

植物学报 ›› 2022, Vol. 57 ›› Issue (3): 288-298.DOI: 10.11983/CBB21194 cstr: 32102.14.CBB21194
支添添1,2,*( ), 周舟1,2, 韩成云1,2, 任春梅1,*(
), 周舟1,2, 韩成云1,2, 任春梅1,*( )
)
收稿日期:2021-11-13
接受日期:2022-03-18
出版日期:2022-05-01
发布日期:2022-05-18
通讯作者:
支添添,任春梅
作者简介:rencm@hunau.net基金资助:
Tiantian Zhi1,2,*( ), Zhou Zhou1,2, Chengyun Han1,2, Chunmei Ren1,*(
), Zhou Zhou1,2, Chengyun Han1,2, Chunmei Ren1,*( )
)
Received:2021-11-13
Accepted:2022-03-18
Online:2022-05-01
Published:2022-05-18
Contact:
Tiantian Zhi,Chunmei Ren
摘要: 程序性细胞死亡(PCD)对于植物生长发育和防御反应均极为重要。拟南芥(Arabidopsis thaliana)酪氨酸降解途径延胡索酰乙酰乙酸水解酶(FAH)缺失突变体sscd1 (short-day sensitive cell death 1)在短日照下(8小时光照/16小时黑暗)发生PCD。前期研究发现, sscd1突变体的PCD与茉莉素(JAs)信号转导有关, 而与水杨酸(SA)信号转导无关。PAD4 (Phytoalexin deficient 4)参与SA和JAs信号转导之间的相互拮抗。该研究发现sscd1突变体PCD伴随着PAD4表达上调; 而PAD4突变导致sscd1突变体PCD加速, 同时上调JAs信号转导途径下游响应基因vegetative storage protein 2、thionin2.1和defensin1.2的表达; sscd1/pad4/coil三突变体中JAs信号转导受阻导致PCD加速现象消失。PAD4突变上调sscd1突变体酪氨酸降解基因homogentisate dioxygenase和maleylacetoacetate isomerase以及单线态氧特异性诱导基因bonzai1-associated protein 1和a putative c2h2 zinc finger transcription factor的表达, 并且上调均依赖JAs信号转导受体COI1。综上所述, PAD4突变通过增强JAs信号转导加速酪氨酸降解, 增加单线态氧的积累, 从而促进sscd1突变体的PCD。
