

植物学报 ›› 2021, Vol. 56 ›› Issue (4): 414-421.DOI: 10.11983/CBB20212 cstr: 32102.14.CBB20212
张一弓1, 张怡1, 阿依白合热木·木台力甫1, 张道远2,*( )
)
收稿日期:2020-12-26
接受日期:2021-05-08
出版日期:2021-07-01
发布日期:2021-06-30
通讯作者:
张道远
作者简介:*E-mail: zhangdy@ms.xjb.ac.cn基金资助:
Yigong Zhang1, Yi Zhang1, Ayibaiheremu Mutailifu1, Daoyuan Zhang2,*( )
)
Received:2020-12-26
Accepted:2021-05-08
Online:2021-07-01
Published:2021-06-30
Contact:
Daoyuan Zhang
摘要: ABI3是ABA信号通路中关键的转录调控因子, 参与种子休眠、质体发育及苔藓耐干等重要生理过程, 在植物抗逆中发挥关键作用。以荒漠耐干苔藓——齿肋赤藓(Syntrichia caninervis)为材料, 克隆了抗逆基因ScABI3并获得3个独立的pCAMBIA1301-ScABI3转基因拟南芥(Arabidopsis thaliana)纯合株系。结果表明, 转基因拟南芥叶片气孔孔径增大, 单位面积气孔数量减少, 植株水分利用效率提高; 在干旱处理14天后转基因拟南芥植株存活率显著高于野生型, 离体叶片失水率显著低于野生型。进一步研究发现, ScABI3转基因拟南芥通过提高自身活性氧(ROS)清除能力增强植株抗旱性。研究结果可为开发利用荒漠植物基因资源培育抗逆作物品种奠定基础。
张一弓, 张怡, 阿依白合热木·木台力甫, 张道远. 异源过表达齿肋赤藓ScABI3基因改变拟南芥气孔表型并提高抗旱性. 植物学报, 2021, 56(4): 414-421.
