

植物学报 ›› 2023, Vol. 58 ›› Issue (5): 701-711.DOI: 10.11983/CBB22223 cstr: 32102.14.CBB22223
张盈川1, 吴晓明玉1, 陶保龙1, 陈丽1,2, 鲁海琴1, 赵伦1, 文静1, 易斌1, 涂金星1, 傅廷栋1, 沈金雄1,*( )
)
收稿日期:2022-09-16
接受日期:2023-02-25
出版日期:2023-09-01
发布日期:2023-09-21
通讯作者:
*E-mail: jxshen@mail.hzau.edu.cn
基金资助:
Zhang Yingchuan1, Wu Xiaomingyu1, Tao Baolong1, Chen Li1,2, Lu Haiqin1, Zhao Lun1, Wen Jing1, Yi Bin1, Tu Jinxing1, Fu Tingdong1, Shen Jinxiong1,*( )
)
Received:2022-09-16
Accepted:2023-02-25
Online:2023-09-01
Published:2023-09-21
Contact:
*E-mail: jxshen@mail.hzau.edu.cn
摘要: miRNA通过调控靶基因的表达参与植物生长发育和响应逆境胁迫等多个方面。对前期鉴定到的1个miRNA——Bna-miR43进行功能研究, 通过构建过表达载体探讨了Bna-miR43在甘蓝型油菜(Brassica napus)响应干旱胁迫中的功能。降解组测序预测到Bna-miR43的4个靶基因均属于F-box蛋白家族。在模拟干旱条件下, 甘蓝型油菜J572根系Bna-miR43的表达量逐渐下降; 靶基因则呈现与Bna-miR43相反的表达模式, 且随着干旱处理时间的增加, 靶基因的表达量逐渐上升。转基因实验表明, 在干旱胁迫下, 油菜Bna-miR43过表达株系表现为对干旱极度敏感, 转基因株系种子在干旱条件下发芽率显著下降, 植株脱水严重, 体内积累了更多的丙二醛(MDA)和过氧化氢(H2O2)。