

植物学报 ›› 2024, Vol. 59 ›› Issue (6): 978-998.DOI: 10.11983/CBB24080 cstr: 32102.14.CBB24080
所属专题: 大食物观; 玉米生物学与分子设计(2024年59卷6期)
收稿日期:2024-05-27
接受日期:2024-08-20
出版日期:2024-11-10
发布日期:2024-08-28
通讯作者:
*李平华, 博士, 教授, 博士生导师。2011年获BTI研究所Lawrence Bogorad分子生物学奖; 2013年入选国家海外重点人才工程青年项目; 2015年入选山东省“泰山学者攀登计划”。研究团队主要以C4作物玉米为材料, 利用正反向遗传学、分子生物学、细胞生物学以及生物信息学等方法, 系统研究密植条件下玉米的株型建成, C4光合启动过程中基因表达的调控机制, 为全面理解并利用C4光合特性进行玉米耐密高光效育种提供理论依据。E-mail: pinghuali@sdau.edu.cn
作者简介:†共同第一作者
基金资助:
Qiang Zhang†, Zhenyu Zhao†, Pinghua Li*( )
)
Received:2024-05-27
Accepted:2024-08-20
Online:2024-11-10
Published:2024-08-28
Contact:
*E-mail: pinghuali@sdau.edu.cn
About author:†These authors contributed equally to this paper
摘要: 基因编辑技术已成为现代农业育种领域的重要工具。玉米(Zea mays)是全球最重要的粮食作物之一, 基因编辑技术在玉米中的应用显示出巨大的潜力。该文综述了基因编辑技术在玉米研究中的应用进展, 重点介绍了CRISPR/Cas等系统在玉米基因组编辑中的最新成果。首先, 介绍了基因编辑技术的基本原理和类型, 特别是CRISPR/Cas系统的工作机制及其在玉米中的应用优势。其次, 总结了基因编辑技术在玉米育种中的研究进展, 涵盖从基础的基因组编辑到复杂的多基因编辑, 旨在改良玉米的产量、品质和抗逆性等关键性状。最后, 文章列举了我国在玉米基因编辑方面的杰出工作, 并讨论了基因编辑技术应用于玉米育种中存在的问题, 同时展望了未来发展方向。
张强, 赵振宇, 李平华. 基因编辑技术在玉米中的研究进展. 植物学报, 2024, 59(6): 978-998.
Qiang Zhang, Zhenyu Zhao, Pinghua Li. Research Progress of Gene Editing Technology in Maize. Chinese Bulletin of Botany, 2024, 59(6): 978-998.
| 基因编辑技术 | 编辑机制 | 功能作用 | 编辑效率 | 脱靶效应 | 设计复杂度 | 应用范围 |
|---|---|---|---|---|---|---|
| CRISPR/Cas9 | 使用sgRNA导向 DNA, 并引入双链断裂 | 所有类型的基因编辑, 主要是通过切割进而引入基因突变 | 编辑效率高, 但取决于目标位点的可访问性和sgRNA的设计 | 脱靶效应较高, 需要精确设计sg- RNA以减少脱靶 | 中等, 需要设计精确的sgRNA | 应用广泛, 包括基因敲除、插入和替换 |
| CRISPR/Cas12 | 与Cas9类似, 但能够识别不同的PAM序列和单链切割 | 所有类型的基因编辑, 相较于Cas9提供更精细的编辑选择 | 编辑效率高, 受目标位点、PAM序列和sgRNA设计影响 | 脱靶效应低于Cas- 9, 提供更精确的编辑 | 中等, 需要考虑PAM兼容性和精确的sgRNA | 应用广泛, 但尤其适用于需要更精确编辑的应用 |