Yigong Zhang, Yi Zhang, Ayibaiheremu Mutailifu, Daoyuan Zhang. Heterologous Overexpression of Desiccation-tolerance Moss ScABI3 Gene Changes Stomatal Phenotype and Improves Drought Resistance in Transgenic Arabidopsis. Chinese Bulletin of Botany, 2021, 56(4): 414-421.
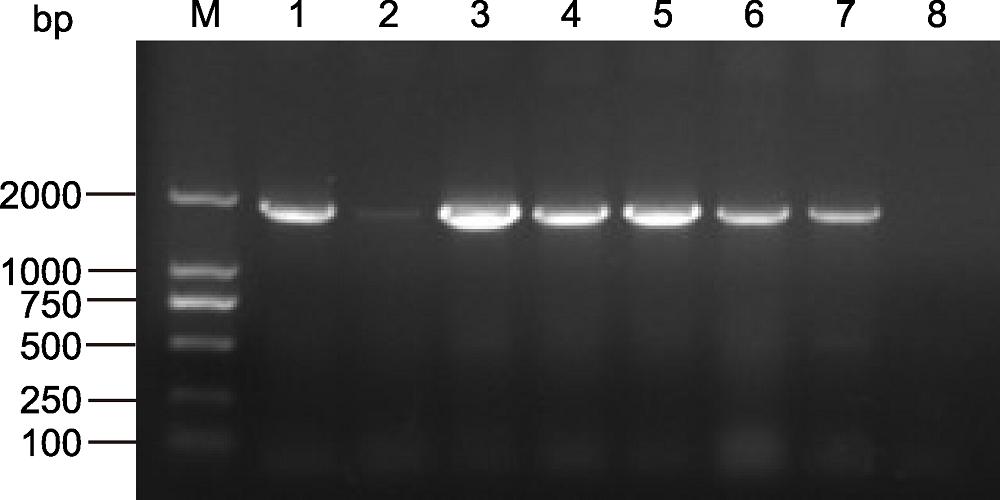
图1 转基因拟南芥植株PCR检测 M: DL2000分子量标准品; 1-6: 转基因株系; 7: 阳性对照; 8:野生型
Figure 1 PCR test of ScABI3 overexpression transgenic Arabidopsis M: DL2000 marker; 1-6: Transgenic lines; 7: Positive control; 8: Wild type
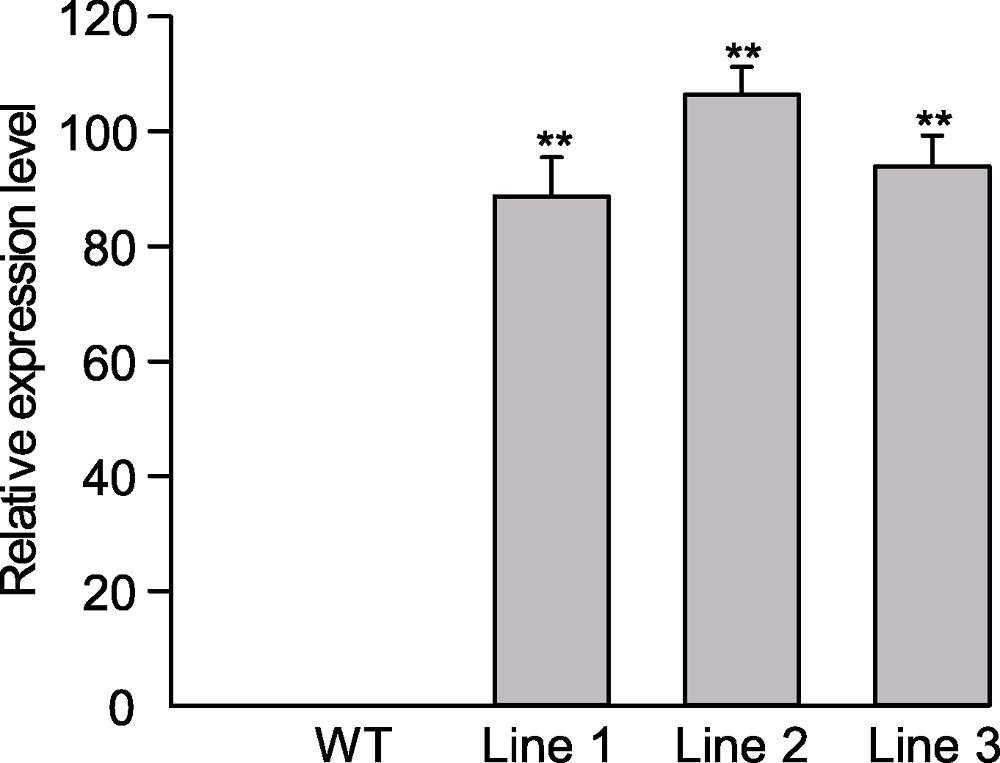
图2 转基因拟南芥叶片ScABI3基因表达水平 ** 表示差异极显著(P<0.01)。
Figure 2 Expression level of ScABI3 in leave of Arabidopsis transgenic lines ** indicate extremely significant differences at P<0.01.
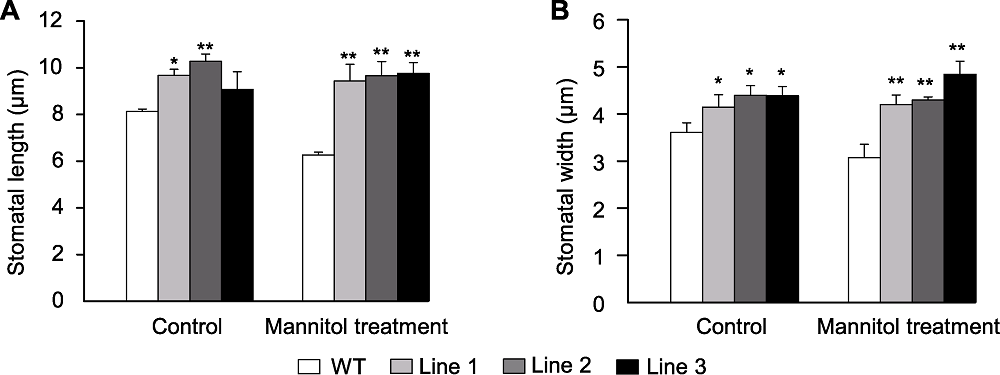
图4 甘露醇处理下转基因和野生型(WT)拟南芥气孔长度(A)和宽度(B) * 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。
Figure 4 Stomata length (A) and width (B) of the transgenic Arabidopsis and wild type (WT) under mannitol treatment * indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01.