支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡. 植物学报, 2022, 57(3): 288-298.
Tiantian Zhi, Zhou Zhou, Chengyun Han, Chunmei Ren. PAD4 Mutation Accelerating Programmed Cell Death in Arabidopsis thaliana Tyrosine Degradation Deficient Mutant sscd1. Chinese Bulletin of Botany, 2022, 57(3): 288-298.
| Primer name | Primer sequence (5′-3′) |
|---|---|
| VSP2-F | GGATTGAACCCATCATACTCAG |
| VSP2-R | CACGAGACTCTTCCTCACCTTT |
| PDF1.2-F | GCTTCCATCATCACCCTTATC |
| PDF1.2-R | TTGGCTTCTCGCACAACTT |
| THI2.1-F | GTTGGGTAAACGCCATTCT |
| THI2.1-R | CATTGTTCCGACGCTCCATT |
| PAD4-F | GACGCTGCCATACTCAAACT |
| PAD4-R | CCAAAGGTGATACAAAAGACGC |
| BAP1-F | ATCGGATCCCACCAGAGATTACGG |
| BAP1-R | AATCTCGGCCTCCACAAACCAG |
| ZP-F | TACGAAGGAAAGAACGGAGGC |
| ZP-R | GGTATCGGCGGTATGTTGAGG |
| HGO-F | GGAGATTGATTTCGTTGATGGGTT |
| HGO-R | GCGGAGTCTTTCATTCCTGTGTTA |
| MAAI-F | GCTGGACTCTGCTACTGCGA |
| MAAI-R | AGGGCGATACGGACACGATG |
| ACTIN2-F | AGCACTTGCACCAAGCAGCATG |
| ACTIN2-R | ACGATTCCTGGACCTGCCTCATC |
表1 qRT-PCR引物序列
Table 1 The primers used for qRT-PCR
| Primer name | Primer sequence (5′-3′) |
|---|---|