| CRISPR/Cas13 | 专门针对RNA编辑, 不涉及DNA, 使用特定的crRNA导向 | 主要用于RNA编辑, 不直接改变 DNA | 由于专门针对RNA, 编辑效率可能依赖于目标RNA的可访问性和crRNA设计 | 主要针对RNA, 几乎没有DNA脱靶风险 | 中等到高, 需要设计特定的crRNA | 主要应用于RNA相关操作 |
| 胞嘧啶单碱基编辑(CBE) | 脱氨基酶将C转化为U, 细胞修复为T | 仅限C·G转换为T·A | 编辑效率较高, 但同样受目标序列和细胞类型影响 | 脱靶效应低于CR- ISPR-Cas9, 但需要仔细设计sgRNA以降低脱靶效应 | 需要精确设计特定的sgRNA | 主要用于精确的单碱基编辑 |
| 腺嘌呤单碱基编辑(ABE) | 脱氨基酶将A转化为I, 细胞将其读作G | 仅限A·T转换为 G·C | 编辑效率较高, 但受目标序列和细胞类型的影响 | 与CBE类似, 脱靶效应低, 但不能忽视 | 需要精确设计特定的sgRNA | 主要用于精确的单碱基编辑 |
| 引导编辑(PE) | 结合nCas9和逆转录酶, 使用由pegRNA提供的RNA模板直接编辑DNA | 支持所有类型的小规模编辑, 包括替换、插入和删除 | 编辑效率从中等到高, 取决于目标序列、细胞类型和pegRNA设计 | 脱靶效应比CRIS- PR-Cas9和BE编辑器显著降低 | 较高, 需要设计包含特定目标识别序列和RNA模板的pegRNA | 应用广泛, 能够实现复杂的基因编辑, 包括各种类型的遗传突变 |
表1 CRISPR/Cas及其衍生技术
Table 1 CRISPR/Cas and its derived technologies
| 基因编辑技术 | 编辑机制 | 功能作用 | 编辑效率 | 脱靶效应 | 设计复杂度 | 应用范围 |
|---|---|---|---|---|---|---|
| CRISPR/Cas9 | 使用sgRNA导向 DNA, 并引入双链断裂 | 所有类型的基因编辑, 主要是通过切割进而引入基因突变 | 编辑效率高, 但取决于目标位点的可访问性和sgRNA的设计 | 脱靶效应较高, 需要精确设计sg- RNA以减少脱靶 | 中等, 需要设计精确的sgRNA | 应用广泛, 包括基因敲除、插入和替换 |
| CRISPR/Cas12 | 与Cas9类似, 但能够识别不同的PAM序列和单链切割 | 所有类型的基因编辑, 相较于Cas9提供更精细的编辑选择 | 编辑效率高, 受目标位点、PAM序列和sgRNA设计影响 | 脱靶效应低于Cas- 9, 提供更精确的编辑 | 中等, 需要考虑PAM兼容性和精确的sgRNA | 应用广泛, 但尤其适用于需要更精确编辑的应用 |
| CRISPR/Cas13 | 专门针对RNA编辑, 不涉及DNA, 使用特定的crRNA导向 | 主要用于RNA编辑, 不直接改变 DNA | 由于专门针对RNA, 编辑效率可能依赖于目标RNA的可访问性和crRNA设计 | 主要针对RNA, 几乎没有DNA脱靶风险 | 中等到高, 需要设计特定的crRNA | 主要应用于RNA相关操作 |
| 胞嘧啶单碱基编辑(CBE) | 脱氨基酶将C转化为U, 细胞修复为T | 仅限C·G转换为T·A | 编辑效率较高, 但同样受目标序列和细胞类型影响 | 脱靶效应低于CR- ISPR-Cas9, 但需要仔细设计sgRNA以降低脱靶效应 | 需要精确设计特定的sgRNA | 主要用于精确的单碱基编辑 |