干旱处理后, Bna-miR43过表达株系中编码超氧化物歧化酶(SOD)、过氧化氢酶(CAT)和乙醇酸氧化酶(GOX)的3个基因下调表达。上述结果表明, Bna-miR43通过调控靶基因的表达调节甘蓝型油菜渗透物质积累和细胞活性氧(ROS)稳态, 在调控植物耐旱性中发挥重要作用。
张盈川, 吴晓明玉, 陶保龙, 陈丽, 鲁海琴, 赵伦, 文静, 易斌, 涂金星, 傅廷栋, 沈金雄. Bna-miR43介导甘蓝型油菜响应干旱胁迫. 植物学报, 2023, 58(5): 701-711.
Zhang Yingchuan, Wu Xiaomingyu, Tao Baolong, Chen Li, Lu Haiqin, Zhao Lun, Wen Jing, Yi Bin, Tu Jinxing, Fu Tingdong, Shen Jinxiong. Bna-miR43 Mediates the Response of Drought Tolerance in Brassica napus. Chinese Bulletin of Botany, 2023, 58(5): 701-711.
| Gene | Annotation | Primer sequence (5'-3') |
|---|---|---|
| BnaC05g39240D | COX1 | F: CAAGAACAGGAAAGTGGTTGAG R: GCAACGTGAACCTGTTCTTAAT |
| BnaA07g11360D | CAT1 | F: AATCGTCTTTGCATCATCCATG R: GTCAAAGAGGAGTTGTTGTTCC |
| BnaC08g42970D | SOD1 | F: GATCACAAAACTATGGCCAAGG R: AAACAGTTCCTGTCACAGTAGT |
| BnaC09g53650D | ABI2 | F: GATGAGTTTGATCCGAGATCGA R: TGAACTCGAACAAGCTTCTACT |