| VSP2-F | GGATTGAACCCATCATACTCAG |
| VSP2-R | CACGAGACTCTTCCTCACCTTT |
| PDF1.2-F | GCTTCCATCATCACCCTTATC |
| PDF1.2-R | TTGGCTTCTCGCACAACTT |
| THI2.1-F | GTTGGGTAAACGCCATTCT |
| THI2.1-R | CATTGTTCCGACGCTCCATT |
| PAD4-F | GACGCTGCCATACTCAAACT |
| PAD4-R | CCAAAGGTGATACAAAAGACGC |
| BAP1-F | ATCGGATCCCACCAGAGATTACGG |
| BAP1-R | AATCTCGGCCTCCACAAACCAG |
| ZP-F | TACGAAGGAAAGAACGGAGGC |
| ZP-R | GGTATCGGCGGTATGTTGAGG |
| HGO-F | GGAGATTGATTTCGTTGATGGGTT |
| HGO-R | GCGGAGTCTTTCATTCCTGTGTTA |
| MAAI-F | GCTGGACTCTGCTACTGCGA |
| MAAI-R | AGGGCGATACGGACACGATG |
| ACTIN2-F | AGCACTTGCACCAAGCAGCATG |
| ACTIN2-R | ACGATTCCTGGACCTGCCTCATC |
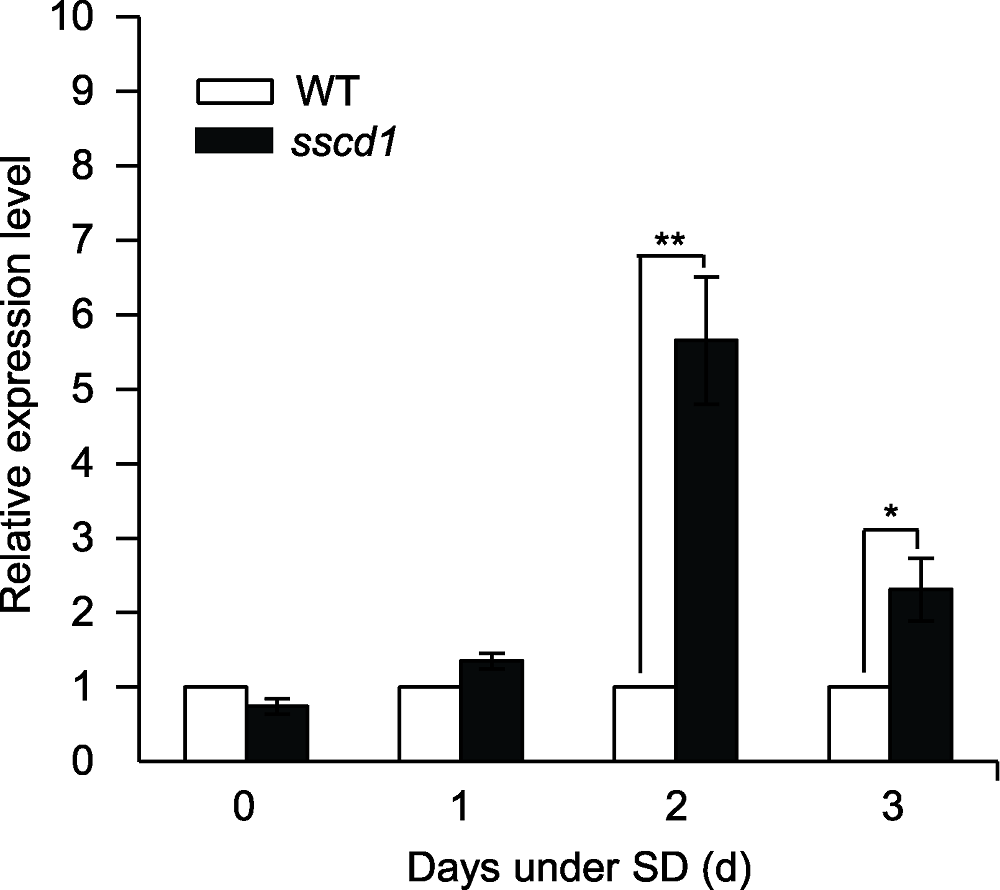
图1 拟南芥sscd1突变体的程序性细胞死亡(PCD)伴随PAD4表达的上调 图中数值和误差线为3次重复的平均值±标准误, 在野生型(作为对照)中的表达量设为1。* 表示差异显著(P<0.05), ** 表示差异极显著(P<0.01) (Student’s t-test)。WT: 野生型; SD: 短日照
Figure 1 Programmed cell death (PCD) in Arabidopsis sscd1 mutant is accompanied by the up-regulation of PAD4 gene The values and error bars in the figure are means ± SE from three biological replicates, expression level in wild type (as control) was set to 1. * indicates significant difference at P<0.05, ** indicates significant difference at P<0.01 (Student’s t-test). WT: Wild type; SD: Short day