| 腺嘌呤单碱基编辑(ABE) | 脱氨基酶将A转化为I, 细胞将其读作G | 仅限A·T转换为 G·C | 编辑效率较高, 但受目标序列和细胞类型的影响 | 与CBE类似, 脱靶效应低, 但不能忽视 | 需要精确设计特定的sgRNA | 主要用于精确的单碱基编辑 |
| 引导编辑(PE) | 结合nCas9和逆转录酶, 使用由pegRNA提供的RNA模板直接编辑DNA | 支持所有类型的小规模编辑, 包括替换、插入和删除 | 编辑效率从中等到高, 取决于目标序列、细胞类型和pegRNA设计 | 脱靶效应比CRIS- PR-Cas9和BE编辑器显著降低 | 较高, 需要设计包含特定目标识别序列和RNA模板的pegRNA | 应用广泛, 能够实现复杂的基因编辑, 包括各种类型的遗传突变 |
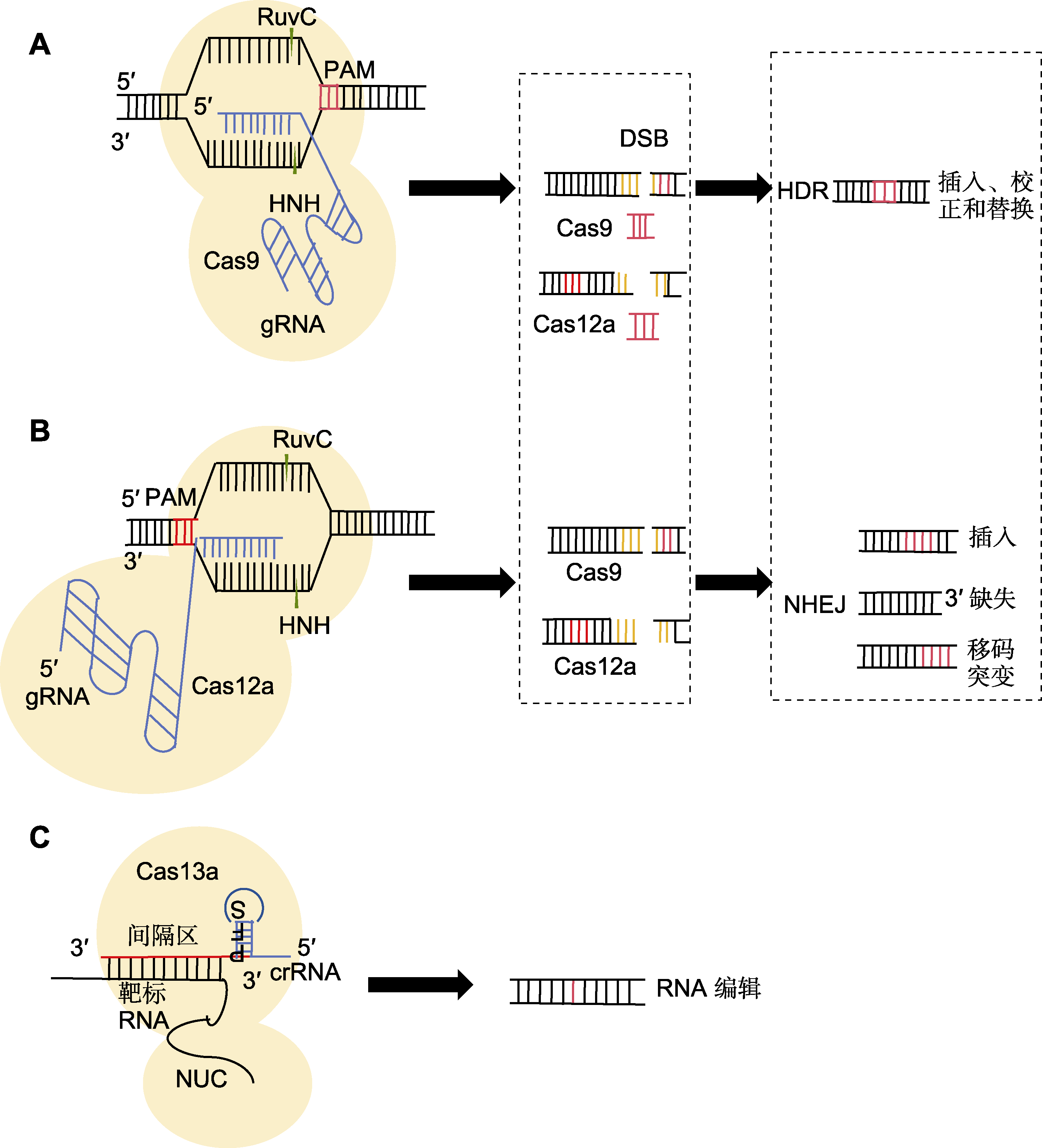
图1 CRISPR/Cas基因编辑系统工作原理 (A) Cas9蛋白利用结构域RuvC在原间隔区邻近基序(PAM)序列附近的特定单链DNA上切割, 随后, HNH的结构域与sgRNA配对的DNA另一条单链进行切割形成双链断裂(DSB), 最终进行同源定向修复(HDR)或非同源末端连接(NHEJ)修复成双链; (B) Cas12a通过crRNA介导识别5'-TTTN或5'-TTN的PAM序列; (C) 当crRNA与靶标RNA通过碱基互补配对结合后, 形成crRNA-Cas13复合物, 导致Cas13蛋白构象发生变化, 从而激活其RNA切割活性, 使其能够特异性识别并结合靶标RNA进行切割
Figure 1 Principle of operation of CRISPR/Cas gene editing system (A) The Cas9 protein utilizes its RuvC domain to cleave a specific single-stranded DNA near the protospacer adjacent motif (PAM) sequence, subsequently, the HNH domain cleaves the other single strand of DNA paired with the sgRNA, resulting in the formation of a double-strand break (DSB), which is ultimately repaired by homology-directed repair (HDR) or non-homologous end joining (NHEJ) to form double-stranded DNA; (B) Cas12a mediates the recognition of the PAM sequence 5'-TTTN or 5'-TTN by crRNA; (C) When the crRNA pairs with the target RNA through base complementarity, a crRNA-Cas13 complex is formed, causing a conformational change in the Cas13 protein, thereby activating its RNA cleavage activity, enabling the target RNA to be specifically recognized, bound, and cleaved.
