

植物学报 ›› 2022, Vol. 57 ›› Issue (5): 596-610.DOI: 10.11983/CBB22022 cstr: 32102.14.CBB22022
刘晓龙1,2,*( ), 季平1, 杨洪涛1,2, 丁永电1,2, 付佳玲1, 梁江霞1, 余聪聪1
), 季平1, 杨洪涛1,2, 丁永电1,2, 付佳玲1, 梁江霞1, 余聪聪1
收稿日期:2022-01-28
接受日期:2022-04-20
出版日期:2022-09-01
发布日期:2022-09-09
通讯作者:
刘晓龙
作者简介:*E-mail: lxl032202@163.com基金资助:
Liu Xiaolong1,2,*( ), Ji Ping1, Yang Hongtao1,2, Ding Yongdian1,2, Fu Jialing1, Liang Jiangxia1, Yu Congcong1
), Ji Ping1, Yang Hongtao1,2, Ding Yongdian1,2, Fu Jialing1, Liang Jiangxia1, Yu Congcong1
Received:2022-01-28
Accepted:2022-04-20
Online:2022-09-01
Published:2022-09-09
Contact:
Liu Xiaolong
About author:*E-mail: lxl032202@163.com摘要: 为探究脱落酸(ABA)对水稻(Oryza sativa)抽穗开花期高温胁迫的诱抗效应, 以江西省主推水稻品种黄华占为材料, 于孕穗期用蒸馏水、ABA溶液(10、50和100 µmol∙L-1)、氟啶酮(FLU)和原花青素(PC) 6种溶液进行叶面喷施, 然后移入对照(CK)和高温胁迫(HS)环境处理8天, 考查籽粒活性氧(ROS)积累、抗氧化防御能力、产量构成及相关基因的表达。结果表明, 高温胁迫下, 水稻的穗长、穗重、结实率、千粒重和产量与超氧阴离子和过氧化氢含量呈显著负相关。高温胁迫下, 喷施ABA显著上调了ABA应答和抗氧化防御基因的表达, 籽粒中活性氧含量下降了8.24%-31.35%; 喷施ABA显著增加了水稻的穗长、穗重、结实率和千粒重, 显著上调了产量形成基因的表达, 增产12.73%-20.77%。高温胁迫下, 喷施FLU可抑制ABA的生物合成, 导致ROS过量积累和水稻减产; 喷施抗氧化剂PC则抑制ROS过量积累, 使产量增加。以上结果表明, 高温胁迫下, 孕穗期喷施ABA不仅能够激发ABA信号通路, 而且上调抗氧化防御能力和产量形成基因的表达, 进而提高水稻在抽穗开花期的耐热性, 达到增产目的。
刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应. 植物学报, 2022, 57(5): 596-610.
Liu Xiaolong, Ji Ping, Yang Hongtao, Ding Yongdian, Fu Jialing, Liang Jiangxia, Yu Congcong. Priming Effect of Abscisic Acid on High Temperature Stress During Rice Heading-flowering Stage. Chinese Bulletin of Botany, 2022, 57(5): 596-610.
| Primer name | Primer sequence (5'-3') |
|---|---|
| SalTF | CGAAATAATGTTCCATGGTGTT |
| SalTR | TGTACTACGGATCGGTGCAA |
| OsWsi18F | TGTGACTCGATCCAGCGTAG |
| OsWsi18R | GTTCCTGCTGAGAAGCCATC |
| OsACT1F | TTCCAGCCTTCCTTCATA |
| OsACT1R | AACGATGTTGCCATATAGAT |
| OsCATBF | GCTGGTGAGAGATACCGGTCA |
| OsCATBR | TCAACCCACCGCTGGAGA |
| OsAPX6F | CCCCAAGATCCCCATGATCTA |
| OsAPX6R | CCTCTGGCGGGCATTG |
| OsFe-SODF | CGACGCCGAGGAATTTCTAG |
| OsFe-SODR | AGGTGGTGTAAGTGTCTCTCATGC |
| OsCu/Zn-SODF | TGTGACGGGACTTACTCCTGG |
| OsCu/Zn-SODR | CACCCATTCGTAGTATCGCCA |
| Ghd7F | AATCCGGTACGCGTCCAGA |
| Ghd7R | CCAAGCTCAAGCCTACTAGG |
| WxF | TTGGGATACCAGCGTTGTGG |
| WxR | CGGTCTTTCCCCAAACCTTCT |
| OsAGPL2F | AAGCCGAGAGCGTGGTAAAA |
| OsAGPL2R | CAGGAGCGCTAGGGTTTTCA |
| OsAGPL3F | TGCCGATGAGCAACTGCATA |
| OsAGPL3R | TTATACGCCCGGGAAAGGTG |
表1 引物序列
Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') |
|---|---|
| SalTF | CGAAATAATGTTCCATGGTGTT |
| SalTR | TGTACTACGGATCGGTGCAA |
| OsWsi18F | TGTGACTCGATCCAGCGTAG |
| OsWsi18R | GTTCCTGCTGAGAAGCCATC |
| OsACT1F | TTCCAGCCTTCCTTCATA |
| OsACT1R | AACGATGTTGCCATATAGAT |
| OsCATBF | GCTGGTGAGAGATACCGGTCA |
| OsCATBR | TCAACCCACCGCTGGAGA |
| OsAPX6F | CCCCAAGATCCCCATGATCTA |
| OsAPX6R | CCTCTGGCGGGCATTG |
