Bashir W, Anwar S, Zhao Q, Hussain I, Xie FT (2019). Interactive effect of drought and cadmium stress on soybean root morphology and gene expression. Ecotoxicol Environ Saf 175, 90-101.
DOI URL |
| [7] |
Cao XC, Zhu CQ, Zhong C, Zhang JH, Wu LH, Jin QY, Ma QX (2019). Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol 19, 108.
DOI URL |
| [8] |
Chakhchar A, Chaguer N, Ferradous A, Filali-Maltouf A, El Modafar C (2018). Root system response in Argania spinosa plants under drought stress and recovery. Plant Signal Behav 13, e1489669.
DOI URL |
| [9] |
Correa-Aragunde N, Graziano M, Chevalier C, Lamattina L (2006). Nitric oxide modulates the expression of cell cycle regulatory genes during lateral root formation in tomato. J Exp Bot 57, 581-588.
PMID |
| [10] |
Correa-Aragunde N, Graziano M, Lamattina L (2004). Nitric oxide plays a central role in determining lateral root development in tomato. Planta 218, 900-905.
PMID |
| [11] |
Dash M, Yordanov YS, Georgieva T, Tschaplinski TJ, Yordanova E, Busov V (2017). Poplar PtabZIP1-like enhances lateral root formation and biomass growth under drought stress. Plant J 89, 692-705.
DOI URL |
| [12] |
Fang Q, Jiang TZ, Xu LX, Liu H, Mao H, Wang XQ, Jiao B, Duan YJ, Wang Q, Dong QN, Yang L, Tian GZ, Zhang C, Zhou YF, Liu XP, Wang HY, Fan D, Wang BJ, Luo KM (2017). A salt-stress-regulator from the poplar R2R3 MYB family integrates the regulation of lateral root emergence and ABA signaling to mediate salt stress tolerance in Arabidopsis. Plant Physiol Biochem 114, 100-110.
DOI URL |
| [13] | Fukaki H, Okushima Y, Tasaka M (2007). Auxin-mediated lateral root formation in higher plants. Int Rev Cytol 256, 111-137. |
| [14] |
Gibbs DJ, Voß U, Harding SA, Fannon J, Moody LA, Yamada E, Swarup K, Nibau C, Bassel GW, Choudhary A, Lavenus J, Bradshaw SJ, Stekel DJ, Bennett MJ, Coates JC (2014). AtMYB93 is a novel negative regulator of lateral root development in Arabidopsis. New Phytol 203, 1194-1207.
DOI URL |
| [15] |
Gu M, Zhang J, Li HH, Meng DQ, Li R, Dai XL, Wang SC, Liu W, Qu HY, Xu GH (2017). Maintenance of phosphate homeostasis and root development are coordinately regulated by MYB1, an R2R3-type MYB transcription factor in rice. J Exp Bot 68, 3603-3615.
DOI URL |
| [16] |
Hu ZR, Wang R, Zheng M, Liu XB, Meng F, Wu HL, Yao YY, Xin MM, Peng HR, Ni ZF, Sun QX (2018). TaWRKY51 promotes lateral root formation through negative regulation of ethylene biosynthesis in wheat (Triticum aes-tivum L.). Plant J 96, 372-388.
DOI URL |
| [17] |
Lee HK, Cho SK, Son O, Xu ZY, Hwang I, Kim WT (2009). Drought stress-induced Rma1H1, a RING membrane- anchor E3 ubiquitin ligase homolog, regulates aquaporin levels via ubiquitination in transgenic Arabidopsis plants. Plant Cell 21, 622-641.
DOI URL |
| [18] |
Nie J, Wen C, Xi L, Lv SH, Zhao QC, Kou YP, Ma N, Zhao LJ, Zhou XF (2018). The AP2/ERF transcription factor CmERF053 of chrysanthemum positively regulates shoot branching, lateral root, and drought tolerance. Plant Cell Rep 37, 1049-1060.
DOI URL |
| [19] |
Romano JM, Dubos C, Prouse MB, Wilkins O, Hong H, Poole M, Kang KY, Li EY, Douglas CJ, Western TL, Mansfield SD, Campbell MM (2012). AtMYB61, an R2R3-MYB transcription factor, functions as a pleiotropic regulator via a small gene network. New Phytol 195, 774-786.
DOI PMID |
| [20] |
Sahay S, Khan E, Gupta M (2019). Nitric oxide and abscisic acid protects against PEG-induced drought stress differentially in Brassica genotypes by combining the role of stress modulators, markers and antioxidants. Nitric Oxide 89, 81-92.