| BnaC01g04330D | ABF | F: GCCGATTTGACTAGATCAACAC R: CGTCCTAGAAAGCAACATCAAG |
| BnaA10g24440D | ABCG22 | F: TTGATGATCTTGATTGACACGC R: CCAAACGCACAACTGTAACTAA |
表1 荧光定量PCR引物序列
Table 1 The primer sequences of qRT-PCR
| Gene | Annotation | Primer sequence (5'-3') |
|---|---|---|
| BnaC05g39240D | COX1 | F: CAAGAACAGGAAAGTGGTTGAG R: GCAACGTGAACCTGTTCTTAAT |
| BnaA07g11360D | CAT1 | F: AATCGTCTTTGCATCATCCATG R: GTCAAAGAGGAGTTGTTGTTCC |
| BnaC08g42970D | SOD1 | F: GATCACAAAACTATGGCCAAGG R: AAACAGTTCCTGTCACAGTAGT |
| BnaC09g53650D | ABI2 | F: GATGAGTTTGATCCGAGATCGA R: TGAACTCGAACAAGCTTCTACT |
| BnaC01g04330D | ABF | F: GCCGATTTGACTAGATCAACAC R: CGTCCTAGAAAGCAACATCAAG |
| BnaA10g24440D | ABCG22 | F: TTGATGATCTTGATTGACACGC R: CCAAACGCACAACTGTAACTAA |
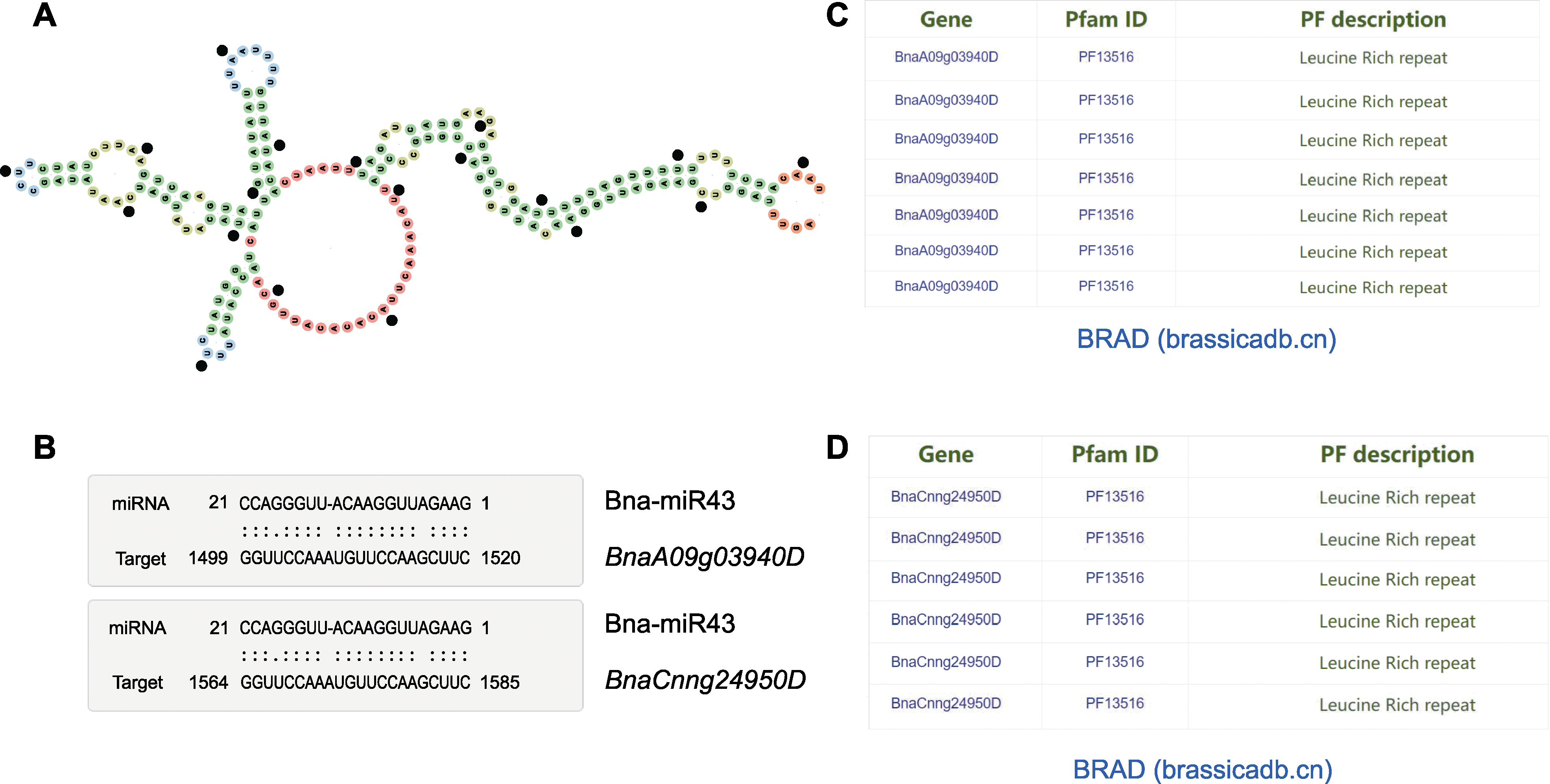
图1 Bna-miR43及其靶基因的基本信息 (A) Bna-miR43前体二级结构预测; (B) Bna-miR43及其靶基因之间的碱基互补配对; (C) BnaA09g03940D的结构域注释; (D) BnaCnng24950D的结构域注释