| OsFe-SODF | CGACGCCGAGGAATTTCTAG |
| OsFe-SODR | AGGTGGTGTAAGTGTCTCTCATGC |
| OsCu/Zn-SODF | TGTGACGGGACTTACTCCTGG |
| OsCu/Zn-SODR | CACCCATTCGTAGTATCGCCA |
| Ghd7F | AATCCGGTACGCGTCCAGA |
| Ghd7R | CCAAGCTCAAGCCTACTAGG |
| WxF | TTGGGATACCAGCGTTGTGG |
| WxR | CGGTCTTTCCCCAAACCTTCT |
| OsAGPL2F | AAGCCGAGAGCGTGGTAAAA |
| OsAGPL2R | CAGGAGCGCTAGGGTTTTCA |
| OsAGPL3F | TGCCGATGAGCAACTGCATA |
| OsAGPL3R | TTATACGCCCGGGAAAGGTG |
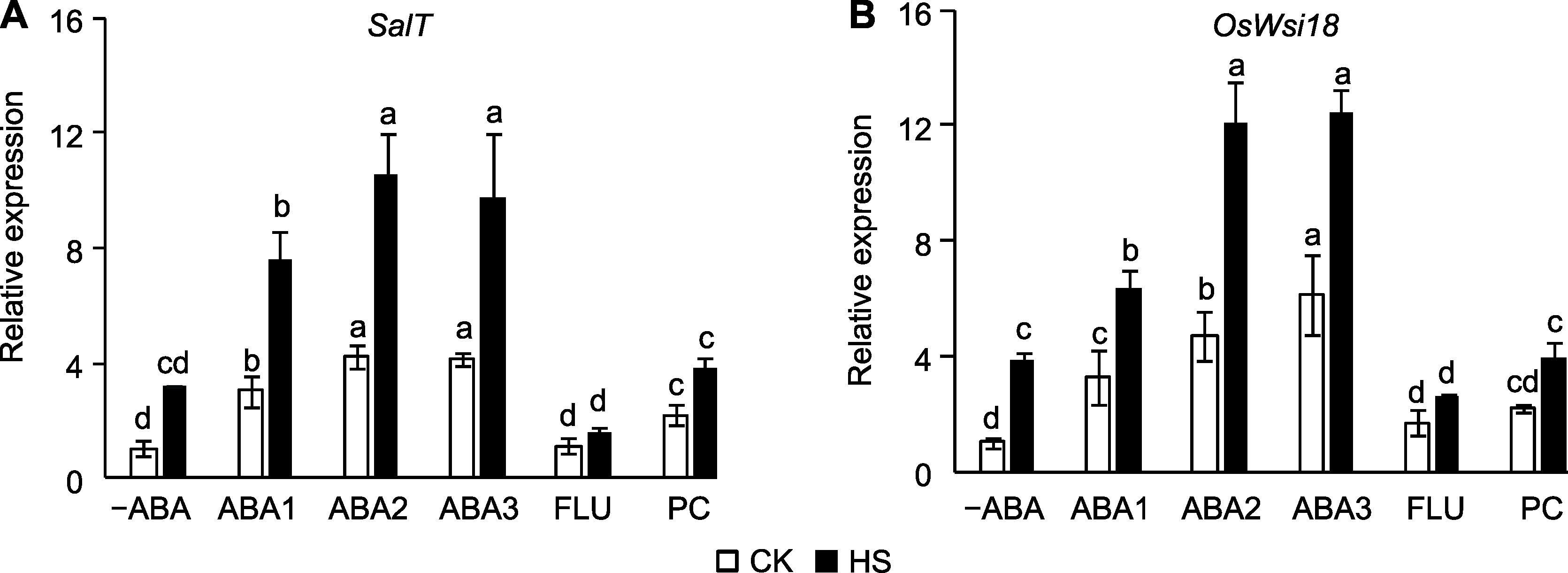
图1 孕穗期喷施ABA对高温胁迫下水稻ABA应答基因表达量的影响 (A) SalT; (B) OsWsi18。 -ABA: 蒸馏水; ABA1: 10 μmol?L-1 ABA; ABA2: 50 μmol?L-1 ABA; ABA3: 100 μmol?L-1 ABA; FLU: 10 μmol?L-1氟啶酮; PC: 1%原花青素; CK: 对照; HS: 高温胁迫。不同小写字母表示在对照和高温胁迫条件下不同处理间差异显著(P<0.05)。
Figure 1 Effect of spraying ABA solution at booting stage on the expression of ABA-responsive genes in rice under high temperature stress (A) SalT; (B) OsWsi18. -ABA: Distilled water; ABA1: 10 μmol?L-1 ABA; ABA2: 50 μmol?L-1 ABA; ABA3: 100 μmol?L-1 ABA; FLU: 10 μmol?L-1 fluridone; PC: 1% proanthocyanidins; CK: Control; HS: High temperature stress. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
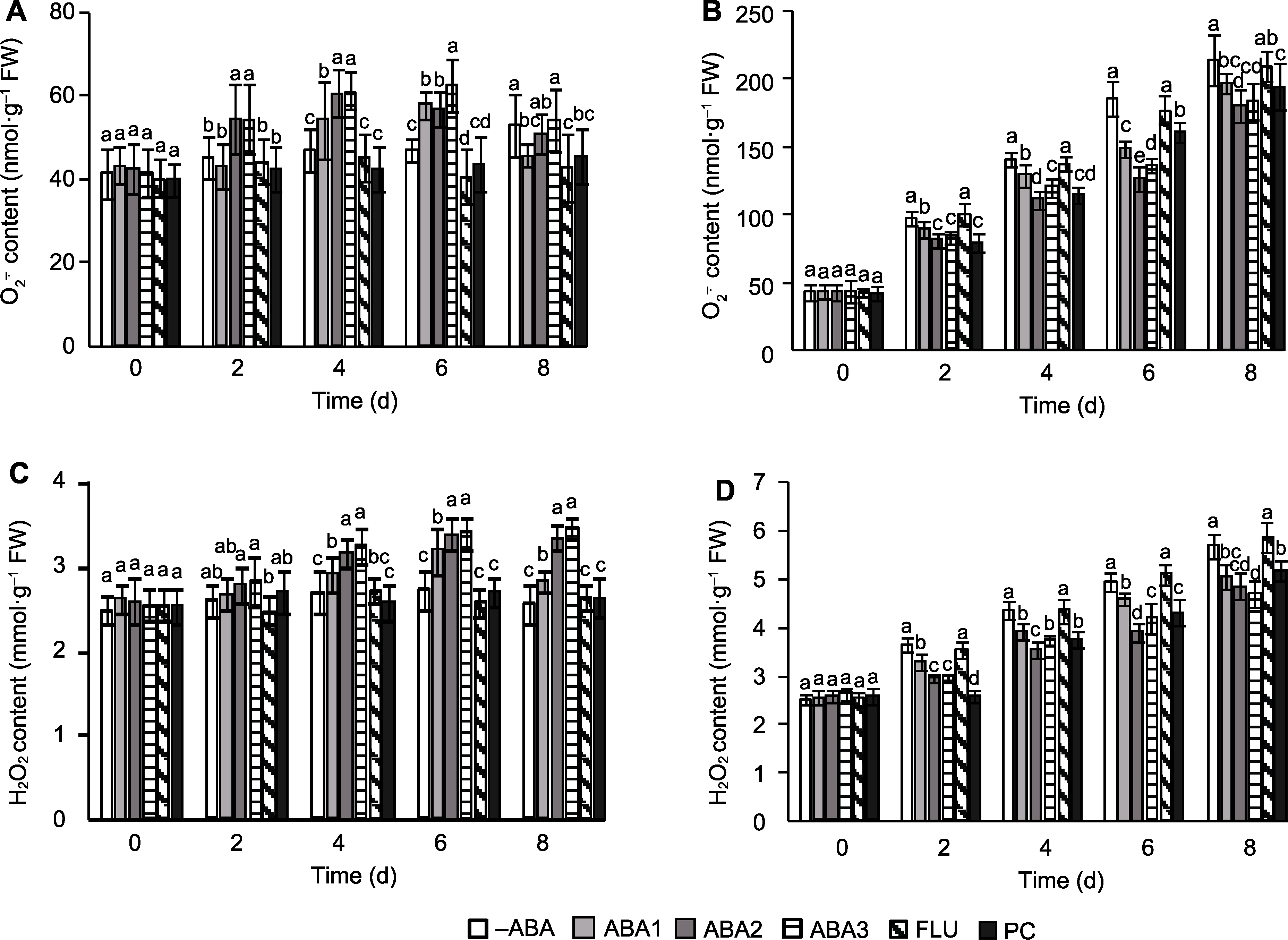
图2 孕穗期喷施ABA对高温胁迫下水稻籽粒活性氧(ROS)积累的影响 (A) 对照超氧阴离子含量; (B) 高温胁迫处理超氧阴离子含量; (C) 对照(CK)过氧化氢含量; (D) 高温胁迫(HS)处理过氧化氢含量。-ABA、ABA1、ABA2、ABA3、FLU、PC、CK和HS同图1。不同小写字母表示在对照和高温胁迫条件下不同处理间差异显著(P<0.05)。
Figure 2 Effect of spraying ABA solution at booting stage on reactive oxygen species (ROS) accumulation in rice spikelets under high temperature stress (A) O2-. content of CK; (B) O2-. content of HS; (C) H2O2 content of CK; (D) H2O2 content of HS. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
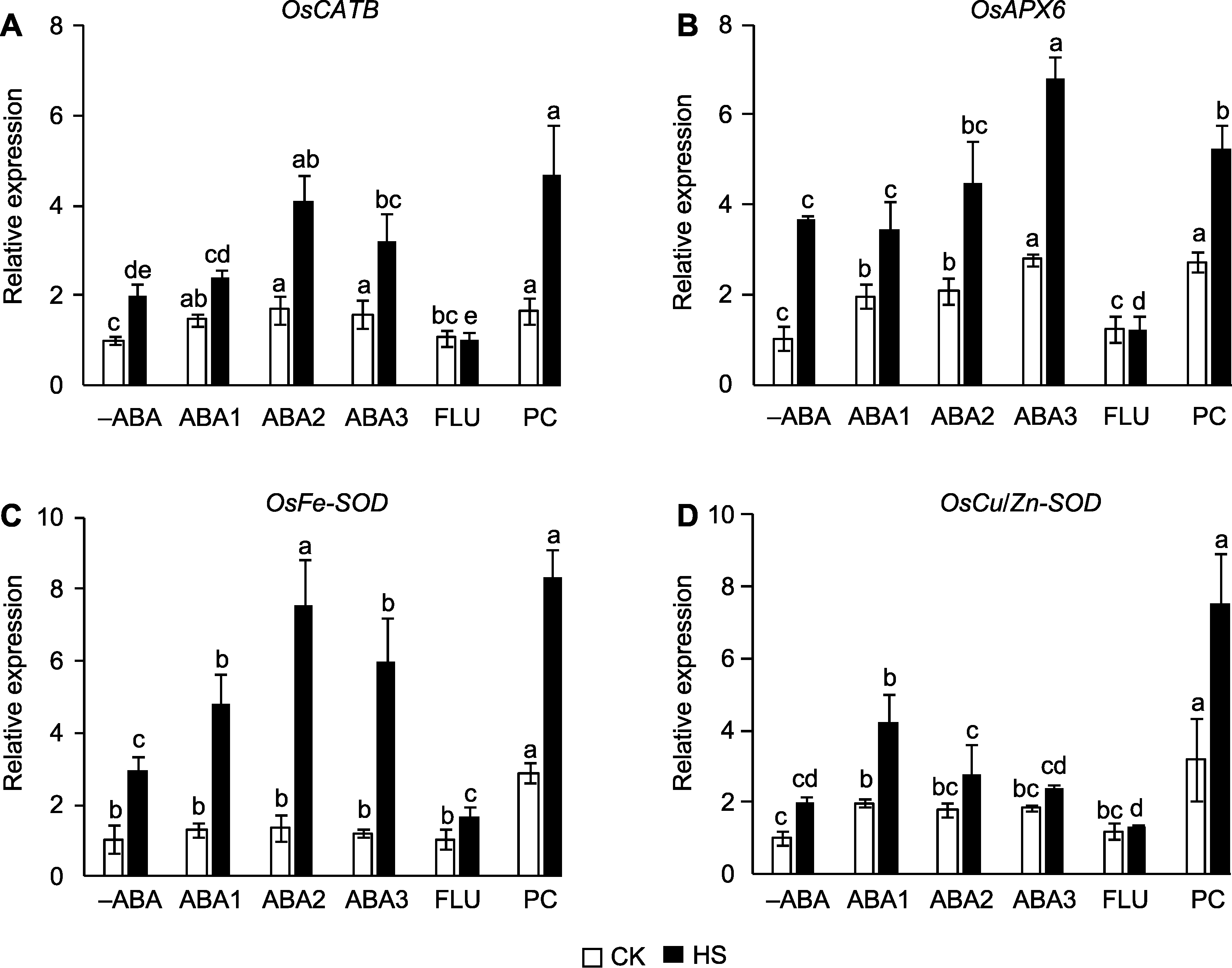
图3 孕穗期喷施ABA对高温胁迫下水稻活性氧(ROS)清除基因表达量的影响 (A) OsCATB; (B) OsAPX6; (C) OsFe-SOD; (D) OsCu/Zn-SOD。-ABA、ABA1、ABA2、ABA3、FLU、PC、CK和HS同图1。不同小写字母表示在对照和高温胁迫条件下不同处理间差异显著(P<0.05)。
Figure 3 Effect of spraying ABA solution at booting stage on the expression of reactive oxygen species (ROS)-scavenging genes in rice under high temperature stress (A) OsCATB; (B) OsAPX6; (C) OsFe-SOD; (D) OsCu/Zn-SOD. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
| Treatments | Shoot length (cm) | Stem dry weight (g) | Panicle length (cm) | Panicle weight (g) | |
|---|---|---|---|---|---|
| CK | -ABA | 85.54±5.46 a | 37.27±5.10 a | 20.96±0.92 b | 3.01±0.18 ab |
| ABA1 | 86.70±5.38 a | 38.19±3.92 a | 21.41±0.94 ab | 3.05±0.38 ab | |
| ABA2 | 86.54±3.47 a | 39.90±4.39 a | 21.64±0.90 ab | 3.03±0.41 ab | |
| ABA3 | 87.88±3.74 a | 41.52±2.66 a | 21.79±0.61 a | 3.12±0.42 a | |
| FLU | 84.10±4.24 a | 37.28±5.77 a | 20.92±0.67 b | 2.75±0.29 b | |
| PC | 87.30±5.50 a | 41.61±2.77 a | 22.03±0.73 a | 3.09±0.18 ab | |
| HS | -ABA | 82.64±3.23 a | 34.79±4.08 a | 19.96±0.86 b | 2.42±0.29 b |