DOI URL |
| [21] |
Santisree P, Bhatnagar-Mathur P, Sharma KK (2015). NO to drought-multifunctional role of nitric oxide in plant drought: do we have all the answers? Plant Sci 239, 44-55.
DOI URL |
| [22] |
Seo PJ, Park CM (2009). Auxin homeostasis during lateral root development under drought condition. Plant Signal Behav 4, 1002-1004.
DOI URL |
| [23] |
Shin R, Burch AY, Huppert KA, Tiwari SB, Murphy AS, Guilfoyle TJ, Schachtman DP (2007). The Arabidopsis transcription factor MYB77 modulates auxin signal transduction. Plant Cell 19, 2440-2453.
DOI URL |
| [24] |
Wang PC, Du YY, Hou YJ, Zhao Y, Hsu CC, Yuan FJ, Zhu XH, Tao WA, Song CP, Zhu JK (2015). Nitric oxide negatively regulates abscisic acid signaling in guard cells by S-nitrosylation of OST1. Proc Natl Acad Sci USA 112, 613-618.
DOI URL |
| [25] |
Willems E, Leyns L, Vandesompele J (2008). Standardization of realtime PCR gene expression data from independent biological replicates. Anal Biochem 379, 127-129.
DOI PMID |
| [26] |
Xie YJ, Mao Y, Lai DW, Zhang W, Zheng TQ, Shen WB (2013). Roles of NIA/NR/NOA1-dependent nitric oxide production and HY1 expression in the modulation of Arabidopsis salt tolerance. J Exp Bot 64, 3045-3060.
DOI URL |
| [27] |
Xing L, Zhao Y, Gao JH, Xiang CB, Zhu JK (2016). The ABA receptor PYL9 together with PYL8 plays an important role in regulating lateral root growth. Sci Rep 6, 27177.
DOI PMID |
| [28] | Zhao Y, Xing L, Wang XG, Hou YJ, Gao JH, Wang PC, Duan CG, Zhu XH, Zhu JK (2014). The ABA receptor PYL8 promotes lateral root growth by enhancing MYB77- dependent transcription of auxin-responsive gene. Sci Signal 7, ra53. |
| [29] |
Zhou GY, Zhou XH, Nie YY, Bai SH, Zhou LY, Shao JJ, Cheng WS, Wang JW, Hu FQ, Fu YL (2018). Drought- induced changes in root biomass largely result from altered root morphological traits: evidence from a synthesis of global field trials. Plant Cell Environ 41, 2589-2599.
DOI URL |
| [30] | Che YM, Sun YJ, Lu SC, Hou LX, Fan XX, Liu X (2021). AtMYB77 involves in lateral root development via regulating nitric oxide biosynthesis under drought stress in Arabidopsis thaliana. Chin Bull Bot 56, 404-413. |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [3] | 王亚萍, 包文泉, 白玉娥. 单细胞转录组学在植物生长发育及胁迫响应中的应用进展[J]. 植物学报, 2025, 60(1): 101-113. |
| [4] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [5] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [6] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [7] | 赵来鹏, 王柏柯, 杨涛, 李宁, 杨海涛, 王娟, 闫会转. SlHVA22l基因调节番茄耐旱性[J]. 植物学报, 2024, 59(4): 558-573. |
| [8] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [9] | 王鹏, 隋佳容, 丁欣瑶, 王伟中, 曹雪倩, 赵海鹏, 王彦平. 郑州城市公园鸟类群落嵌套分布格局及其影响因素[J]. 生物多样性, 2024, 32(3): 23359-. |
| [10] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [11] | 张盈川, 吴晓明玉, 陶保龙, 陈丽, 鲁海琴, 赵伦, 文静, 易斌, 涂金星, 傅廷栋, 沈金雄. Bna-miR43介导甘蓝型油菜响应干旱胁迫[J]. 植物学报, 2023, 58(5): 701-711. |
| [12] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [13] | 李成, 江建平, 谢锋, 赵天, 车静, 李义明, 杜卫国, 杨维康, 徐峰. 中国两栖爬行动物多样性监测进展与展望[J]. 生物多样性, 2023, 31(12): 23382-. |
| [14] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [15] | 姚萌, 康荣华, 王盎, 马方园, 李靳, 台子晗, 方运霆. 利用15N示踪技术研究木荷与马尾松幼苗叶片对NO2的吸收与分配[J]. 植物生态学报, 2023, 47(1): 114-122. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||