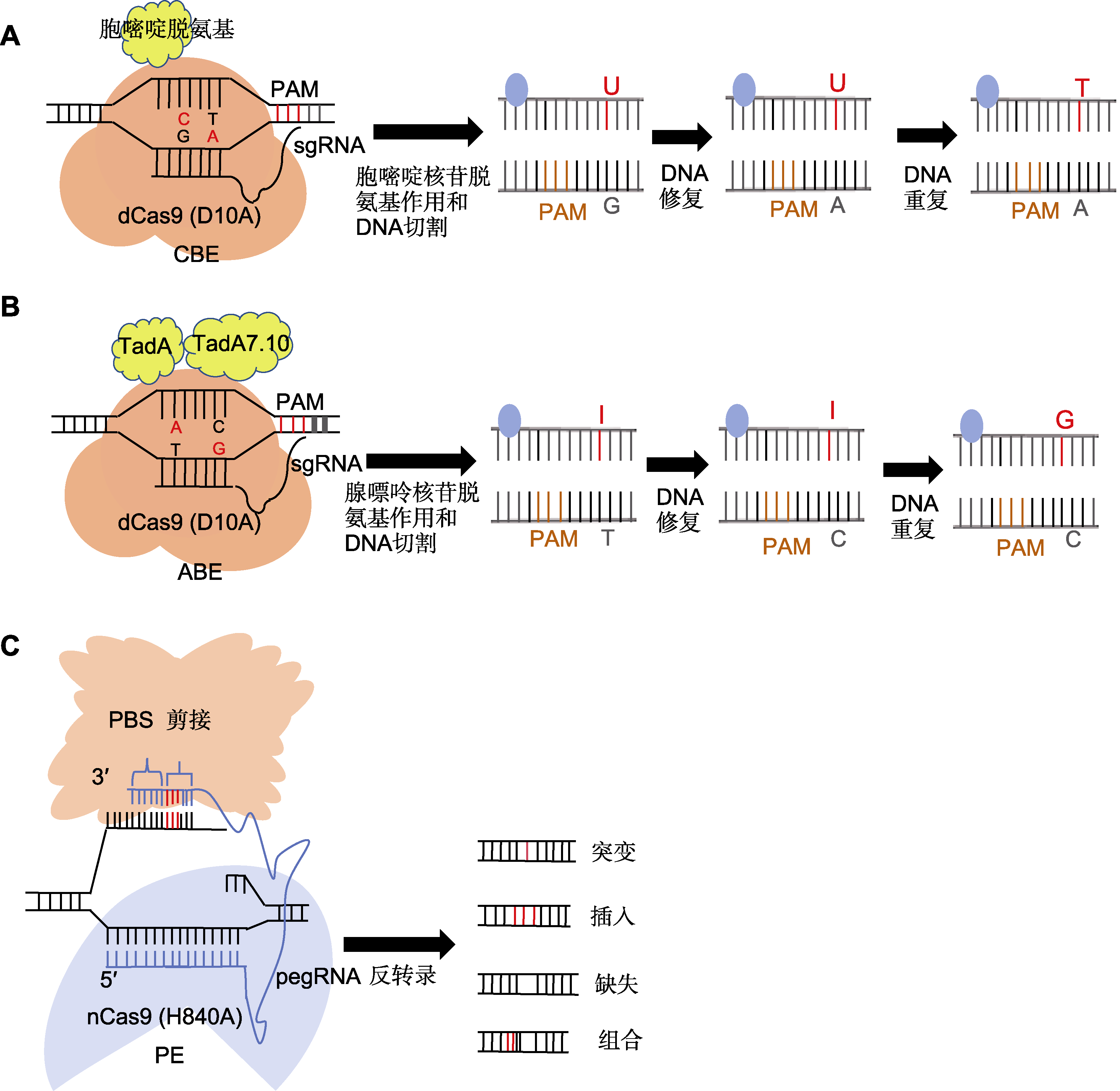
图2 碱基编辑器技术工作原理 (A) sgRNA识别并结合到目标DNA序列特定的原间隔区邻近基序(PAM)位点, dCas9蛋白则与sgRNA结合, 形成复合物并结合于目标DNA上, 但不切割DNA双链, 仅使其单链化; 随后胞嘧啶脱氨酶在sgRNA的引导下, 接触到暴露的单链DNA上的胞嘧啶, 并催化其脱氨反应, 将胞嘧啶转化为尿嘧啶; 最后在DNA重复或修复过程中, 尿嘧啶被视为胸腺嘧啶的类似物, 从而纳入到新合成的DNA链中, 实现C-G碱基对到T-A碱基对的直接替换; (B) 利用CRISPR-Cas9系统中的sgRNA识别并结合到目标DNA序列的PAM位点, Cas9蛋白与sgRNA结合形成复合物, 并定位于目标DNA上; ABE中的腺嘌呤脱氨酶在sgRNA的引导下, 接触到暴露的单链DNA上的腺嘌呤, 并催化其脱氨反应, 将腺嘌呤转化为次黄嘌呤或脱氧次黄嘌呤; 最后在DNA修复过程中, 识别到次黄嘌呤后, 启动修复过程, 中间产物通常被替换为鸟嘌呤, 从而实现A-T碱基对到G-C碱基对的直接替换; (C) 利用pegRNA作为引导分子结合sgRNA, 并在其3'末端增加引物结合位点(PBS)序列和逆转录模板(RTT); 在pegRNA的引导下, 部分失活的Cas9切口酶切断含PAM序列的DNA单链; 切割后的DNA单链与pegRNA的3'末端PBS序列互补并结合, 随后逆转录酶沿RTT模板序列开始逆转录反应, 将目标编辑序列直接引到DNA切口处; 随后细胞内DNA修复机制识别并处理切口处的DNA结构, 最终保留携带目标编辑的DNA链。CBE、ABE和PE同表1。
Figure 2 Principle of operation of base editor technology (A) The sgRNA recognizes and binds to the specific protospacer adjacent motif (PAM) site on the target DNA sequence, and the dCas9 protein binds to the sgRNA, forming a complex that attaches to the target DNA and rendering it single-stranded; subsequently, the cytidine deaminase, guided by the sgRNA, contacts the exposed cytidine on the single-stranded DNA and catalyzes its deamination, converting cytosine to uracil; finally, during DNA replication or repair, uracil is recognized as an analog of thymine and thymine is incorporated into the newly synthesized DNA strand, thereby achieving a direct replacement of the C-G base pair with a T-A base