| ABA1 | 85.01±6.15 a | 36.08±3.69 a | 20.42±0.66 ab | 2.73±0.32 a | |
| ABA2 | 85.09±6.20 a | 37.74±5.74 a | 20.94±1.96 ab | 2.86±0.21 a | |
| ABA3 | 87.02±3.37 a | 38.55±5.34 a | 20.85±0.44 ab | 2.81±0.23 a | |
| FLU | 83.00±4.00 a | 36.96±3.34 a | 19.84±0.73 b | 2.36±0.19 b | |
| PC | 85.03±3.98 a | 37.56±2.99 a | 21.28±1.03 a | 2.89±0.40 a | |
表2 孕穗期喷施ABA对高温胁迫下水稻植株生长发育的影响
Table 2 Effect of spraying ABA solution at booting stage on the growth and development of rice plants under high temperature stress
| Treatments | Shoot length (cm) | Stem dry weight (g) | Panicle length (cm) | Panicle weight (g) | |
|---|---|---|---|---|---|
| CK | -ABA | 85.54±5.46 a | 37.27±5.10 a | 20.96±0.92 b | 3.01±0.18 ab |
| ABA1 | 86.70±5.38 a | 38.19±3.92 a | 21.41±0.94 ab | 3.05±0.38 ab | |
| ABA2 | 86.54±3.47 a | 39.90±4.39 a | 21.64±0.90 ab | 3.03±0.41 ab | |
| ABA3 | 87.88±3.74 a | 41.52±2.66 a | 21.79±0.61 a | 3.12±0.42 a | |
| FLU | 84.10±4.24 a | 37.28±5.77 a | 20.92±0.67 b | 2.75±0.29 b | |
| PC | 87.30±5.50 a | 41.61±2.77 a | 22.03±0.73 a | 3.09±0.18 ab | |
| HS | -ABA | 82.64±3.23 a | 34.79±4.08 a | 19.96±0.86 b | 2.42±0.29 b |
| ABA1 | 85.01±6.15 a | 36.08±3.69 a | 20.42±0.66 ab | 2.73±0.32 a | |
| ABA2 | 85.09±6.20 a | 37.74±5.74 a | 20.94±1.96 ab | 2.86±0.21 a | |
| ABA3 | 87.02±3.37 a | 38.55±5.34 a | 20.85±0.44 ab | 2.81±0.23 a | |
| FLU | 83.00±4.00 a | 36.96±3.34 a | 19.84±0.73 b | 2.36±0.19 b | |
| PC | 85.03±3.98 a | 37.56±2.99 a | 21.28±1.03 a | 2.89±0.40 a | |
| Treatments | Panicle number | Spikelets per panicle | Percentage of filled spikelets (%) | 1000-kernel weight (g) | Harvest index (%) | Grain yield per hole (g) | |
|---|---|---|---|---|---|---|---|
| CK | -ABA | 14.22±1.86 a | 124.73±14.91 a | 84.89±2.11 a | 22.97±1.21 a | 49.41±1.79 a | 34.31±4.49 a |
| ABA1 | 14.44±1.74 a | 121.55±12.92 a | 85.21±1.76 a | 22.80±1.45 a | 48.77±1.99 a | 33.90±4.47 a | |
| ABA2 | 14.78±1.64 a | 124.81±13.85 a | 84.63±2.78 a | 22.43±1.06 a | 48.39±1.72 a | 34.73±3.50 a | |
| ABA3 | 15.33±1.32 a | 128.67±13.16 a | 84.00±2.63 a | 21.85±0.91 a | 48.53±1.23 a | 35.92±1.81 a | |
| FLU | 14.44±1.42 a | 123.27±14.02 a | 83.02±3.71 a | 22.61±0.91 a | 49.43±3.72 a | 33.31±4.41 a | |
| PC | 15.78±1.56 a | 124.53±12.76 a | 85.26±2.58 a | 21.93±0.58 a | 48.58±2.21 a | 36.46±2.65 a | |
| HS | -ABA | 13.56±1.67 a | 119.76±16.90 a | 78.02±1.96 b | 19.76±0.54 c | 44.83±2.73 a | 24.84±3.69 bc |
| ABA1 | 14.22±1.39 a | 119.04±11.58 a | 81.93±1.63 a | 20.72±0.81 abc | 46.70±2.03 a | 28.59±2.84 a | |
| ABA2 | 14.56±1.88 a | 121.99±8.02 a | 81.12±2.48 a | 20.98±1.45 a | 47.04±2.78 a | 30.00±3.14 a | |
| ABA3 | 14.44±2.07 a | 115.09±18.40 a | 81.10±3.45 a | 21.19±0.93 a | 44.76±3.97 a | 28.01±2.41 ab | |
| FLU | 14.22±1.99 a | 113.38±16.50 a | 75.43±3.33 b | 19.81±0.50 bc | 42.69±2.39 a | 23.74±2.29 c | |