Figure 1 Basic information of Bna-miR43 and its target gene (A) Bna-miR43 precursor’s secondary structure prediction; (B) Base pairing characterization between Bna-miR43 and its target genes; (C) The Pfam domain annotation of BnaA09g03940D; (D) The Pfam domain annotation of BnaCnng24950D
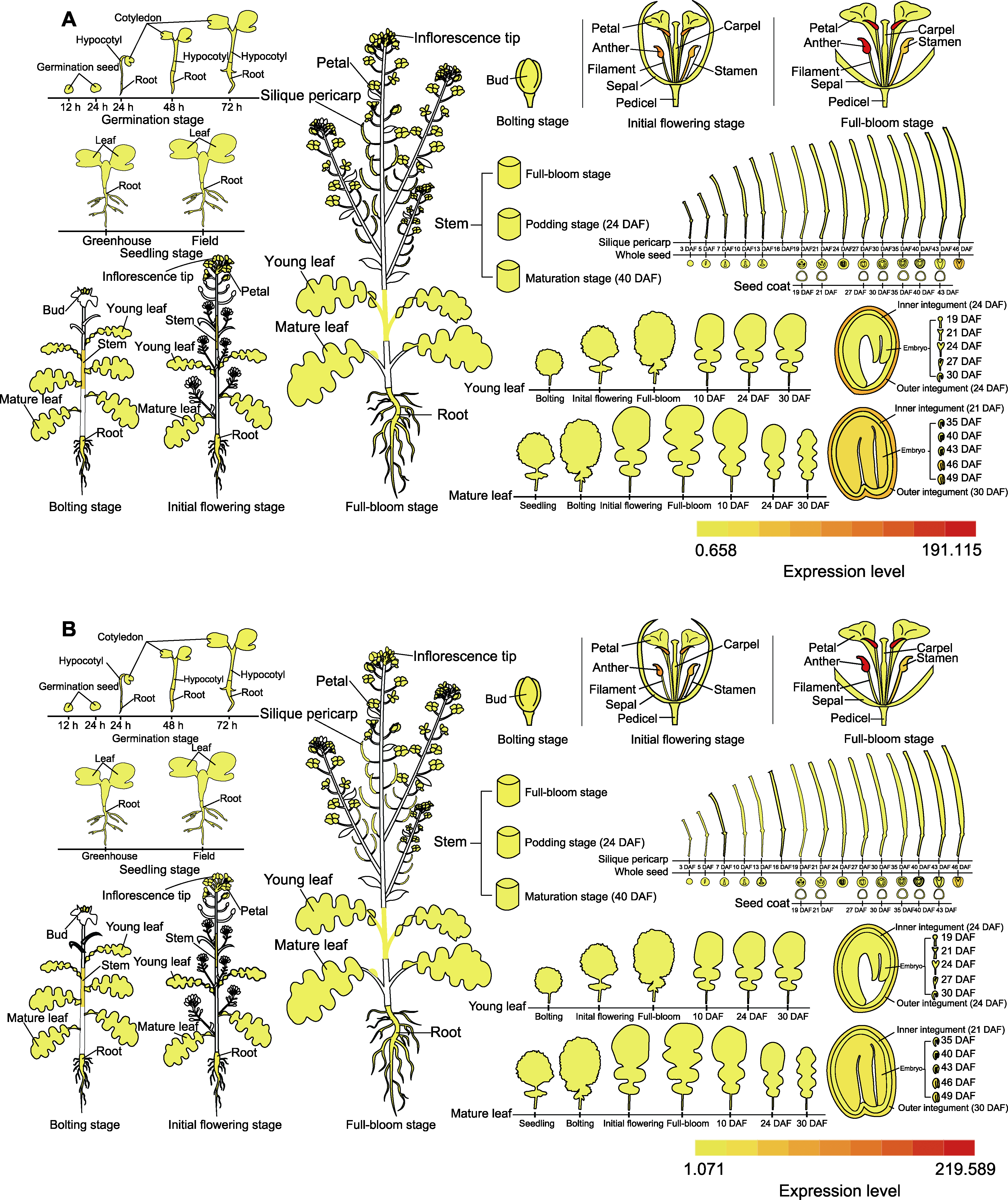
图3 靶基因在甘蓝型油菜中的表达模式 (A) BnaA09g03940D的表达模式; (B) BnaCnng24950D的表达模式
Figure 3 Expression patterns of target genes in Brassica napus (A) The expression pattern of BnaA09g03940D; (B) The expression pattern of BnaCnng24950D
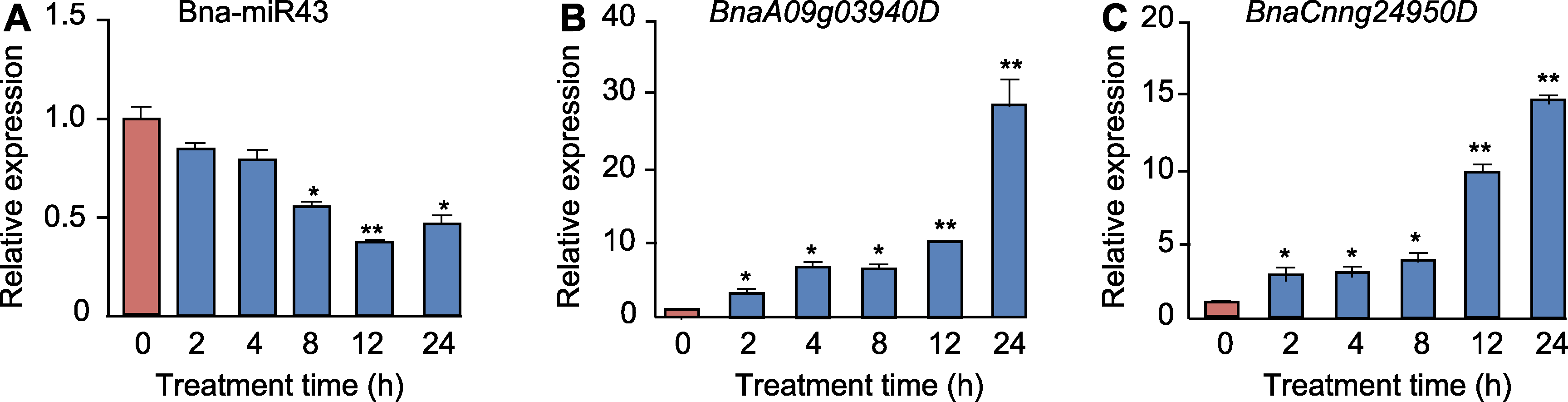
图4 干旱胁迫下甘蓝型油菜J572中Bna-miR43及其靶基因的表达模式 (A) Bna-miR43的表达模式; (B) BnaA09g03940D的表达模式; (C) BnaCnng24950D的表达模式。实验设3次生物学重复, 3次技术重复。* P<0.05; **P<0.01
Figure 4 Expression patterns of Bna-miR43 and its target genes of Brassica napus under drought stress (A) The expression pattern of