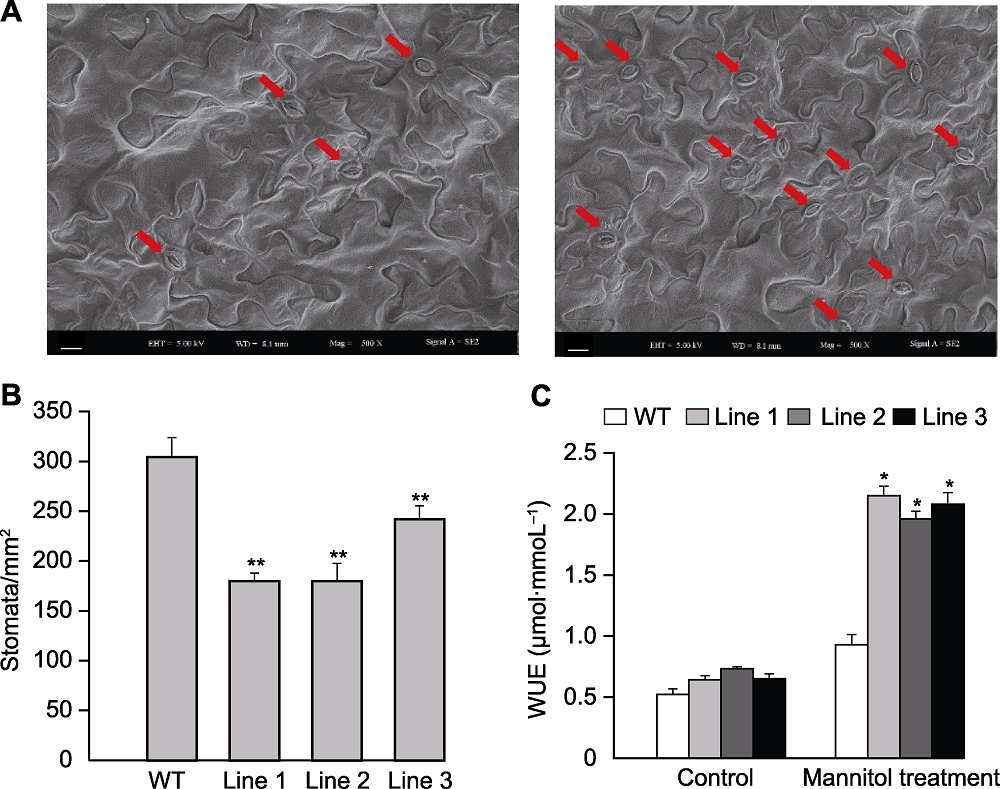
图5 转基因和野生型(WT)拟南芥气孔密度和水分利用率(WUE) (A) 转基因(左)和野生型(右)拟南芥单位面积上的气孔数目; (B) 转基因和野生型拟南芥单位面积气孔数目统计; (C) 甘露醇处理下转基因和野生型拟南芥水分利用率。红色箭头指示气孔; * 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。(A) Bars=10 μm
Figure 5 The stomatal density and water use efficiency (WUE) in the transgenic Arabidopsis and wild type (WT) (A) Stomatal number of transgenic (left) Arabidopsis and WT (right); (B) Stomatal number statistics of the transgenic Arabidopsis and WT; (C) WUE of transgenic Arabidopsis and WT under mannitol treatment. The red arrows indicate stomatas; * indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01. (A) Bars=10 μm
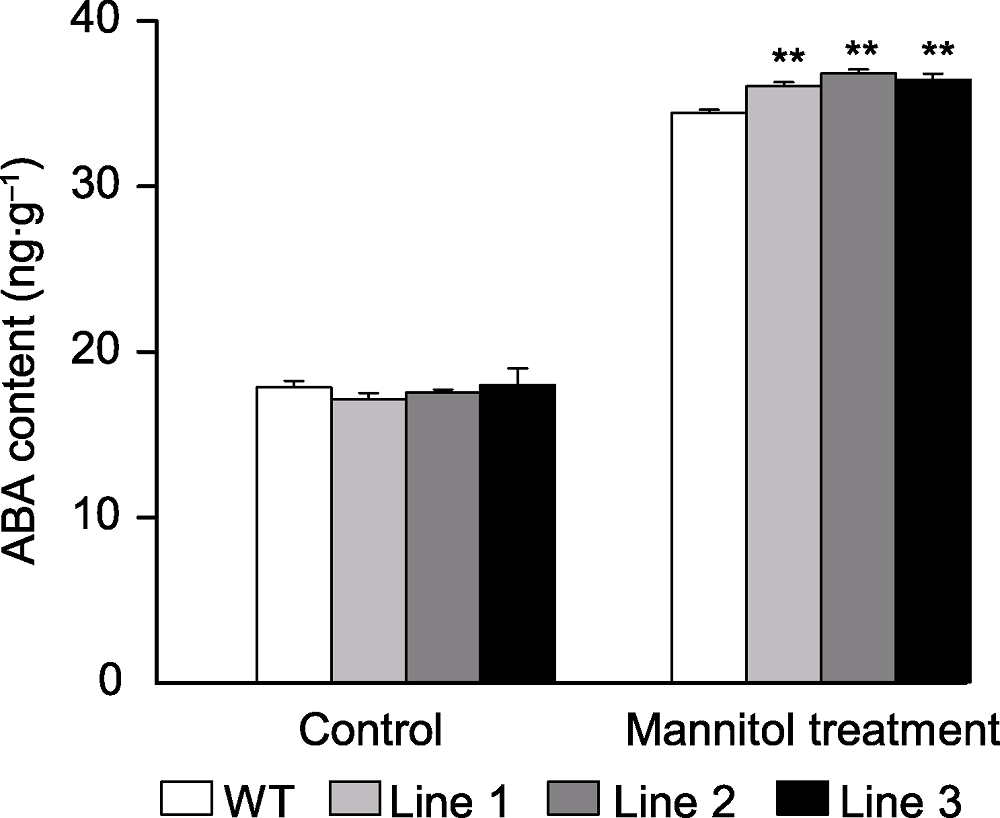
图6 甘露醇处理下转基因和野生型(WT)拟南芥ABA含量 ** 表示差异极显著(P<0.01)。
Figure 6 The content of ABA in the transgenic Arabidopsis and wild type (WT) under mannitol treatment ** indicate extremely significant differences at P<0.01.