pair; (B) The sgRNA in the CRISPR-Cas9 system recognizes and binds to the PAM site on the target DNA sequence, and the Cas9 protein binds to the sgRNA to form a complex which is localized to the target DNA; under the direction of the sgRNA, adenine deaminase in the ABE system contacts the adenine on the single-stranded DNA, catalyzing its deamination reaction and converting adenine to hypoxanthine or deoxyhypoxanthine; during the DNA repair process, recognition of hypoxanthine triggers the initiation of the repair mechanism, typically resulting in the substitution of the intermediate product with guanine; this process facilitates the direct replacement of an A-T base pair with a G-C base pair; (C) Utilizing pegRNA as a guide molecule, it binds to the sgRNA and incorporates a primer binding site (PBS) sequence and reverse transcription template (RTT) at its 3' end; directed by the pegRNA, the partially deactivated Cas9 nickase cleaves the DNA single strand containing the PAM sequence; subsequently, the complementary PBS sequence at the 3' end of the pegRNA binds to the cleaved DNA strand; a reverse transcriptase then initiates a reverse transcription reaction along the RTT template sequence, directly incorporating the desired editing sequence into the DNA nick; finally, the intracellular DNA repair mechanisms are activated, ultimately retaining the DNA strand carrying the intended edit. CBE, ABE, and PE are the same as shown in Table 1.