Bna-miR43; (B) The expression pattern of BnaA09g03940D; (C) The expression pattern of BnaCnng24950D. Three biological replicates and three technical replicates per experiment. * P<0.05; ** P<0.01
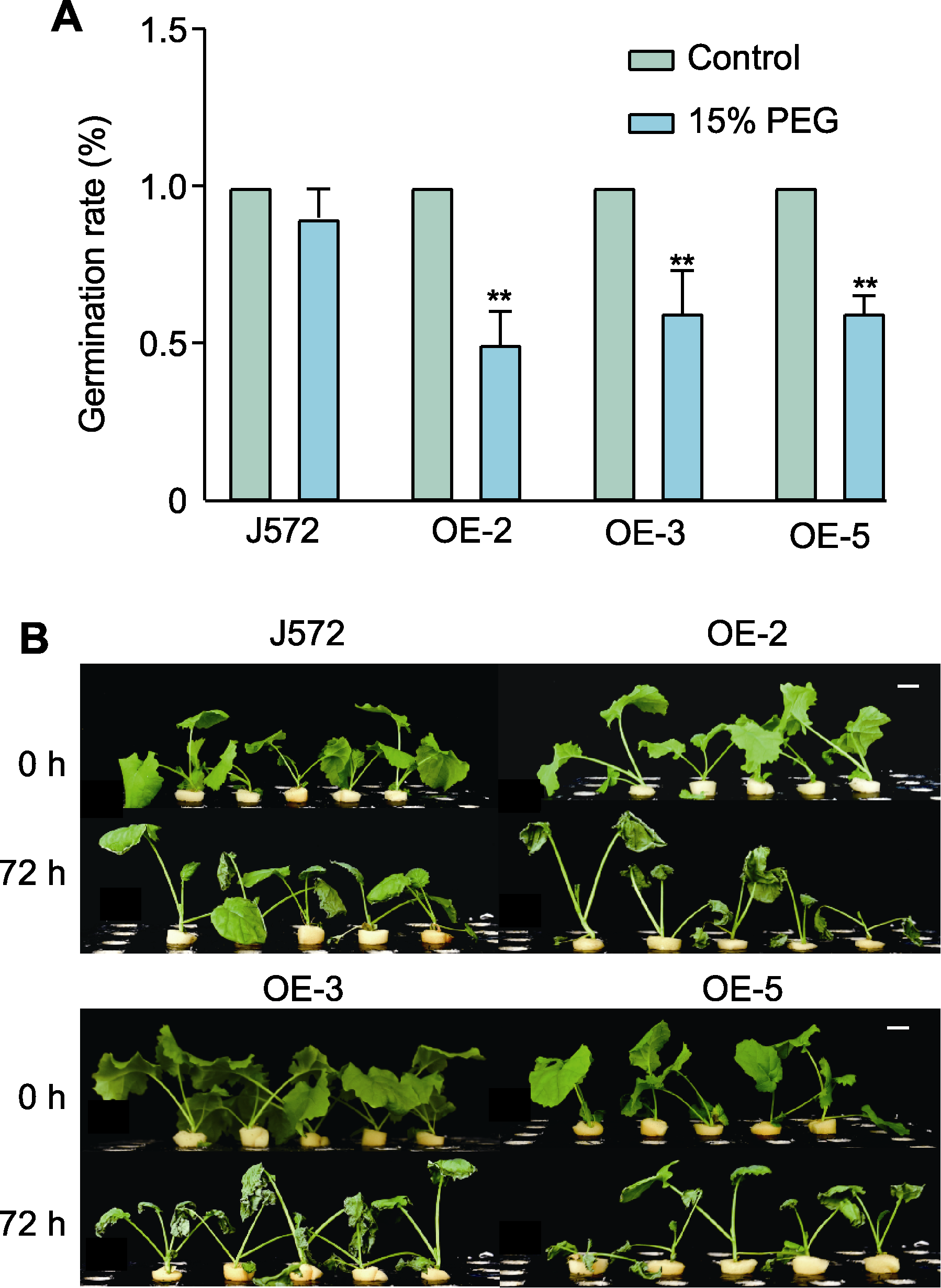
图5 干旱胁迫下甘蓝型油菜种子发芽率统计(A)及PEG模拟干旱处理下过表达Bna-miR43转基因植株地上部表型(B) J572: 对照; OE-2、OE-3和OE-5为不同转基因株系; ** P<0.01; Bars=1 cm
Figure 5 Germination rate of Brassica napus under drought stress (A) and phenotypic analysis of Bna-miR43 overexpressed transgenic plants under PEG simulated drought treatment (B) J572: Control; OE-2, OE-3, and OE-5 indicate different transgenic individuals; ** P<0.01; Bars=1 cm
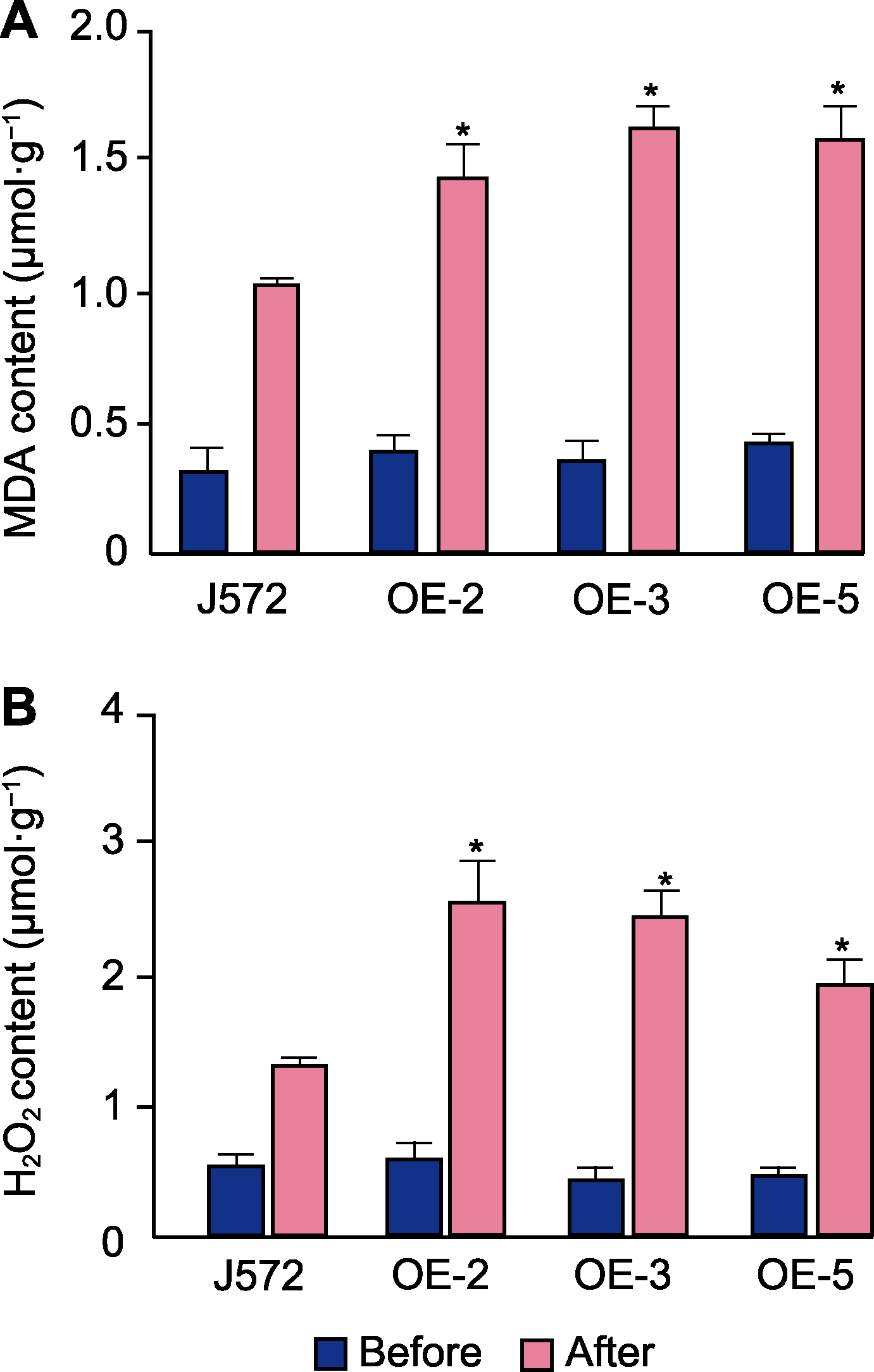
图6 过表达Bna-miR43油菜根系干旱胁迫后丙二醛(MDA) (A)和过氧化氢(H2O2) (B)含量分析 Before: 干旱胁迫处理前; After: 干旱胁迫处理后。实验设3次生物学重复。J572、OE-2、OE-3和OE-5同图5。* P<0.05