| PC | 14.78±1.30 a | 116.31±14.12 a | 81.89±4.24 a | 20.79±1.32 ab | 46.01±3.50 a | 29.08±3.47 a | |
表3 孕穗期喷施ABA对高温胁迫下水稻产量构成因素及产量的影响
Table 3 Effect of spraying ABA at booting stage on yield components and grain yield of rice under high temperature stress
| Treatments | Panicle number | Spikelets per panicle | Percentage of filled spikelets (%) | 1000-kernel weight (g) | Harvest index (%) | Grain yield per hole (g) | |
|---|---|---|---|---|---|---|---|
| CK | -ABA | 14.22±1.86 a | 124.73±14.91 a | 84.89±2.11 a | 22.97±1.21 a | 49.41±1.79 a | 34.31±4.49 a |
| ABA1 | 14.44±1.74 a | 121.55±12.92 a | 85.21±1.76 a | 22.80±1.45 a | 48.77±1.99 a | 33.90±4.47 a | |
| ABA2 | 14.78±1.64 a | 124.81±13.85 a | 84.63±2.78 a | 22.43±1.06 a | 48.39±1.72 a | 34.73±3.50 a | |
| ABA3 | 15.33±1.32 a | 128.67±13.16 a | 84.00±2.63 a | 21.85±0.91 a | 48.53±1.23 a | 35.92±1.81 a | |
| FLU | 14.44±1.42 a | 123.27±14.02 a | 83.02±3.71 a | 22.61±0.91 a | 49.43±3.72 a | 33.31±4.41 a | |
| PC | 15.78±1.56 a | 124.53±12.76 a | 85.26±2.58 a | 21.93±0.58 a | 48.58±2.21 a | 36.46±2.65 a | |
| HS | -ABA | 13.56±1.67 a | 119.76±16.90 a | 78.02±1.96 b | 19.76±0.54 c | 44.83±2.73 a | 24.84±3.69 bc |
| ABA1 | 14.22±1.39 a | 119.04±11.58 a | 81.93±1.63 a | 20.72±0.81 abc | 46.70±2.03 a | 28.59±2.84 a | |
| ABA2 | 14.56±1.88 a | 121.99±8.02 a | 81.12±2.48 a | 20.98±1.45 a | 47.04±2.78 a | 30.00±3.14 a | |
| ABA3 | 14.44±2.07 a | 115.09±18.40 a | 81.10±3.45 a | 21.19±0.93 a | 44.76±3.97 a | 28.01±2.41 ab | |
| FLU | 14.22±1.99 a | 113.38±16.50 a | 75.43±3.33 b | 19.81±0.50 bc | 42.69±2.39 a | 23.74±2.29 c | |
| PC | 14.78±1.30 a | 116.31±14.12 a | 81.89±4.24 a | 20.79±1.32 ab | 46.01±3.50 a | 29.08±3.47 a | |
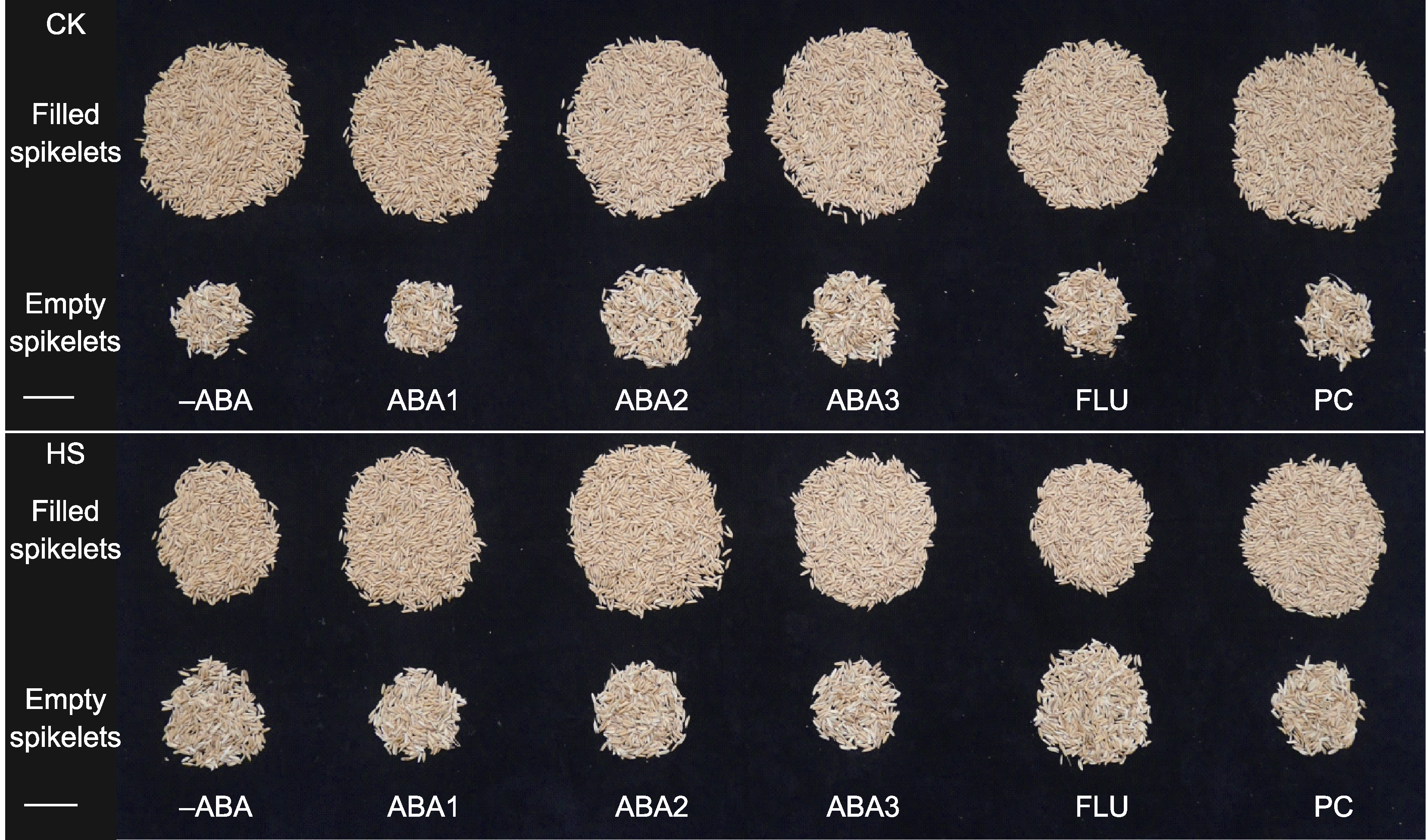
图4 不同处理下成熟期水稻的籽粒 -ABA、ABA1、ABA2、ABA3、FLU、PC、CK和HS同图1。Bars=3 cm
Figure 4 Rice grains at mature stage under different treatments -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Bars=3 cm
| Indexes | Treatment time (d) | Shoot length | Stem dry weight | Panicle length | Panicle weight | Panicle number | Spikelets per panicle | Percentage of filled spikelets | 1000-kernel weight | Harvest index | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents of O2- . | CK | 0 | 0.059 | 0.135 | 0.173 | 0.063 | 0.161 | -0.115 | 0.006 | 0.135 | -0.023 | 0.128 |
| 2 | -0.028 | -0.071 | -0.081 | -0.053 | -0.143 | 0.134 | -0.233 | -0.138 | -0.033 | -0.151 | ||
| 4 | 0.097 | -0.02 | -0.083 | 0.030 | -0.176 | 0.196 | -0.062 | -0.181 | -0.379* | -0.102 | ||
| 6 | 0.209 | 0.150 | 0.152 | 0.257 | 0.040 | 0.053 | 0.019 | -0.329* | -0.107 | 0.069 | ||
| 8 | -0.049 | 0.052 | -0.066 | 0.061 | -0.05 | 0.052 | 0.002 | -0.104 | -0.147 | -0.072 | ||