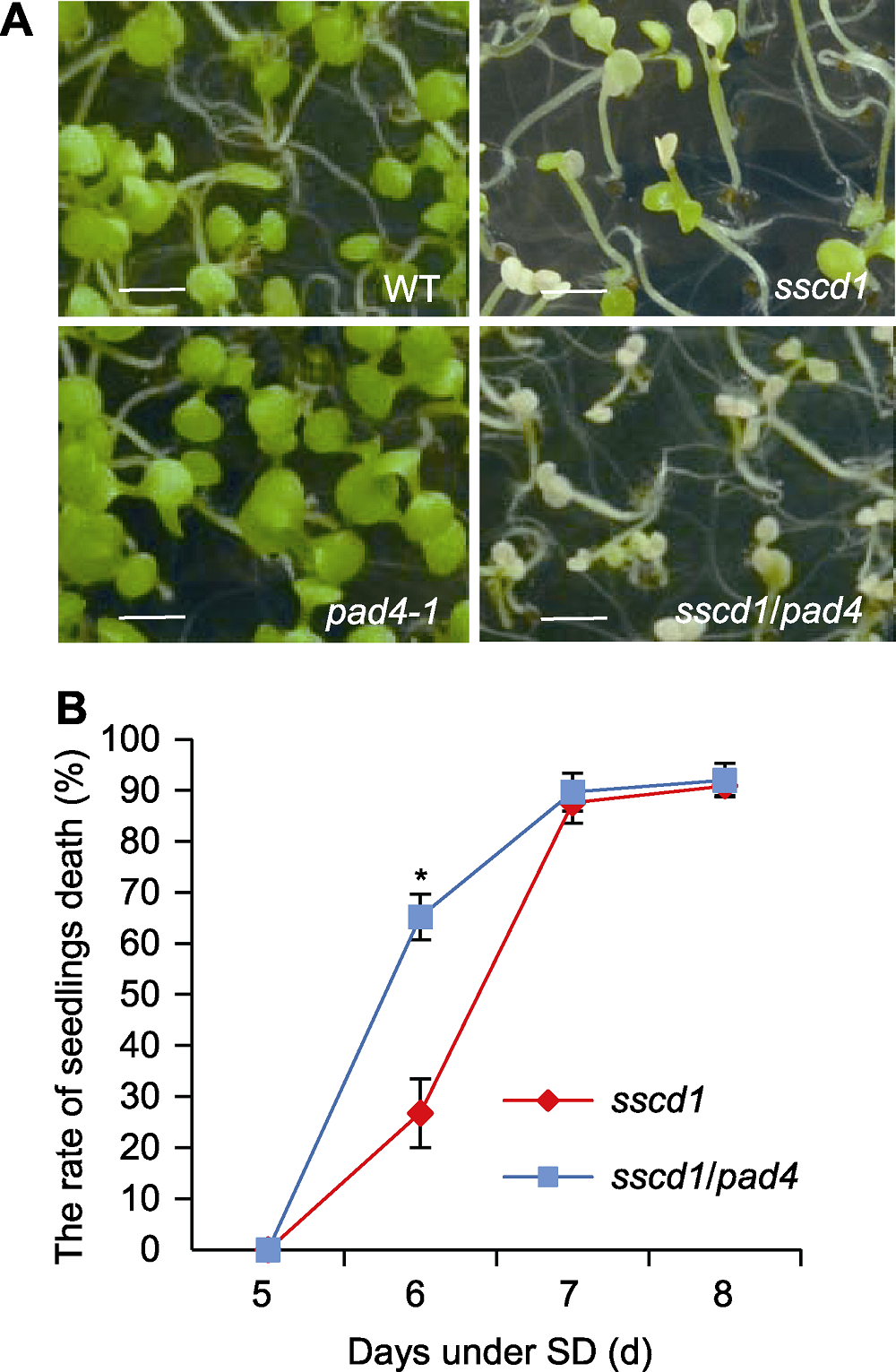
图2 PAD4突变加速拟南芥sscd1突变体的程序性细胞死亡(PCD) (A) 短日照(SD)下6天野生型(WT)和突变体的表型(bars= 0.2 cm); (B) SD下sscd1和sscd1/pad4的死亡率统计。图中数值和误差线为3次重复的平均值±标准误。* 表示差异显著(P<0.05) (Student’s t-test)。
Figure 2 Mutation of PAD4 gene accelerated the programmed cell death (PCD) in Arabidopsis sscd1 mutant (A) The phenotype of wild type (WT) and mutant seedlings grown under short day (SD) for 6 d (bars=0.2 cm); (B) The rate of seedlings death in sscd1 and sscd1/pad4 seedlings grown under SD. The values and error bars in the figure are means ± SE from three biological replicates. * indicates significant difference at P<0.05 (Student’s t-test).
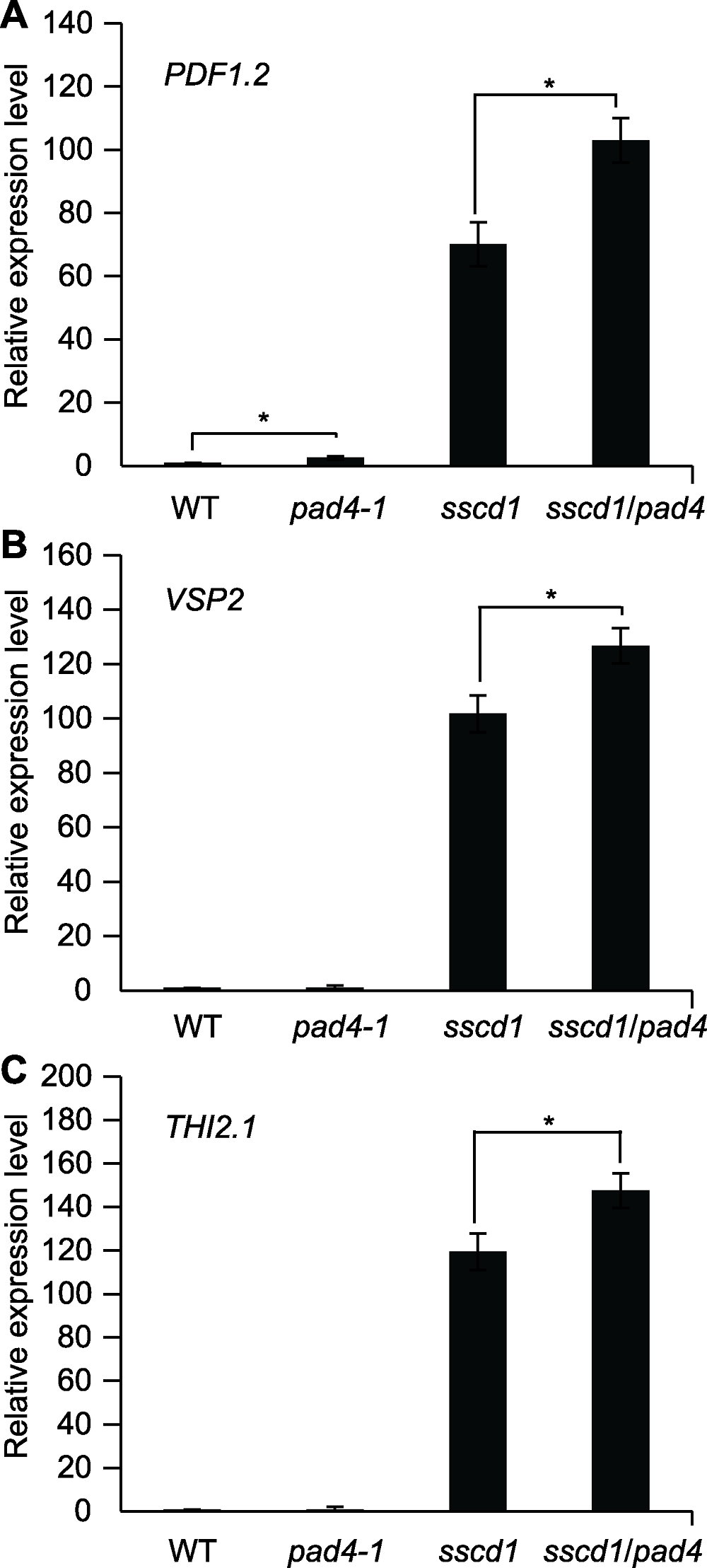
图3 茉莉酸(JAs)响应基因在拟南芥野生型(WT)和突变体中的相对表达量 图中数值和误差线为3次重复的平均值±标准误。* 表示差异显著(P<0.05) (Student’s t-test)。WT: 野生型
Figure 3 Relative expression level of jasmonates (JAs) responsive genes in Arabidopsis wild type (WT) and mutant seedlings The values and error bars in the figure are means ± SE from three biological replicates. * indicate significant differences at P<0.05 (Student’s t-test). WT: Wild type