Figure 6 Analysis of malondialdehyde (MDA) (A) and hydrogen peroxide (H2O2) (B) content in roots of Bna-miR43 overexpressed transgenic rapeseed after drought stress Before: Before drought treatment; After: After drought treatment. Three biological replicates per experiment. J572, OE-2, OE-3, and OE-5 are the same as shown in Figure 5. * P<0.05
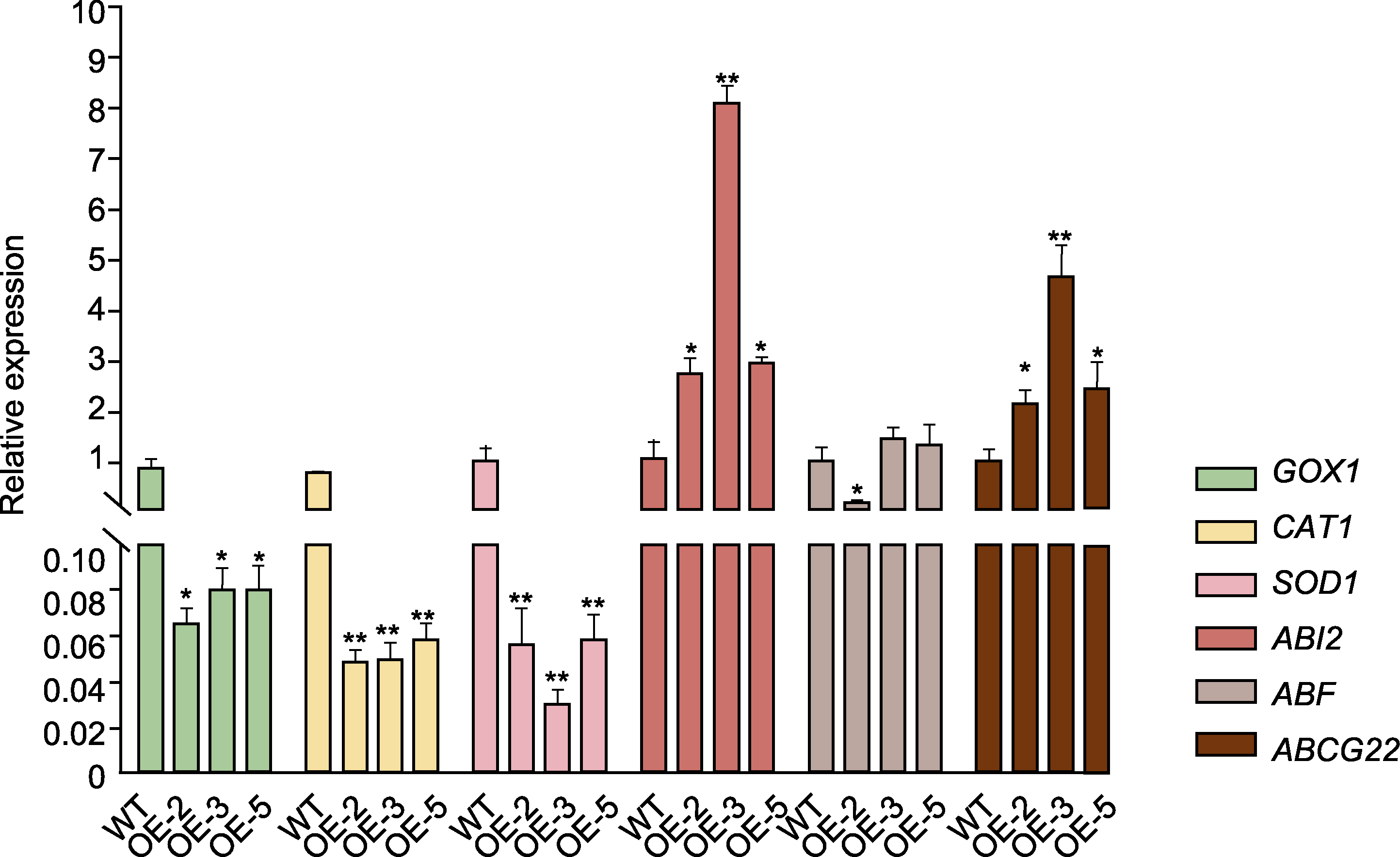
图7 过表达Bna-miR43油菜中氧化应激反应和ABA信号通路基因表达量分析 WT: 野生型; OE-2、OE-3和OE-5同图5。* P<0.05; ** P<0.01
Figure 7 Analysis of the expression of oxidative stress-responsive genes and genes involved in ABA signaling pathway in Bna-miR43 overexpressed transgenic rapeseed WT: Wild type; OE-2, OE-3, and OE-5 are the same as shown in Figure 5. * P<0.05; ** P<0.01
| [1] | 陈丽 (2018). 甘蓝型油菜株型及角果长度相关miRNA和靶基因的挖掘. 博士论文. 武汉: 华中农业大学. pp. 21-59. |
| [2] | 宋凝曦, 谢寅峰, 李霞 (2020). 干旱胁迫下表观遗传机制对转C4型PEPC基因水稻种子萌发的影响. 植物学报 55, 677-692. |
| [3] |
王劲东, 周豫, 余佳雯, 范晓磊, 张昌泉, 李钱峰, 刘巧泉 (2020). MiR172-AP2模块调控植物生长发育及逆境响应的研究进展. 植物学报 55, 205-215.