| 应用 | 靶标基因 | 基因功能 | 性状改良 | 参考文献 |
|---|---|---|---|---|
| 单倍体诱导 | ZmPLA1 | 编码特异性磷脂酶 | 单倍体诱导 | Dong et al., |
| ZmDMP | 单倍体诱导 | 单倍体诱导 | Liu et al., | |
| CENH3 | 单倍体诱导 | 单倍体诱导 | Wang et al., | |
| 雄性不育 | MS45 | 编码异胡豆苷合成酶类似蛋白 | 花粉发育异常, 雄性不育 | Svitashev et al., |
| MS8 | 编码β-1,3-半乳糖基转移酶 | 花粉发育异常, 雄性不育 | Chen et al., | |
| ZmABCG2和ZmFAR1 | 角质层减少和蜡质含量增加 | 花粉发育异常, 雄性不育 | Jiang et al., | |
| ZmTMS5 | 编码RNase Z蛋白 | 温敏雄性不育植株 | Li et al., | |
| Dcl5 | 产生多样化的24 nt phasiRNAs | 温敏雄性不育植株 | Teng et al., | |
| 株型 | CLE7和FCP1 | 控制分生组织大小 | 果穗行数和籽粒产量增加, 果穗变大, 叶夹角减小 | Liu et al., |
| ZmLg1 | 编码调控SBP结构域蛋白 | 叶夹角减小, 种植密度增大 | Li et al., | |
| SAMBA | 影响有丝分裂期推进复合体 | 节间缩短, 上部叶片缩小、直 立, 叶片整体缩小 | Gong et al., | |
| ZmRAVL1 | 影响油菜素内酯信号通路 | 叶夹角变小 | 刘杰和严建兵, | |
| 激素 | GA20ox3 | 赤霉素合成 | 半矮生 | Zhang et al., |
| ZmACO2 | 乙烯合成 | 促进花序和花发育, 增加穗重 | Ning et al., | |
| ZmCEP1 | 肽激素合成 | 降低植株和穗高度、穗长、籽 粒大小和百粒重 | Xu et al., | |
| CKX | 细胞分裂素氧化酶合成 | 细胞分裂和植物器官的形成 | 刘超等, | |
| 果穗 | Zm079、Zm080和Zm081 | 调控百粒重 | 增加穗重、穗长和穗粗 | 穆路遥, |
| 光合作用 | Zmcst1 | 影响气孔开放和光合作用 | 叶片提前衰老 | Wang et al., |
| qkw9 | 光合作用减弱 | 为籽粒充实提供的母体光合产 物减少 | Huang et al., | |
| ZmSWEET13 | 光合作用受损 | 叶片中可溶性糖和淀粉含量 增加 | Bezrutczyk et al., | |
| ANT1 | 光合作用受损 | 相互遮荫 | Liu et al., | |
| Zmpif3、Zmpif4和Zmpif5 | 调控光信号和光形态发生 | 减弱植株对遮荫环境的响应 | Wu et al., | |
| 香味 | ZmBADH2a和mBADH2b | 控制2-乙酰基-1-吡咯烷酮合成 | 挥发物含量增加, 香气增加 | Wang et al., |
| 甜味 | Zmsh2 | 编码AGPase酶 | 甜玉米 | Dong et al., |
| 糯性 | SH2和WX | 编码AGPase酶和GBSS酶 | 甜玉米和糯玉米 | Gao et al., |
| 抗倒伏 | ZmWx1 | 编码GBSS酶 | 纤维素和木质素含量增加 | Li et al., |
| stiff1 | 控制纤维素和木质素含量 | 秸秆强度增加 | Zhang et al., | |
| qpa1 | 半矮化 | 株高和穗位降低, 茎粗增加, 叶片更直立 | Wei et al., | |
| ZmPHYCs | 减弱避荫综合症 | 降低植株高度和穗位高度 | Li et al., | |
| 干旱 | ARGOS8 | 乙烯响应负调控因子 | 提高耐旱能力 | Shi et al., |
| ZmHDT103 | 编码乙酰化酶 | 提高耐旱能力 | Wang et al., | |