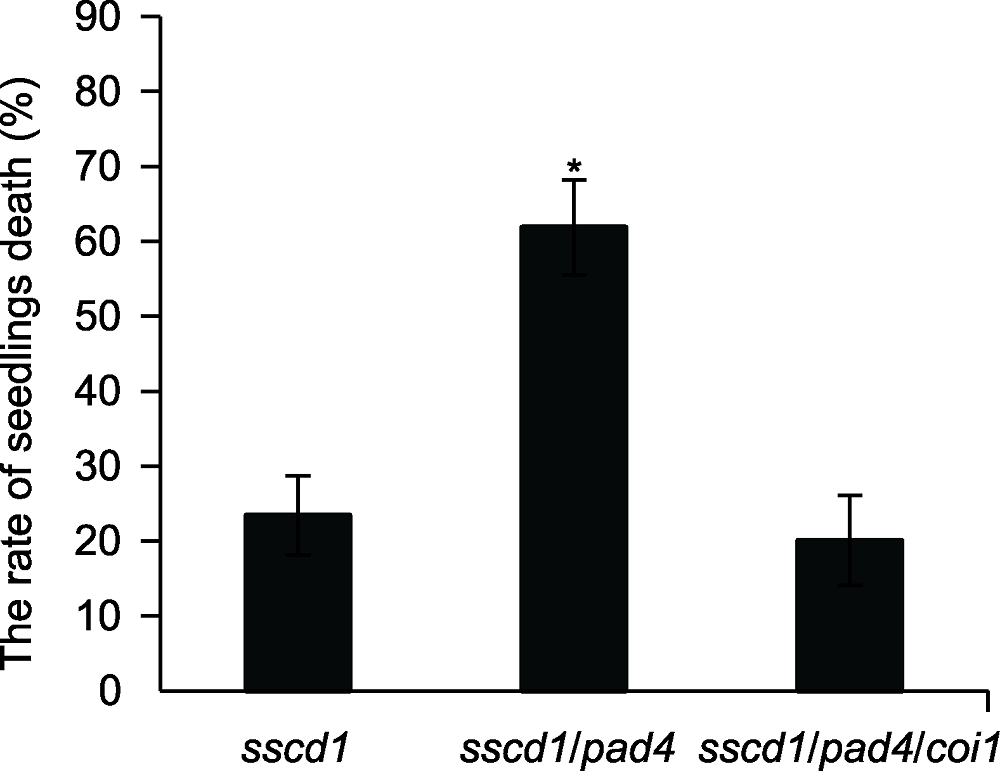
图4 拟南芥突变体幼苗死亡率统计 图中数值和误差线为3次重复的平均值±标准误。* 表示差异显著(P<0.05) (Student’s t-test)。
Figure 4 The rate of seedlings death in Arabidopsis mutants The values and error bars in the figure are means ± SE from three biological replicates. * indicates significant difference at P<0.05 (Student’s t-test).
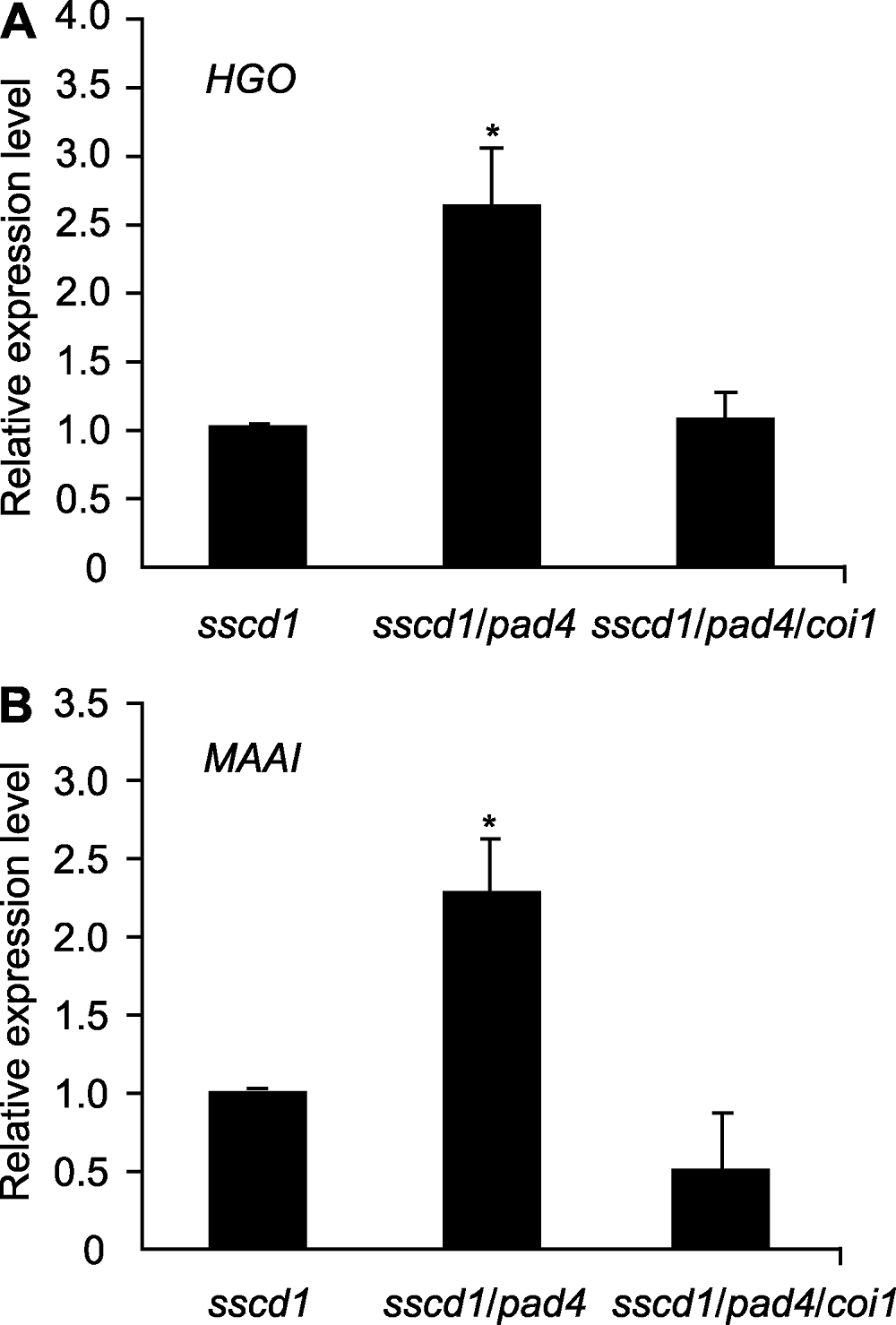
图5 拟南芥突变体中酪氨酸(Tyr)降解基因的相对表达量 图中数值和误差线为3次重复的平均值±标准误。* 表示差异显著(P<0.05) (Student’s t-test)。
Figure 5 Relative expression level of Tyrosine (Tyr) degradation gene in Arabidopsis mutant seedlings The values and error bars in the figure are means ± SE from three biological replicates. * indicate significant differences at P<0.05 (Student’s t-test).
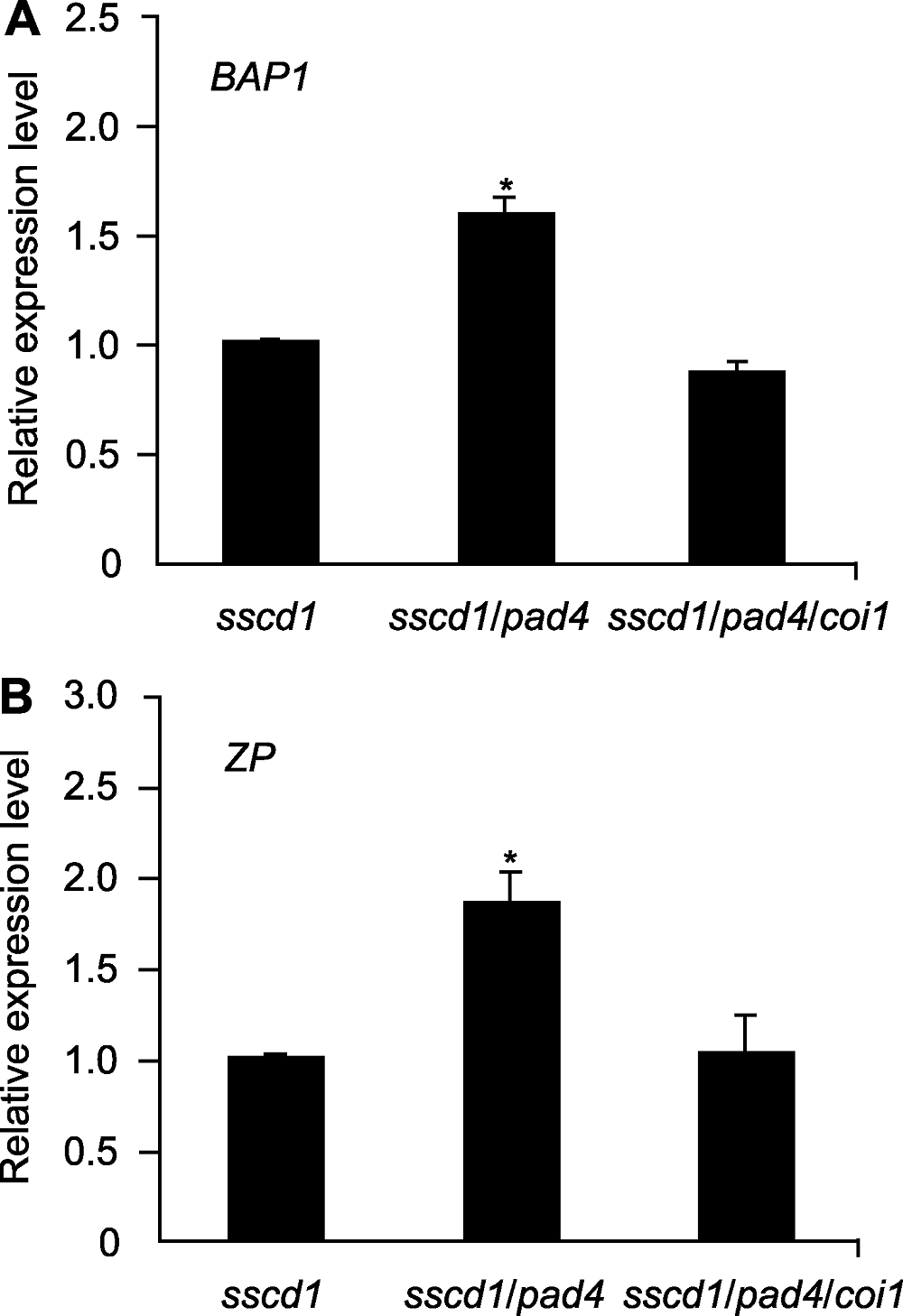
图6 拟南芥突变体中单线态氧特异性诱导基因的相对表达量 图中数值和误差线为3次重复的平均值±标准误,* 表示差异显著(P<0.05) (Student’s t-test)。
Figure 6 Relative expression level of singlet oxygen specific induced gene in Arabidopsis mutant seedlings The values and error bars in the figure are means ± SE from three biological replicates. * indicate significant differences at P<0.05 (Student’s t-test).