| HS | 0 | -0.075 | -0.348** | -0.359** | -0.117 | -0.331* | 0.076 | 0.004 | 0.055 | -0.047 | -0.203 | |
| 2 | -0.099 | -0.093 | -0.463** | -0.510** | -0.138 | -0.173 | -0.448** | -0.421** | -0.413** | -0.600** | ||
| 4 | -0.059 | -0.195 | -0.373** | -0.441** | -0.142 | -0.029 | -0.384** | -0.390** | -0.174 | -0.435** | ||
| 6 | -0.215 | -0.249 | -0.339* | -0.487** | -0.140 | -0.070 | -0.408** | -0.404** | -0.205 | -0.450** | ||
| 8 | 0.101 | -0.191 | -0.257 | -0.393** | -0.138 | -0.106 | -0.241 | -0.330* | -0.275* | -0.375** | ||
| Contents of H2O2 | CK | 0 | -0.181 | -0.021 | 0.086 | 0.035 | 0.047 | -0.163 | 0.222 | -0.070 | -0.109 | -0.061 |
| 2 | 0.070 | 0.024 | 0.134 | 0.171 | 0.030 | 0.061 | -0.045 | -0.066 | 0.028 | 0.048 | ||
| 4 | 0.077 | 0.194 | 0.374* | 0.353* | 0.089 | -0.046 | -0.160 | 0.066 | -0.188 | 0.018 | ||
| 6 | 0.196 | 0.228 | 0.174 | 0.339* | 0.070 | 0.034 | 0.068 | -0.329* | -0.322* | 0.050 | ||
| 8 | 0.225 | 0.195 | 0.221 | 0.135 | 0.093 | 0.070 | -0.107 | -0.152 | -0.123 | 0.065 | ||
| HS | 0 | 0.097 | 0.168 | 0.130 | 0.290* | 0.227 | -0.251 | 0.151 | 0.166 | -0.174 | 0.071 | |
| 2 | -0.208 | -0.210 | -0.439** | -0.569** | -0.206 | -0.033 | -0.477** | -0.400** | -0.201 | -0.502** | ||
| 4 | -0.160 | -0.051 | -0.317* | -0.400** | -0.076 | -0.063 | -0.499** | -0.412** | -0.182 | -0.435** | ||
| 6 | -0.196 | -0.252 | -0.372** | -0.642** | -0.250 | 0.009 | -0.493** | -0.505** | -0.169 | -0.531** | ||
| 8 | -0.120 | -0.231 | -0.346* | -0.510** | -0.121 | -0.090 | -0.500** | -0.533** | -0.212 | -0.500** | ||
表4 水稻生长和产量构成因素与超氧阴离子和过氧化氢含量的相关系数
Table 4 Correlation index between the growth indexes, yield component indexes and contents of O2-. and H2O2 in rice
| Indexes | Treatment time (d) | Shoot length | Stem dry weight | Panicle length | Panicle weight | Panicle number | Spikelets per panicle | Percentage of filled spikelets | 1000-kernel weight | Harvest index | Yield | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Contents of O2- . | CK | 0 | 0.059 | 0.135 | 0.173 | 0.063 | 0.161 | -0.115 | 0.006 | 0.135 | -0.023 | 0.128 |
| 2 | -0.028 | -0.071 | -0.081 | -0.053 | -0.143 | 0.134 | -0.233 | -0.138 | -0.033 | -0.151 | ||
| 4 | 0.097 | -0.02 | -0.083 | 0.030 | -0.176 | 0.196 | -0.062 | -0.181 | -0.379* | -0.102 | ||
| 6 | 0.209 | 0.150 | 0.152 | 0.257 | 0.040 | 0.053 | 0.019 | -0.329* | -0.107 | 0.069 | ||
| 8 | -0.049 | 0.052 | -0.066 | 0.061 | -0.05 | 0.052 | 0.002 | -0.104 | -0.147 | -0.072 | ||
| HS | 0 | -0.075 | -0.348** | -0.359** | -0.117 | -0.331* | 0.076 | 0.004 | 0.055 | -0.047 | -0.203 | |
| 2 | -0.099 | -0.093 | -0.463** | -0.510** | -0.138 | -0.173 | -0.448** | -0.421** | -0.413** | -0.600** | ||
| 4 | -0.059 | -0.195 | -0.373** | -0.441** | -0.142 | -0.029 | -0.384** | -0.390** | -0.174 | -0.435** | ||