DOI |
| [4] |
吴丹丹, 陈永坤, 杨宇, 孔春艳, 龚明 (2021). 小桐子半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对低温锻炼的响应. 植物学报 56, 544-558.
DOI |
| [5] |
张盈川, 吴晓明玉, 陶保龙, 陈丽, 鲁海琴, 赵伦, 文静, 易斌, 涂金星, 傅廷栋, 沈金雄 (2023). Bna-miR43-FBXL调控模块参与甘蓝型油菜铝胁迫的功能分析. 作物学报 49, 1211-1221.
DOI |
| [6] |
Akram NA, Iqbal M, Muhammad A, Ashraf M, Al-Qurainy F, Shafiq S (2018). Aminolevulinic acid and nitric oxide regulate oxidative defense and secondary metabolisms in canola (Brassica napus L.) under drought stress. Protoplasma 255, 163-174.
DOI URL |
| [7] |
An J, Li QX, Yang JJ, Zhang GQ, Zhao ZX, Wu YZ, Wang Y, Wang W (2019). Wheat F-box protein TaFBA1 positively regulates plant drought tolerance but negatively regulates stomatal closure. Front Plant Sci 10, 1242.
DOI PMID |
| [8] | An JP, Rui L, Qu FJ, You CX, Wang XF, Hao YJ (2016). Apple F-Box protein MdMAX2 regulates plant photomorphogenesis and stress response. Front Plant Sci 7, 1685. |
| [9] |
Bai QQ, Wang XY, Chen X, Shi GQ, Liu ZP, Guo CJ, Xiao K (2018). Wheat miRNA TaemiR408 acts as an essential mediator in plant tolerance to Pi deprivation and salt stress via modulating stress-associated physiological processes. Front Plant Sci 9, 499.
DOI PMID |
| [10] |
Baldoni E, Genga A, Cominelli E (2015). Plant MYB transcription factors: their role in drought response mechanisms. Int J Mol Sci 16, 15811-15851.
DOI PMID |
| [11] |
Batool T, Ali S, Seleiman MF, Naveed NH, Ali A, Ahmed K, Abid M, Rizwan M, Shahid MR, Alotaibi M, Al-Ashkar I, Mubushar M (2020). Plant growth promoting rhizobacteria alleviates drought stress in potato in response to suppressive oxidative stress and antioxidant enzymes activities. Sci Rep 10, 16975.
DOI PMID |
| [12] |
Bu Q, Lv T, Shen H, Luong P, Wang J, Wang Z, Huang Z, Xiao L, Engineer C, Kim TH, Schroeder JI, Huq E (2014). Regulation of drought tolerance by the F-Box protein MAX2 in Arabidopsis. Plant Physiol 164, 424-439.
DOI URL |
| [13] |
Danquah A, De Zelicourt A, Colcombet J, Hirt H (2014). The role of ABA and MAPK signaling pathways in plant abiotic stress responses. Biotechnol Adv 32, 40-52.
DOI PMID |
| [14] |
Hu Y, Wang B, Hu TX, Chen H, Li H, Zhang W, Zhong Y, Hu HL (2015). Combined action of an antioxidant defence system and osmolytes on drought tolerance and post- drought recovery of Phoebe zhennan S. Lee saplings. Acta Physiol Plant 37, 84.
DOI URL |
| [15] |
Ji XM, Dong BD, Shiran B, Talbot MJ, Edlington JE, Hughes T, White RG, Gubler F, Dolferus R (2011). Control of abscisic acid catabolism and abscisic acid homeostasis is important for reproductive stage stress tolerance in cereals. Plant Physiol 156, 647-662.
DOI PMID |
| [16] |
Kaur G, Asthir B (2017). Molecular responses to drought stress in plants. Biol Plant 61, 201-209.
DOI URL |
| [17] |
Kim H, Lee K, Hwang H, Bhatnagar N, Kim DY, Yoon IS, Byun MO, Kim ST, Jung KH, Kim BG (2014). Overexpression of PYL5 in rice enhances drought tolerance, inhibits growth, and modulates gene expression. J Exp Bot 65, 453-464.
DOI URL |
| [18] |
Li WX, Oono Y, Zhu JH, He XJ, Wu JM, Iida K, Lu XY, Cui XP, Jin HL, Zhu JK (2008). The Arabidopsis NFYA5 transcription factor is regulated transcriptionally and posttranscriptionally to promote drought resistance. Plant Cell 20, 2238-2251.
DOI URL |
| [19] |
Liu HH, Tian X, Li YJ, Wu CA, Zheng CC (2008). Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14, 836-843.