| [1] |
张宪省 (2018). 我国科学家在程序性细胞死亡机制研究领域取得重大突破. 植物学报 53, 445-446.
DOI |
| [2] |
赵曦娟, 钱礼超, 刘玉乐 (2018). 中国科学家在植物程序性细胞死亡领域取得重要成果. 植物学报 53, 447-450.
DOI |
| [3] |
Alvarez ME (2000). Salicylic acid in the machinery of hypersensitive cell death and disease resistance. Plant Mol Biol 44, 429-442.
PMID |
| [4] |
Asai T, Stone JM, Heard JE, Kovtun Y, Yorgey P, Sheen J, Ausubel FM (2000). Fumonisin B1-induced cell death in Arabidopsis protoplasts requires jasmonate-, ethylene-, and salicylate-dependent signaling pathways. Plant Cell 12, 1823-1835.
PMID |
| [5] |
Attaran E, Major IT, Cruz JA, Rosa BA, Koo AJK, Chen J, Kramer DM, He SY, Howe GA (2014). Temporal dynamics of growth and photosynthesis suppression in response to jasmonate signaling. Plant Physiol 165, 1302-1314.
PMID |
| [6] |
Benedetti CE, Xie D, Turner JG (1995). COI1-dependent expression of an Arabidopsis vegetative storage protein in flowers and siliques and in response to coronatine or methyl jasmonate. Plant Physiol 109, 567-572.
PMID |
| [7] |
Brodersen P, Malinovsky FG, Hématy K, Newman MA, Mundy J (2005). The role of salicylic acid in the induction of cell death in Arabidopsis acd11. Plant Physiol 138, 1037- 1045.
PMID |
| [8] |
Brodersen P, Petersen M, Nielsen HB, Zhu SJ, Newman MA, Shokat KM, Rietz S, Parker J, Mundy J (2006). Arabidopsis MAP kinase 4 regulates salicylic acid- and jasmonic acid/ethylene-dependent responses via EDS1 and PAD4. Plant J 47, 532-546.
PMID |
| [9] |
Campos ML, Kang JH, Howe GA (2014). Jasmonate-triggered plant immunity. J Chem Ecol 40, 657-675.
DOI URL |
| [10] |
Cohen S, Flescher E (2009). Methyl jasmonate: a plant stress hormone as an anti-cancer drug. Phytochemistry 70, 1600-1609.
DOI URL |
| [11] |
Cui HT, Gobbato E, Kracher B, Qiu JD, Bautor J, Parker JE (2017). A core function of EDS1 with PAD4 is to protect the salicylic acid defense sector in Arabidopsis immunity. New Phytol 213, 1802-1817.
DOI URL |
| [12] |
Cui HT, Qiu JD, Zhou Y, Bhandari DD, Zhao CH, Bautor J, Parker JE (2018). Antagonism of transcription factor MYC2 by EDS1/PAD4 complexes bolsters salicylic acid defense in Arabidopsis effector-triggered immunity. Mol Plant 11, 1053-1066.
DOI URL |
| [13] |
Daneva A, Gao Z, Van Durme M, Nowack MK (2016). Functions and regulation of programmed cell death in plant development. Annu Rev Cell Dev Biol 32, 441-468.
PMID |
| [14] |
Devoto A, Nieto-Rostro M, Xie DX, Ellis C, Harmston R, Patrick E, Davis J, Sherratt L, Coleman M, Turner JG (2002). COI1 links jasmonate signaling and fertility to the SCF ubiquitin-ligase complex in Arabidopsis. Plant J 32, 457-466.
DOI URL |
| [15] |
Dickman M, Williams B, Li YR, De Figueiredo P, Wolpert T (2017). Reassessing apoptosis in plants. Nat Plants 3, 773-779.
DOI PMID |
| [16] |
Dickman MB, Fluhr R (2013). Centrality of host cell death in plant-microbe interactions. Annu Rev Phytopathol 51, 543- 570.
DOI PMID |
| [17] |
Dixon DP, Edwards R (2006). Enzymes of tyrosine catabolism in Arabidopsis thaliana. Plant Sci 171, 360-366.
DOI URL |
| [18] |
Feys BJ, Moisan LJ, Newman MA, Parker JE (2001). Direct interaction between the Arabidopsis disease resistance signaling proteins, EDS1 and PAD4. EMBO J 20, 5400-5411.
PMID |
| [19] | Grompe M, Al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P (1993). Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes Dev 7, 2298-2307. |
| [20] |
Gupta V, Willits MG, Glazebrook J (2000). Arabidopsis thaliana EDS4 contributes to salicylic acid (SA)-dependent expression of defense responses: evidence for inhibition of jasmonic acid signaling by SA. Mol Plant Microbe Interact 13, 503-511.
DOI URL |
| [21] |
Han CY, Ren CM, Zhi TT, Zhou Z, Liu Y, Chen F, Peng W, Xie DX (2013). Disruption of fumarylacetoacetate hydrolase causes spontaneous cell death under short-day conditions in Arabidopsis. Plant Physiol 162, 1956-1964.