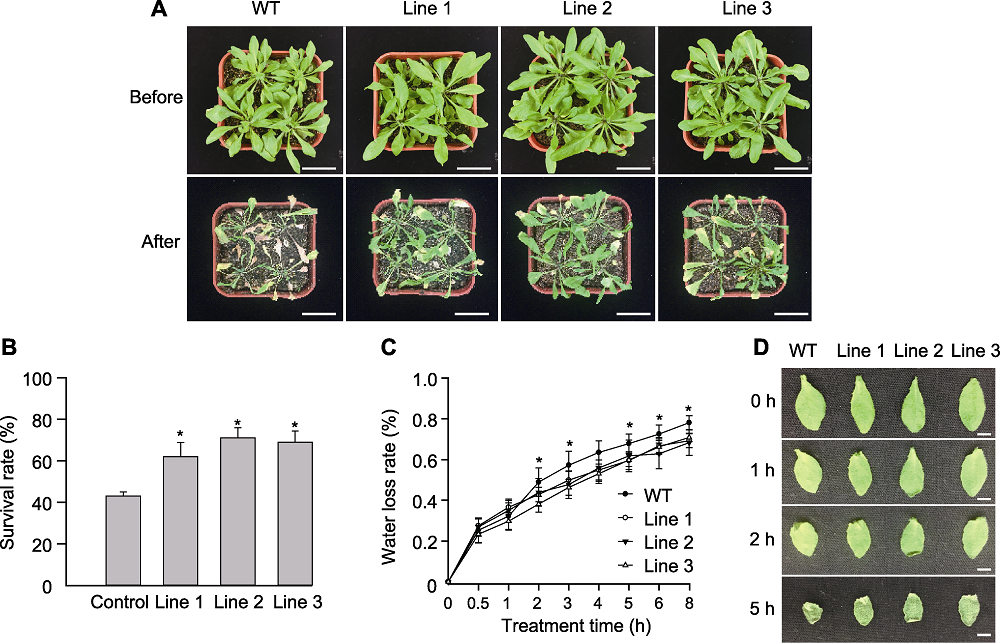
图7 干旱条件下转基因和野生型(WT)拟南芥表型比较 自然干旱下转基因和野生型拟南芥表型(A)、存活率(B)、失水率(C)以及离体叶片失水表型(D)。* 表示差异显著(P<0.05)。(A) Bars=2 cm; (D) Bars=10 mm
Figure 7 Comparisons of the transgenic Arabidopsis and wild type (WT) under drought stress Phenotype (A), survival rate (B), water loss rate (C) and the phenotype of water loss to detached leaves (D) of the transgenic plant and WT under drought stress. * indicate significant differences at P<0.05. (A) Bars=2 cm; (D) Bars=10 mm
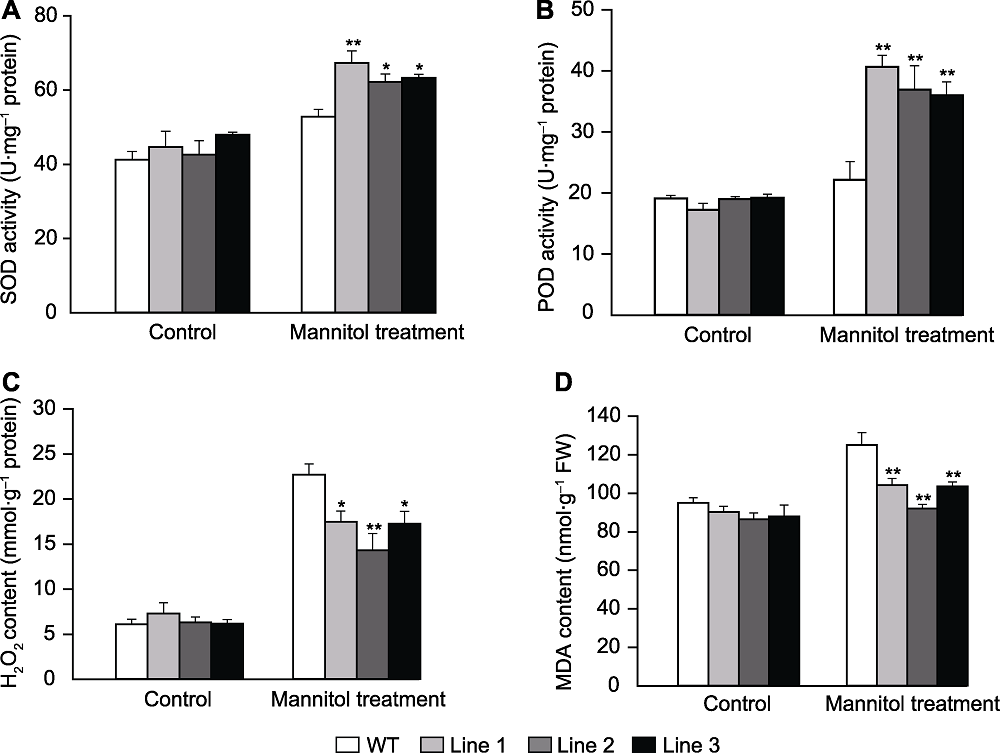
图8 甘露醇处理下转基因和野生型拟南芥生理指标 (A) 超氧化物歧化酶(SOD)活性; (B) 过氧化物酶(POD)活性; (C) H2O2含量; (D) 丙二醛(MDA)含量。* 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01)。
Figure 8 Physiological indicators of the transgenic Arabidopsis and wild type (WT) under mannitol stress (A) Superoxide dismutase (SOD) activity; (B) Peroxidase (POD) activity; (C) H2O2 contents; (D) Malondialdehyde (MDA) contents. * indicate significant differences at P<0.05, ** indicate extremely significant differences at P<0.01.
| [1] | 王宏亮, 郭思义, 王棚涛, 宋纯鹏 (2018). 植物气孔发育机制研究进展. 植物学报 53, 164-174. |
| [2] |
王雅静, 张欣莹, 黄桂荣, 刘晓英, 郭瑞, 顾峰雪, 钟秀丽, 梅旭荣 (2019). 植物磷脂酸的特性及其在ABA诱导气孔运动中的作用. 植物学报 54, 245-254.
DOI |
| [3] |
Bedi S, Sengupta S, Ray A, Nag Chaudhuri R (2016). ABI3 mediates dehydration stress recovery response in Arabidopsis thaliana by regulating expression of downstream genes. Plant Sci 250, 125-140.
DOI URL |
| [4] |
Brady SM, Sarkar SF, Bonetta D, McCourt P (2003). The ABSCISIC ACID INSENSITIVE 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34, 67-75.
DOI URL |
| [5] |
Chen K, Li GJ, Bressan RA, Song CP, Zhu JK, Zhao Y (2020). Abscisic acid dynamics, signaling, and functions in plants. J Integr Plant Biol 62, 25-54.