| 6 | -0.215 | -0.249 | -0.339* | -0.487** | -0.140 | -0.070 | -0.408** | -0.404** | -0.205 | -0.450** | ||
| 8 | 0.101 | -0.191 | -0.257 | -0.393** | -0.138 | -0.106 | -0.241 | -0.330* | -0.275* | -0.375** | ||
| Contents of H2O2 | CK | 0 | -0.181 | -0.021 | 0.086 | 0.035 | 0.047 | -0.163 | 0.222 | -0.070 | -0.109 | -0.061 |
| 2 | 0.070 | 0.024 | 0.134 | 0.171 | 0.030 | 0.061 | -0.045 | -0.066 | 0.028 | 0.048 | ||
| 4 | 0.077 | 0.194 | 0.374* | 0.353* | 0.089 | -0.046 | -0.160 | 0.066 | -0.188 | 0.018 | ||
| 6 | 0.196 | 0.228 | 0.174 | 0.339* | 0.070 | 0.034 | 0.068 | -0.329* | -0.322* | 0.050 | ||
| 8 | 0.225 | 0.195 | 0.221 | 0.135 | 0.093 | 0.070 | -0.107 | -0.152 | -0.123 | 0.065 | ||
| HS | 0 | 0.097 | 0.168 | 0.130 | 0.290* | 0.227 | -0.251 | 0.151 | 0.166 | -0.174 | 0.071 | |
| 2 | -0.208 | -0.210 | -0.439** | -0.569** | -0.206 | -0.033 | -0.477** | -0.400** | -0.201 | -0.502** | ||
| 4 | -0.160 | -0.051 | -0.317* | -0.400** | -0.076 | -0.063 | -0.499** | -0.412** | -0.182 | -0.435** | ||
| 6 | -0.196 | -0.252 | -0.372** | -0.642** | -0.250 | 0.009 | -0.493** | -0.505** | -0.169 | -0.531** | ||
| 8 | -0.120 | -0.231 | -0.346* | -0.510** | -0.121 | -0.090 | -0.500** | -0.533** | -0.212 | -0.500** | ||
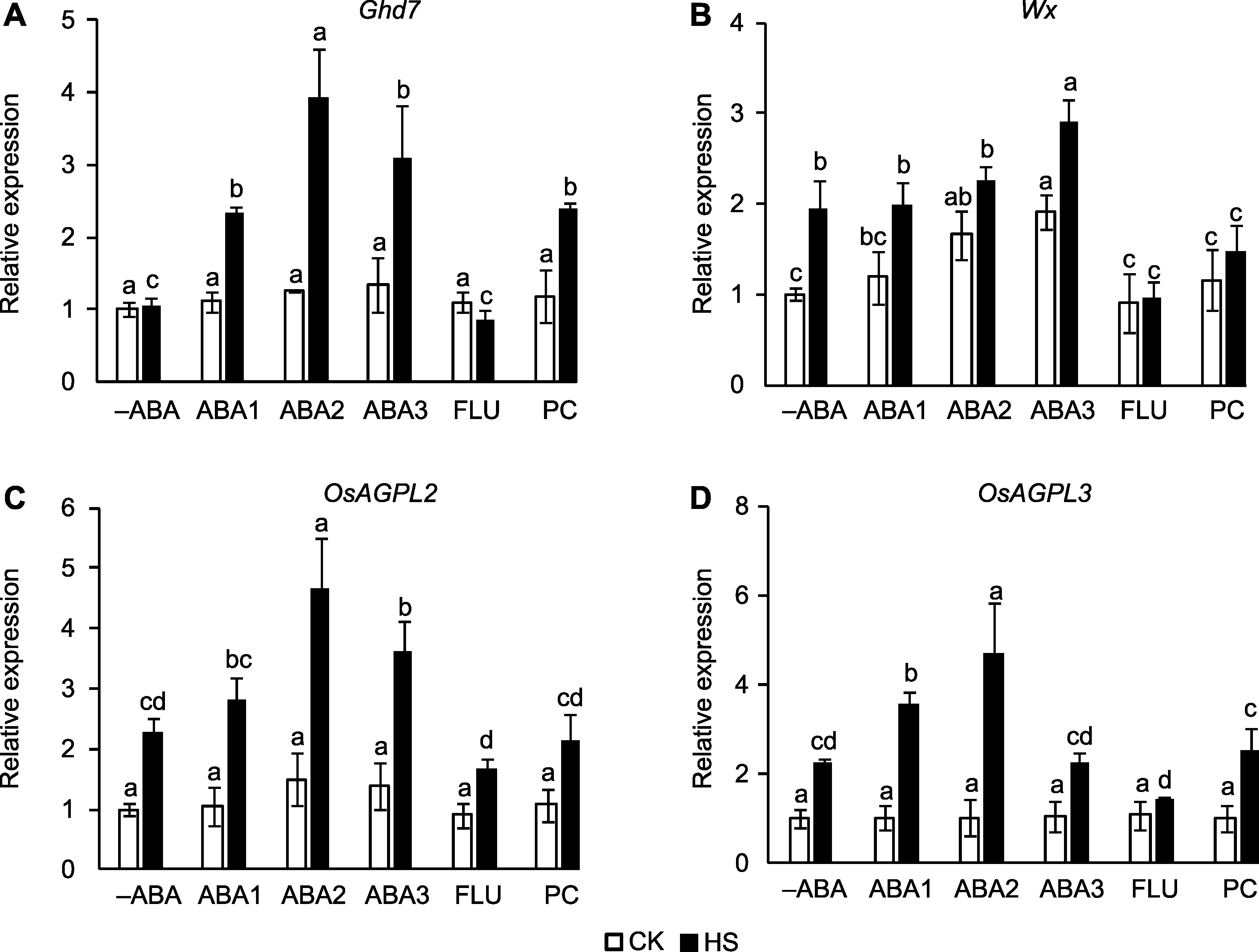
图5 孕穗期喷施ABA对高温胁迫下水稻产量形成基因表达量的影响 (A) Ghd7; (B) Wx; (C) OsAGPL2; (D) OsAGPL3。-ABA、ABA1、ABA2、ABA3、FLU、PC、CK和HS同图1。不同小写字母表示在对照和高温胁迫条件下不同处理间差异显著(P<0.05)。
Figure 5 Effect of spraying ABA solution at booting stage on the expression of yield related genes in rice under high temperature stress (A) Ghd7; (B) Wx; (C) OsAGPL2; (D) OsAGPL3. -ABA, ABA1, ABA2, ABA3, FLU, PC, CK, and HS are the same as shown in Figure 1. Different lowercase letters indicate significance differences among different treatments under control and high temperature stress (P<0.05).
| [1] | 陈唯, 曾晓贤, 谢楚萍, 田长恩, 周玉萍 (2019). 植物内源ABA水平的动态调控机制. 植物学报 54, 677-687. |
| [2] | 段骅, 傅亮, 剧成欣, 刘立军, 杨建昌 (2013). 氮素穗肥对高温胁迫下水稻结实和稻米品质的影响. 中国水稻科学 27, 591-602. |
| [3] | 郭贵华, 刘海艳, 李刚华, 刘明, 李岩, 王绍华, 刘正辉, 唐设, 丁艳锋 (2014). ABA缓解水稻孕穗期干旱胁迫生理特性的分析. 中国农业科学 47, 4380-4391. |
| [4] | 雷娅伟, 白小明, 王婷, 吕优伟, 雷舒涵 (2015). 脱落酸对3个野生草地早熟禾种质高温胁迫的缓解效应. 草地学报 23, 89-94, 100. |
| [5] | 李迎春, 帅细强, 杨蓉, 刘丹 (2019). 高温热害对江西省早稻产量影响的定量分析. 湖北农业科学 58(20), 79-83. |
| [6] | 刘晓龙, 季平, 杨洪涛, 邵勤, 任珺怡, 祝佳滢, 张超毅, 陆晨旭 (2021). 高温胁迫对水稻内源脱落酸含量及相关基因表达的影响. 分子植物育种 1-12. [2021-12-20].https://kns.cnki.net/kcms/detail/detail.aspx?dbcode=CAPJ&dbname=CAPJLAST&filename=FZZW20211215000&uniplatform=NZKPT&v=_MB1PS_Ch2BhePRwLHu5Uh4VVaM4GNe8EyhVd2rEe7ln_AJgZxEiEWU6WlC2bx-z. |
| [7] | 隆春艳, 古洪辉, 汪正香, 蒋雄, 杨翠芹, 秦耀国 (2017). 外源脱落酸对高温胁迫下菠菜光合与叶绿素荧光参数的影响. 四川农业大学学报 35, 24-30. |
| [8] |
王强, 陈雷, 张晓丽, 唐茂艳, 吕荣华, 陶伟, 梁天锋 (2015). 化学调控对水稻高温热害的缓解作用研究. 中国稻米 21 (4), 80-82.