DOI URL |
| [22] |
Hildebrandt TM, Nesi AN, Araújo WL, Braun HP (2015). Amino acid catabolism in plants. Mol Plant 8, 1563-1579.
DOI PMID |
| [23] |
Huysmans M, Buono RA, Skorzinski N, Radio MC, De Winter F, Parizot B, Mertens J, Karimi M, Fendrych M, Nowack MK (2018). NAC transcription factors ANAC087 and ANAC046 control distinct aspects of programmed cell death in the Arabidopsis Columella and lateral root cap. Plant Cell 30, 2197-2213.
DOI URL |
| [24] |
Huysmans M, Saul LA, Coll NS, Nowack MK (2017). Dying two deaths-programmed cell death regulation in development and disease. Curr Opin Plant Biol 35, 37-44.
DOI PMID |
| [25] |
Jirage D, Zhou N, Cooper B, Clarke JD, Dong XN, Glazebrook J (2001). Constitutive salicylic acid-dependent signaling in cpr1 and cpr6mutants requires PAD4. Plant J 26, 395-407.
PMID |
| [26] |
Jorquera R, Tanguay RM (2001). Fumarylacetoacetate, the metabolite accumulating in hereditary tyrosinemia, activates the ERK pathway and induces mitotic abnormalities and genomic instability. Hum Mol Genet 10, 1741-1752.
DOI PMID |
| [27] |
Kabbage M, Kessens R, Bartholomay LC, Williams B (2017). The life and death of a plant cell. Annu Rev Plant Biol 68, 375-404.
DOI PMID |
| [28] |
Katsir L, Chung HS, Koo AJK, Howe GA (2008). Jasmonate signaling: a conserved mechanism of hormone sensing. Curr Opin Plant Biol 11, 428-435.
DOI PMID |
| [29] |
Kurusu T, Kuchitsu K (2017). Autophagy, programmed cell death and reactive oxygen species in sexual reproduction in plants. J Plant Res 130, 491-499.
DOI URL |
| [30] |
Lindblad B, Lindstedt S, Steen G (1977). On the enzymic defects in hereditary tyrosinemia. Proc Natl Acad Sci USA 74, 4641-4645.
DOI URL |
| [31] | Locato V, De Gara L (2018). Programmed cell death in plants: an overview. Methods Mol Biol 1743, 1-8. |
| [32] |
Lock EA, Gaskin P, Ellis MK, Provan WM, Robinson M, Smith LL, Prisbylla MP, Mutter LC (1996). Tissue distribution of 2-(2-nitro-4-trifluoromethylbenzoyl) cyclohexane-1,3-dione (NTBC): effect on enzymes involved in tyrosine catabolism and relevance to ocular toxicity in the rat. Toxicol Appl Pharmacol 141, 439-447.
DOI URL |
| [33] |
Louis J, Gobbato E, Mondal HA, Feys BJ, Parker JE, Shah J (2012). Discrimination of Arabidopsis PAD4 activities in defense against green peach aphid and pathogens. Plant Physiol 158, 1860-1872.
DOI PMID |
| [34] |
Maekawa T, Kufer TA, Schulze-Lefert P (2011). NLR functions in plant and animal immune systems: so far and yet so close. Nat Immunol 12, 817-826.
DOI PMID |
| [35] |
Maizel A (2015). A view to a kill: markers for developmentally regulated cell death in plants. Plant Physiol 169, 2341.
DOI URL |
| [36] |
Mur LAJ, Kenton P, Atzorn R, Miersch O, Wasternack C (2006). The outcomes of concentration-specific interactions between salicylate and jasmonate signaling include synergy, antagonism, and oxidative stress leading to cell death. Plant Physiol 140, 249-262.
DOI URL |
| [37] |
Olvera-Carrillo Y, Van Bel M, Van Hautegem T, Fendrych M, Huysmans M, Simaskova M, Van Durme M, Buscaill P, Rivas S, Coll NS, Coppens F, Maere S, Nowack MK (2015). A conserved core of programmed cell death indicator genes discriminates developmentally and environmentally induced programmed cell death in plants. Plant Physiol 169, 2684-2699.
DOI PMID |
| [38] | Petrov V, Hille J, Mueller-Roeber B, Gechev TS (2015). ROS-mediated abiotic stress-induced programmed cell death in plants. Front Plant Sci 6, 69. |
| [39] |
Rao MV, Lee HI, Creelman RA, Mullet JE, Davis KR (2000). Jasmonic acid signaling modulates ozone-induced hypersensitive cell death. Plant Cell 12, 1633-1646.
PMID |
| [40] |
Reape TJ, Molony EM, McCabe PF (2008). Programmed cell death in plants: distinguishing between different modes. J Exp Bot 59, 435-444.
DOI URL |
| [41] | Reinbothe C, Springer A, Samol I, Reinbothe S (2009). Plant oxylipins: role of jasmonic acid during programmed cell death, defence and leaf senescence. FEBS J 276, 4666- 4681. |
| [42] |
Repka V, Fischerová I, Šilhárová K (2004). Methyl jasmonate is a potent elicitor of multiple defense responses in grapevine leaves and cell-suspension cultures. Biol Plant 48, 273-283.
DOI URL |
| [43] |
Ruppert S, Kelsey G, Schedl A, Schmid E, Thies E, Schütz G (1992). Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev 6, 1430-1443.
DOI URL |
| [44] |
Russo PA, Mitchell GA, Tanguay RM (2001). Tyrosinemia: a review. Pediatr Dev Pathol 4, 212-221.