DOI URL |
| [6] |
Duff RJ, Oliver MJ, Wood AJ (1999). A Tortula ruralis cDNA encoding small-subunit ribosomal protein S3a: polysomal retention of transcript in response to desiccation and rehydration. Bryologist 102, 418-425.
DOI URL |
| [7] |
Khandelwal A, Cho SH, Marella H, Sakata Y, Perroud PF, Pan A, Quatrano RS (2010). Role of ABA and ABI3 in desiccation tolerance. Science 327, 546.
DOI PMID |
| [8] |
Marella HH, Sakata Y, Quatrano RS (2006). Characterization and functional analysis of ABSCISIC ACID INSENSITIVE3-like genes from Physcomitrella patens. Plant J 46, 1032-1044.
DOI URL |
| [9] |
Mittal A, Gampala SSL, Ritchie GL, Payton P, Burke JJ, Rock CD (2014). Related to ABA-insensitive3 (ABI3)/Viviparous1 and AtABI5 transcription factor coexpression in cotton enhances drought stress adaptation. Plant Biotechnol J 12, 578-589.
DOI URL |
| [10] |
Mönke G, Seifert M, Keilwagen J, Mohr M, Grosse I, Hähnel U, Junker A, Weisshaar B, Conrad U, Bäumlein H, Altschmied L (2012). Toward the identification and regulation of the Arabidopsis thaliana ABI3 regulon. Nucleic Acids Res 40, 8240-8254.
DOI URL |
| [11] |
Oliver MJ, Dowd SE, Zaragoza J, Mauget SA, Payton PR (2004). The rehydration transcriptome of the desiccation-tolerant bryophyte Tortula ruralis: transcript classification and analysis. BMC Genomics 5, 89.
DOI URL |
| [12] |
Pan Z, Pitt WG, Zhang YM, Wu N, Tao Y, Truscott TT (2016). The upside-down water collection system of Syntrichia caninervis. Nat Plants 2, 16076.
DOI URL |
| [13] |
Rohde A, Prinsen E, De Rycke R, Engler G, Van Montagu M, Boerjan W (2002). PtABI3 impinges on the growth and differentiation of embryonic leaves during bud set in poplar. Plant Cell 14, 1885-1901.
DOI URL |
| [14] |
Shinde S, Nurul Islam M, Ng CKY (2012). Dehydration stress-induced oscillations in LEA protein transcripts involves abscisic acid in the moss, Physcomitrella patens. New Phytol 195, 321-328.
DOI PMID |
| [15] |
Tamminen I, Mäkelä P, Heino P, Palva ET (2001). Ectopic expression of ABI3 gene enhances freezing tolerance in response to abscisic acid and low temperature in Arabidopsis thaliana. Plant J 25, 1-8.
PMID |
| [16] |
Tanaka Y, Nose T, Jikumaru Y, Kamiya Y (2013). ABA inhibits entry into stomatal-lineage development in Arabidopsis leaves. Plant J 74, 448-457.
DOI URL |
| [17] | Wood AJ, Oliver MJ (2004). Molecular biology and genomics of the desiccation tolerant moss Tortula ruralis. In: Wood AJ, Oliver MJ, Cove DJ, eds. New Frontiers in Bryology. Dordrecht: Springer. pp. 71-89. |
| [18] |
Wu N, Zhang YM, Downing A, Zhang J, Yang CH (2012). Membrane stability of the desert moss Syntrichia caninervis Mitt. during desiccation and rehydration. J Bryol 34, 1-8.
DOI URL |
| [19] |
Xia J, Wang XQ, Perroud PF, He YK, Quatrano R, Zhang WX (2016). Endogenous small-noncoding RNAs and potential functions in desiccation tolerance in Physcomitrella patens. Sci Rep 6, 30118.
DOI URL |
| [20] |
Xiao LH, Yobi A, Koster KL, He YK, Oliver MJ (2018). Desiccation tolerance in Physcomitrella patens: rate of dehydration and the involvement of endogenous abscisic acid (ABA). Plant Cell Environ 41, 275-284.
DOI URL |
| [21] |
Yotsui I, Serada S, Naka T, Saruhashi M, Taji T, Hayashi T, Quatrano RS, Sakata Y (2016). Large-scale proteome analysis of abscisic acid and ABSCISIC ACID INSENSITIVE3-dependent proteins related to desiccation tolerance in Physcomitrella patens. Biochem Biophys Res Commun 471, 589-595.