DOI |
| [9] | 杨建莹, 霍治国, 王培娟, 邬定荣 (2020). 江西早稻高温热害等级动态判识及时空变化特征. 应用生态学报 31, 199-207. |
| [10] | 杨卫丽, 黄福灯, 曹珍珍, 雷炳婷, 胡东维, 程方民 (2013). 高温胁迫对水稻光合PSII系统伤害及其与叶绿体D1蛋白间关系. 作物学报 39, 1060-1068. |
| [11] |
杨雲雲, 陈鑫, 陈启洲, 卢芳, 徐晨, 杨洪涛, 苏佩佩, 刘晓龙 (2021). 脱落酸对水稻种子萌发期耐高温胁迫的诱抗效应. 华北农学报 36(3), 185-194.
DOI |
| [12] | 张桂莲, 陈立云, 张顺堂, 肖应辉, 贺治洲, 雷东阳 (2006). 高温胁迫对水稻剑叶保护酶活性和膜透性的影响. 作物学报 32, 1306-1310. |
| [13] | Brennan T, Frenkel C (1977). Involvement of hydrogen peroxide in the regulation of senescence in pear. Plant Physiol 59, 411-416. |
| [14] | Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J 90, 856-867. |
| [15] | Dar NA, Amin I, Wani W, Wani SA, Shikari AB, Wani SH, Masoodi KZ (2017). Abscisic acid: a key regulator of abiotic stress tolerance in plants. Plant Gene 11, 106-111. |
| [16] | Dwivedi SK, Basu S, Kumar S, Kumari S, Kumar A, Jha S, Mishra JS, Bhatt BP, Kumar G (2019). Enhanced antioxidant enzyme activities in developing anther contributes to heat stress alleviation and sustains grain yield in wheat. Funct Plant Biol 46, 1090-1102. |
| [17] | Elstner EF, Heupel A (1976). Inhibition of nitrite formation from hydroxylammoniumchloride: a simple assay for superoxide dismutase. Anal Biochem 70, 616-620. |
| [18] | Feng BH, Zhang CX, Chen TT, Zhang XF, Tao LX, Fu GF (2018). Salicylic acid reverses pollen abortion of rice caused by heat stress. BMC Plant Biol 18, 245. |
| [19] | Fu GF, Feng BH, Zhang CX, Yang YJ, Yang XQ, Chen TT, Zhao X, Zhang XF, Jin QY, Tao LX (2016). Heat stress is more damaging to superior spikelets than inferiors of rice (Oryza sativa L.) due to their different organ temperatures. Front Plant Sci 7, 1637. |
| [20] | Hirose T, Ohdan T, Nakamura Y, Terao T (2006). Expression profiling of genes related to starch synthesis in rice leaf sheaths during the heading period. Physiol Plant 128, 425-435. |
| [21] | Huang J, Zhang FM, Xue Y, Lin J (2017). Recent changes of rice heat stress in Jiangxi province, southeast China. Int J Biometeorol 61, 623-633. |
| [22] | Huang YC, Niu CY, Yang CR, Jinn TL (2016). The heat stress factor HSFA6b connects ABA signaling and ABA-mediated heat responses. Plant Physiol 172, 1182-1199. |
| [23] | Janni M, Gullì M, Maestri E, Marmiroli M, Valliyodan B, Nguyen HT, Marmiroli N (2020). Molecular and genetic bases of heat stress responses in crop plants and breeding for increased resilience and productivity. J Exp Bot 71, 3780-3802. |
| [24] | Jiang CJ, Liu XL, Liu XQ, Zhang H, Yu YJ, Liang ZW (2017). Stunted growth caused by blast disease in rice seedlings is associated with changes in phytohormone signaling pathways. Front Plant Sci 8, 1558. |
| [25] | Joshee N, Kisaka H, Kitagawa Y (1998). Isolation and characterization of a water stress-specific genomic gene, pwsi 18, from rice. Plant Cell Physiol 39, 64-72. |
| [26] | Li N, Euring D, Cha JY, Lin Z, Lu MZ, Huang LJ, Kim WY (2021). Plant hormone-mediated regulation of heat tolerance in response to global climate change. Front Plant Sci 11, 627969. |
| [27] | Liu XL, Xie XZ, Zheng CK, Wei LX, Li XW, Jin YY, Zhang GH, Jiang CJ, Liang ZW (2022). RNAi-mediated suppression of the abscisic acid catabolism gene OsABA- 8ox1 increases abscisic acid content and tolerance to saline-alkaline stress in rice (Oryza sativa L.). Crop J 10, 354-367. |
| [28] | Liu XL, Zhang H, Jin YY, Wang MM, Yang HY, Ma HY, Jiang CJ, Liang ZW (2019). Abscisic acid primes rice seedlings for enhanced tolerance to alkaline stress by upregulating antioxidant defense and stress tolerance- related genes. Plant Soil 438, 39-55. |
| [29] | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 25, 402-408. |
| [30] | Qiao B, Zhang Q, Liu DL, Wang HQ, Yin JY, Wang R, He ML, Cui M, Shang ZL, Wang DK, Zhu ZG (2015). A calcium-binding protein, rice annexin OsANN1, enhances heat stress tolerance by modulating the production of H2O2. J Exp Bot 66, 5853-5866. |
| [31] | Qin P, Zhang GH, Hu BH, Wu J, Chen WL, Ren ZJ, Liu YL, Xie J, Yuan H, Tu B, Ma BT, Wang YP, Ye LM, Li LG, Xiang CB, Li SG (2021). Leaf-derived ABA regulates rice seed development via a transporter-mediated and temperature-sensitive mechanism. Sci Adv 7, eabc8873. |
| [32] | Qiu ZN, Zhu L, He L, Chen DD, Zeng DL, Chen G, Hu J, Zhang GH, Ren DY, Dong GJ, Gao ZY, Shen L, Zhang Q, Guo LB, Qian Q (2019). DNA damage and reactive oxygen species cause cell death in the rice local lesions 1 mutant under high light and high temperature. New Phytol 222, 349-365. |
| [33] | Rabbani MA, Maruyama K, Abe H, Khadri MA, Katsura K, Ito Y, Yoshiwara K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2003). Monitoring expression profiles of rice genes under cold, drought, and high-salinity stresses and abscisic acid application using cDNA microarray and RNA gel-blot analyses. Plant Physiol 133, 1755-1767. |
| [34] | Rezaul IM, Feng BH, Chen TT, Fu WM, Zhang CX, Tao LX, Fu GF (2019). Abscisic acid prevents pollen abortion under high-temperature stress by mediating sugar metabolism in rice spikelets. Physiol Plant 165, 644-663. |
| [35] | Sato H, Suzuki Y, Sakai M, Imbe T (2002). Molecular characterization of Wx-mq, a novel mutant gene for low- amylose content in endosperm of rice (Oryza sativa L.). Breed Sci 52, 131-135. |