DOI URL |
| [20] | Liu XY, Zhang XJ, Sun BC, Hao LY, Liu C, Zhang DF, Tang HJ, Li CH, Li YX, Shi YS, Xie XQ, Song YC, Wang TY, Li Y (2019). Genome-wide identification and comparative analysis of drought-related microRNAs in two maize inbred lines with contrasting drought tolerance by deep sequencing. PLoS One 14, e0219176. |
| [21] |
Nadarajah K, Kumar IS (2019). Drought response in rice: the miRNA story. Int J Mol Sci 20, 3766.
DOI URL |
| [22] |
Nadeem M, Li JJ, Yahya M, Sher A, Ma CX, Wang XB, Qiu LJ (2019). Research progress and perspective on drought stress in legumes: a review. Int J Mol Sci 20, 2541.
DOI URL |
| [23] | Shah SMS, Ullah F (2021). A comprehensive overview of miRNA targeting drought stress resistance in plants. Braz J Biol 83, e242708. |
| [24] |
Wei LY, Zhang DF, Xiang F, Zhang ZX (2009). Differentially expressed miRNAs potentially involved in the regulation of defense mechanism to drought stress in maize seedlings. Int J Plant Sci 170, 979-989.
DOI URL |
| [25] |
Wu JD, Jiang YL, Liang YN, Chen L, Chen WJ, Cheng BJ (2019). Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol Biochem 137, 179-188.
DOI URL |
| [26] |
Wu MF, Tian Q, Reed JW (2006). Arabidopsis microRNA167 controls patterns of ARF6 and ARF8 expression, and regulates both female and male reproduction. Development 133, 4211-4218.
DOI URL |
| [27] | Xiong HY, Li JJ, Liu PL, Duan JZ, Zhao Y, Guo X, Li Y, Zhang HL, Ali J, Li ZC (2014). Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice. PLoS One 9, e92913. |
| [28] |
Xu D, Duan X, Wang B, Hong B, Ho THD, Wu R (1996). Expression of a late embryogenesis abundant protein gene, HVA1, from barley confers tolerance to water deficit and salt stress in transgenic rice. Plant Physiol 110, 249-257.
DOI URL |
| [29] |
Zhao ZX, Zhang GQ, Zhou SM, Ren YQ, Wang W (2017). The improvement of salt tolerance in transgenic tobacco by overexpression of wheat F-box gene TaFBA1. Plant Sci 259, 71-85.
DOI URL |
| [30] |
Zhou SM, Sun XD, Yin SH, Kong XZ, Zhou S, Xu Y, Luo Y, Wang W (2014). The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol Biochem 84, 213-223.
DOI URL |
| [1] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [2] | 杨柳卿, 王劲, 燕敬利, 陈芹芹, 程浩坤, 李春, 赵培玉, 杨博, 江元清. 甘蓝型油菜转录因子BnaABF2的表征分析及互作蛋白鉴定[J]. 植物学报, 2025, 60(1): 49-61. |
| [3] | 李青洋, 刘翠, 何李, 彭姗, 马嘉吟, 胡子祎, 刘宏波. 甘蓝型油菜BnaA02.CPSF6基因的克隆及功能分析(长英文摘要)[J]. 植物学报, 2025, 60(1): 62-73. |
| [4] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [5] | 赵来鹏, 王柏柯, 杨涛, 李宁, 杨海涛, 王娟, 闫会转. SlHVA22l基因调节番茄耐旱性[J]. 植物学报, 2024, 59(4): 558-573. |
| [6] | 吴楠, 覃磊, 崔看, 李海鸥, 刘忠松, 夏石头. 甘蓝型油菜EXA1的克隆及其对植物抗病的调控作用[J]. 植物学报, 2023, 58(3): 385-393. |
| [7] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [8] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [9] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [10] | 李佳馨, 李霞, 谢寅峰. 外源海藻糖增强高表达转玉米C4型PEPC水稻耐旱性的机制[J]. 植物学报, 2021, 56(3): 296-314. |
| [11] | 刘丽燕, 冯锦霞, 刘文鑫, 万贤崇. 干旱胁迫对转PtPIP2;8基因84K杨苗木光合、生长和根系结构的影响[J]. 植物生态学报, 2020, 44(6): 677-686. |
| [12] | 张彤,郭亚璐,陈悦,马金姣,兰金苹,燕高伟,刘玉晴,徐珊,李莉云,刘国振,窦世娟. 水稻OsPR10A的表达特征及其在干旱胁迫应答过程中的功能[J]. 植物学报, 2019, 54(6): 711-722. |
| [13] | 宋敏,张瑶,王丽莹,彭向永. 甘蓝型油菜ZF-HD基因家族的鉴定与系统进化分析[J]. 植物学报, 2019, 54(6): 699-710. |
| [14] | 李伟滔, 贺闽, 陈学伟. ZmFBL41 Chang7-2: 玉米抗纹枯病的关键利器[J]. 植物学报, 2019, 54(5): 547-549. |
| [15] | 郜怀峰,张亚飞,王国栋,孙希武,贺月,彭福田,肖元松. 钼在桃树干旱胁迫响应中的作用解析[J]. 植物学报, 2019, 54(2): 227-236. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||