DOI URL |
| [22] |
Yu H, Chen X, Hong YY, Wang Y, Xu P, Ke SD, Liu HY, Zhu JK, Oliver DJ, Xiang CB (2008). Activated expression of an Arabidopsis HD-START protein confers drought tolerance with improved root system and reduced stomatal density. Plant Cell 20, 1134-1151.
DOI PMID |
| [23] |
Zhang J, Zhang YM, Downing A, Wu N, Zhang BC (2011). Photosynthetic and cytological recovery on remoistening Syntrichia caninervis Mitt., a desiccation-tolerant moss from Northwestern China. Photosynthetica 49, 13-20.
DOI URL |
| [1] | 王堃莹, 邱贵福, 刘子赫, 孟君, 刘宇轩, 贾国栋. 气候变化对不同退化程度小叶杨林分生长和内在水分利用效率的调节[J]. 植物生态学报, 2025, 49(2): 343-355. |
| [2] | 张小雨, 贾国栋, 余新晓, 孙立博, 蒋涛. 不同退化程度小叶杨人工林冠层气孔导度特征及其环境响应[J]. 植物生态学报, 2024, 48(9): 1143-1156. |
| [3] | 王子阳, 刘升学, 杨志蕊, 秦峰. 玉米抗旱性的遗传解析[J]. 植物学报, 2024, 59(6): 883-902. |
| [4] | 廖星鑫, 牛祎, 多兴武, 阿克也得力·居玛哈孜, 买热哈巴·阿不都克尤木, 热孜瓦尼姑丽·胡甫尔, 兰海燕, 曹婧. 异源表达异子蓬SaPEPC2基因提高烟草抗旱性和光合特性(长英文摘要)[J]. 植物学报, 2024, 59(4): 585-599. |
| [5] | 王贺萍, 孙震, 刘雨辰, 苏彦龙, 杜锦瑜, 赵彦, 赵竑博, 王召明, 苑峰, 刘亚玲, 吴振映, 何峰, 付春祥. 蒙古冰草肉桂醇脱氢酶基因序列鉴定及功能分析[J]. 植物学报, 2024, 59(2): 204-216. |
| [6] | 宋毅, 陈航航, 崔鑫, 陆志峰, 廖世鹏, 张洋洋, 李小坤, 丛日环, 任涛, 鲁剑巍. 钾营养状况介导的油菜叶片生长及其对叶际微生物的影响[J]. 植物学报, 2024, 59(1): 54-65. |
| [7] | 王嘉仪, 王襄平, 徐程扬, 夏新莉, 谢宗强, 冯飞, 樊大勇. 北京市行道树绒毛梣的水力结构对城市不透水表面比例的响应[J]. 植物生态学报, 2023, 47(7): 998-1009. |
| [8] | 蒋海港, 曾云鸿, 唐华欣, 刘伟, 李杰林, 何国华, 秦海燕, 王丽超, 姚银安. 三种藓类植物固碳耗水节律调节作用[J]. 植物生态学报, 2023, 47(7): 988-997. |
| [9] | 周文期, 周玉乾, 李永生, 何海军, 杨彦忠, 王晓娟, 连晓荣, 刘忠祥, 胡筑兵. 玉米ZmICE2基因调控气孔发育[J]. 植物学报, 2023, 58(6): 866-881. |
| [10] | 张蕾, 姜鹏飞, 王一鸣, 兰婷, 刘妍婧, 曾庆银. 苦杨×小叶杨杂交F1代苗期抗旱性比较研究[J]. 植物学报, 2023, 58(4): 519-534. |
| [11] | 周玉萍, 颜嘉豪, 田长恩. 保卫细胞中ABA信号调控机制研究进展[J]. 植物学报, 2022, 57(5): 684-696. |
| [12] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| [13] | 马艳泽, 杨熙来, 徐彦森, 冯兆忠. 四种常见树木叶片光合模型关键参数对臭氧浓度升高的响应[J]. 植物生态学报, 2022, 46(3): 321-329. |
| [14] | 罗丹丹, 王传宽, 金鹰. 木本植物水力系统对干旱胁迫的响应机制[J]. 植物生态学报, 2021, 45(9): 925-941. |
| [15] | 叶子飘, 于冯, 安婷, 王复标, 康华靖. 植物气孔导度对CO2响应模型的构建[J]. 植物生态学报, 2021, 45(4): 420-428. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||