DOI URL |
| [45] |
Rustérucci C, Aviv DH, Holt III BF, Dangl JL, Parker JE (2001). The disease resistance signaling components EDS1 and PAD4 are essential regulators of the cell death pathway controlled by LSD1 in Arabidopsis. Plant Cell 13, 2211-2224.
PMID |
| [46] |
Schenck CA, Maeda HA (2018). Tyrosine biosynthesis, metabolism, and catabolism in plants. Phytochemistry 149, 82-102.
DOI PMID |
| [47] |
Sparnins VL, Chapman PJ (1976). Catabolism of L-tyrosine by the homoprotocatechuate pathway in gram-positive bac teria. J Bacteriol 127, 362-366.
DOI PMID |
| [48] |
St-Louis M, Tanguay RM (1997). Mutations in the fumarylacetoacetate hydrolase gene causing hereditary tyrosinemia type I: overview. Hum Mutat 9, 291-299.
PMID |
| [49] |
Van Hautegem T, Waters AJ, Goodrich J, Nowack MK (2015). Only in dying, life: programmed cell death during plant development. Trends Plant Sci 20, 102-113.
DOI PMID |
| [50] |
Vogelmann K, Drechsel G, Bergler J, Subert C, Philippar K, Soll J, Engelmann JC, Engelsdorf T, Voll LM, Hoth S (2012). Early senescence and cell death in Arabidopsis saul1 mutants involves the PAD4-dependent salicylic acid pathway. Plant Physiol 159, 1477-1487.
DOI PMID |
| [51] |
Xie DX, Feys BF, James S, Nieto-Rostro M, Turner JG (1998). COI1: an Arabidopsis gene required for jasmonate- regulated defense and fertility. Science 280, 1091-1094.
PMID |
| [52] |
Xu LH, Liu FQ, Lechner E, Genschik P, Crosby WL, Ma H, Peng W, Huang DF, Xie DX (2002). The SCFCOI1 ubiquitin-ligase complexes are required for jasmonate response in Arabidopsis. Plant Cell 14, 1919-1935.
DOI URL |
| [53] |
Yan JB, Zhang C, Gu M, Bai ZY, Zhang WG, Qi TC, Cheng ZW, Peng W, Luo HB, Nan FJ, Wang Z, Xie DX (2009). The Arabidopsis CORONATINE INSENSITIVE 1 protein is a jasmonate receptor. Plant Cell 21, 2220-2236.
DOI URL |
| [54] |
Yoon J, Chung WI, Choi D (2009). NbHB1, Nicotiana benthamiana homeobox 1, is a jasmonic acid-dependent positive regulator of pathogen-induced plant cell death. New Phytol 184, 71-84.
DOI URL |
| [55] |
Zeng HY, Liu Y, Chen DK, Bao HN, Huang LQ, Yin J, Chen YL, Xiao S, Yao N (2021). The immune components ENHANCED DISEASE SUSCEPTIBILITY 1 and PHYTOALEXIN DEFICIENT 4 are required for cell death caused by overaccumulation of ceramides in Arabidopsis. Plant J 107, 1447-1465.
DOI URL |
| [56] |
Zhang LR, Xing D (2008). Methyl jasmonate induces production of reactive oxygen species and alterations in mitochondrial dynamics that precede photosynthetic dysfunction and subsequent cell death. Plant Cell Physiol 49, 1092-1111.
DOI URL |
| [57] |
Zhi TT, Zhou Z, Huang Y, Han CY, Liu Y, Zhu Q, Ren CM (2016). Sugar suppresses cell death caused by disruption of fumarylacetoacetate hydrolase in Arabidopsis. Planta 244, 557-571.
DOI URL |
| [58] |
Zhi TT, Zhou Z, Qiu B, Zhu Q, Xiong XY, Ren CM (2019). Loss of fumarylacetoacetate hydrolase causes light-dependent increases in protochlorophyllide and cell death in Arabidopsis. Plant J 98, 622-638.
DOI URL |
| [59] |
Zhou LZ, Yang ZG, Zhi TT, Zhou Z, Wang XC, Ren CM, Qiu B (2016). A GC/MS method for determination of succinylacetone in Arabidopsis thaliana. Anal Bioanal Chem 408, 4661-4667.
DOI URL |
| [60] |
Zhou N, Tootle TL, Tsui F, Klessig DF, Glazebrook J (1998). PAD4 functions upstream from salicylic acid to control defense responses in Arabidopsis. Plant Cell 10, 1021-1030.
PMID |
| [61] |
Zhou Z, Zhi TT, Han CY, Peng ZH, Wang RZ, Tong JH, Zhu Q, Ren CM (2020). Cell death resulted from loss of fumarylacetoacetate hydrolase in Arabidopsis is related to phytohormone jasmonate but not salicylic acid. Sci Rep 10, 13714.
DOI PMID |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [3] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [4] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [5] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [6] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [7] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [8] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [9] | 李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展[J]. 植物学报, 2021, 56(4): 462-469. |
| [10] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [11] | 王婷, 羊欢欢, 赵弘巍, JosefVoglmeir, 刘丽. 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021, 56(3): 262-274. |
| [12] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| [13] | 杜斐, 焦雨铃. WUSCHEL介导的固有免疫: 植物干细胞抵御病毒侵害的新机制[J]. 植物学报, 2020, 55(5): 537-540. |
| [14] | 马龙, 李桂林, 李师鹏, 蒋苏. 根尖整体透明技术改良[J]. 植物学报, 2020, 55(5): 596-604. |
| [15] | 张楠,刘自广,孙世臣,刘圣怡,林建辉,彭疑芳,张晓旭,杨贺,岑曦,吴娟. 拟南芥AtR8 lncRNA对盐胁迫响应及其对种子萌发的调节作用[J]. 植物学报, 2020, 55(4): 421-429. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||