| [36] | Shi WJ, Yin XY, Struik PC, Solis C, Xie FM, Schmidt RC, Huang M, Zou YB, Ye CR, Jagadish SVK (2017). High day- and night-time temperatures affect grain growth dynamics in contrasting rice genotypes. J Exp Bot 68, 5233-5245. |
| [37] | Suriyasak C, Harano K, Tanamachi K, Matsuo K, Tamada A, Iwaya-Inoue M, Ishibashi Y (2017). Reactive oxygen species induced by heat stress during grain filling of rice (Oryza sativa L.) are involved in occurrence of grain chalkiness. J Plant Physiol 216, 52-57. |
| [38] | Tang RS, Zheng JC, Jin ZQ, Zhang DD, Huang YH (2008). Possible correlation between high temperature-induced floret sterility and endogenous levels of IAA, GAs and ABA in rice (Oryza sativa L.). Plant Growth Regul 54, 37-43. |
| [39] | Vishwakarma K, Upadhyay N, Kumar N, Yadav G, Singh J, Mishra RK, Kumar V, Verma R, Upadhyay RG, Pandey M, Sharma S (2017). Abscisic acid signaling and abiotic stress tolerance in plants: a review on current knowledge and future prospects. Front Plant Sci 8, 161. |
| [40] | Wang YL, Wang L, Zhou JX, Hu SB, Chen HZ, Xiang J, Zhang YK, Zeng YJ, Shi QH, Zhu DF, Zhang YP (2019). Research progress on heat stress of rice at flowering stage. Rice Sci 26, 1-10. |
| [41] | Wei LX, Lv BS, Li XW, Wang MM, Ma HY, Yang HY, Yang RF, Piao ZZ, Wang ZH, Lou JH, Jiang CJ, Liang ZW (2017a). Priming of rice (Oryza sativa L.) seedlings with abscisic acid enhances seedling survival, plant growth, and grain yield in saline-alkaline paddy fields. Field Crop Res 203, 86-93. |
| [42] | Wei LX, Lv BS, Wang MM, Ma HY, Yang HY, Liu XL, Jiang CJ, Liang ZW (2015). Priming effect of abscisic acid on alkaline stress tolerance in rice (Oryza sativa L.) seedlings. Plant Physiol Biochem 90, 50-57. |
| [43] | Wei XJ, Jiao GA, Lin HY, Sheng ZH, Shao GN, Xie LH, Tang SQ, Xu QG, Hu PS (2017b). GRAIN INCOMPLETE FILLING 2 regulates grain filling and starch synthesis during rice caryopsis development. J Integr Plant Biol 59, 134-153. |
| [44] | Xu YF, Chu CC, Yao SG (2021). The impact of high-temperature stress on rice: challenges and solutions. Crop J 9, 963-976. |
| [45] | Xu YY, Ramanathan V, Victor DG (2018). Global warming will happen faster than we think. Nature 564, 30-32. |
| [46] | Ye NH, Jia LG, Zhang JH (2012). ABA signal in rice under stress conditions. Rice 5, 1. |
| [47] | Zhang CX, Feng BH, Chen TT, Fu WM, Li HB, Li GY, Jin QY, Tao LX, Fu GF (2018). Heat stress-reduced kernel weight in rice at anthesis is associated with impaired source-sink relationship and sugars allocation. Environ Exp Bot 155, 718-733. |
| [48] | Zhang CX, Fu GF, Yang XQ, Yang YJ, Zhao X, Chen TT, Zhang XF, Jin QY, Tao LX (2016). Heat stress effects are stronger on spikelets than on flag leaves in rice due to differences in dissipation capacity. J Agron Crop Sci 202, 394-408. |
| [49] | Zhao C, Liu B, Piao SL, Wang XH, Lobell DB, Huang Y, Huang MT, Yao YT, Bassu S, Ciais P, Durand JL, Elliott J, Ewert F, Janssens IA, Li T, Lin ED, Liu Q, Martre P, Müller C, Peng SS, Peñuelas J, Ruane AC, Wallach D, Wang T, Wu DH, Liu Z, Zhu Y, Zhu ZC, Asseng S (2017). Temperature increase reduces global yields of major crops in four independent estimates. Proc Natl Acad Sci USA 114, 9326-9331. |
| [50] | Zhao Q, Zhou LJ, Liu JC, Du XX, Asad MAU, Huang FD, Pan G, Cheng FM (2018). Relationship of ROS accumulation and superoxide dismutase isozymes in developing anther with floret fertility of rice under heat stress. Plant Physiol Biochem 122, 90-101. |
| [51] | Zong WB, Ren D, Huang MH, Sun KL, Feng JL, Zhao J, Xiao DD, Xie WH, Liu SQ, Zhang H, Qiu R, Tang WJ, Yang RQ, Chen HY, Xie XR, Chen LT, Liu YG, Guo JX (2021). Strong photoperiod sensitivity is controlled by cooperation and competition among Hd1, Ghd7 and DTH8 in rice heading. New Phytol 229, 1635-1649. |
| [1] | 唐远翔, 熊仕臣, 朱洪锋, 张新生, 游成铭, 刘思凝, 谭波, 徐振锋. 长期氮添加对四川盆地西缘常绿阔叶林优势树种凋落叶产量及碳氮磷归还的影响[J]. 植物生态学报, 2025, 49(5): 720-731. |
| [2] | 叶灿, 姚林波, 金莹, 高蓉, 谭琪, 李旭映, 张艳军, 陈析丰, 马伯军, 章薇, 张可伟. 水稻水杨酸代谢突变体高通量筛选方法的建立与应用[J]. 植物学报, 2025, 60(4): 1-0. |
| [3] | 许庭旸, 刘雨辰, 王万鹏, 苏航, 苏昆龙, 吴振映, 吕明, 李福利, 王小山, 付春祥. 喷施不同植物生长调节剂对盐碱地小麦生长发育的影响[J]. 植物学报, 2025, 60(3): 354-362. |
| [4] | 赵凌, 管菊, 梁文化, 张勇, 路凯, 赵春芳, 李余生, 张亚东. 基于高密度Bin图谱的水稻苗期耐热性QTL定位[J]. 植物学报, 2025, 60(3): 342-353. |
| [5] | 李新宇, 谷月, 徐非非, 包劲松. 水稻胚乳淀粉合成相关蛋白的翻译后修饰研究进展[J]. 植物学报, 2025, 60(2): 256-270. |
| [6] | 李建国, 张怡, 张文君. 水稻根系铁膜形成及对磷吸收的影响[J]. 植物学报, 2025, 60(1): 132-143. |
| [7] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| [8] | 姚瑞枫, 谢道昕. 水稻独脚金内酯信号感知的激活和终止[J]. 植物学报, 2024, 59(6): 873-877. |
| [9] | 闫恒宇, 李朝霞, 李玉斌. 高温对玉米生长的影响及中国耐高温玉米筛选研究进展[J]. 植物学报, 2024, 59(6): 1007-1023. |
| [10] | 连锦瑾, 唐璐瑶, 张伊诺, 郑佳兴, 朱超宇, 叶语涵, 王跃星, 商文楠, 傅正浩, 徐昕璇, 吴日成, 路梅, 王长春, 饶玉春. 水稻抗氧化性状遗传位点挖掘及候选基因分析[J]. 植物学报, 2024, 59(5): 738-751. |
| [11] | 黄佳慧, 杨惠敏, 陈欣雨, 朱超宇, 江亚楠, 胡程翔, 连锦瑾, 芦涛, 路梅, 张维林, 饶玉春. 水稻突变体pe-1对弱光胁迫的响应机制[J]. 植物学报, 2024, 59(4): 574-584. |
| [12] | 周俭民. 收放自如的明星战车[J]. 植物学报, 2024, 59(3): 343-346. |
| [13] | 夏婧, 饶玉春, 曹丹芸, 王逸, 柳林昕, 徐雅婷, 牟望舒, 薛大伟. 水稻中乙烯生物合成关键酶OsACS和OsACO调控机制研究进展[J]. 植物学报, 2024, 59(2): 291-301. |
| [14] | 朱超宇, 胡程翔, 朱哲楠, 张芷宁, 汪理海, 陈钧, 李三峰, 连锦瑾, 唐璐瑶, 钟芊芊, 殷文晶, 王跃星, 饶玉春. 水稻穗部性状QTL定位及候选基因分析[J]. 植物学报, 2024, 59(2): 217-230. |
| [15] | 方妍力, 田传玉, 苏如意, 刘亚培, 王春连, 陈析丰, 郭威, 纪志远. 水稻抗细菌性条斑病基因挖掘与初定位[J]. 植物学报, 2024, 59(1): 1-9. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||