| ZmSRL5 | 编码CALSP蛋白 | 提高耐旱能力 | Pan et al., | |
| 抗除草剂 | ZmEPSPS | 抑制叶绿体中5-烯醇丙酮酰莽 草酸-3-磷酸合酶的作用 | 抗除草剂 | Kaul et al., |
| ALS2 | 编码乙酰乳酸合成酶 | 抗除草剂 | Svitashev et al., | |
| 病原侵染 | ZmLox3 | 编码脂肪氧化酶 | 抗黑粉菌 | Pathi et al., |
| ZmGDlα | 编码RabGDP解离抑制因子 | 抗粗缩病 | Liu et al., | |
| ZmCOI1a和ZmJAZ15 | 茉莉酸合成 | 抗茎腐病 | Ma et al., | |
| ZmFBL41 | E3泛素连接酶复合体的成员之一 | 抗纹枯病 | 李伟滔等, |
表2 基因编辑技术在玉米改良中的应用
Table 2 Applications of gene editing technology in maize improvement
| 应用 | 靶标基因 | 基因功能 | 性状改良 | 参考文献 |
|---|---|---|---|---|
| 单倍体诱导 | ZmPLA1 | 编码特异性磷脂酶 | 单倍体诱导 | Dong et al., |
| ZmDMP | 单倍体诱导 | 单倍体诱导 | Liu et al., | |
| CENH3 | 单倍体诱导 | 单倍体诱导 | Wang et al., | |
| 雄性不育 | MS45 | 编码异胡豆苷合成酶类似蛋白 | 花粉发育异常, 雄性不育 | Svitashev et al., |
| MS8 | 编码β-1,3-半乳糖基转移酶 | 花粉发育异常, 雄性不育 | Chen et al., | |
| ZmABCG2和ZmFAR1 | 角质层减少和蜡质含量增加 | 花粉发育异常, 雄性不育 | Jiang et al., | |
| ZmTMS5 | 编码RNase Z蛋白 | 温敏雄性不育植株 | Li et al., | |
| Dcl5 | 产生多样化的24 nt phasiRNAs | 温敏雄性不育植株 | Teng et al., | |
| 株型 | CLE7和FCP1 | 控制分生组织大小 | 果穗行数和籽粒产量增加, 果穗变大, 叶夹角减小 | Liu et al., |
| ZmLg1 | 编码调控SBP结构域蛋白 | 叶夹角减小, 种植密度增大 | Li et al., | |
| SAMBA | 影响有丝分裂期推进复合体 | 节间缩短, 上部叶片缩小、直 立, 叶片整体缩小 | Gong et al., | |
| ZmRAVL1 | 影响油菜素内酯信号通路 | 叶夹角变小 | 刘杰和严建兵, | |
| 激素 | GA20ox3 | 赤霉素合成 | 半矮生 | Zhang et al., |
| ZmACO2 | 乙烯合成 | 促进花序和花发育, 增加穗重 | Ning et al., | |
| ZmCEP1 | 肽激素合成 | 降低植株和穗高度、穗长、籽 粒大小和百粒重 | Xu et al., | |
| CKX | 细胞分裂素氧化酶合成 | 细胞分裂和植物器官的形成 | 刘超等, | |
| 果穗 | Zm079、Zm080和Zm081 | 调控百粒重 | 增加穗重、穗长和穗粗 | 穆路遥, |
| 光合作用 | Zmcst1 | 影响气孔开放和光合作用 | 叶片提前衰老 | Wang et al., |
| qkw9 | 光合作用减弱 | 为籽粒充实提供的母体光合产 物减少 | Huang et al., | |
| ZmSWEET13 | 光合作用受损 | 叶片中可溶性糖和淀粉含量 增加 | Bezrutczyk et al., | |
| ANT1 | 光合作用受损 | 相互遮荫 | Liu et al., | |
| Zmpif3、Zmpif4和Zmpif5 | 调控光信号和光形态发生 | 减弱植株对遮荫环境的响应 | Wu et al., | |
| 香味 | ZmBADH2a和mBADH2b | 控制2-乙酰基-1-吡咯烷酮合成 | 挥发物含量增加, 香气增加 | Wang et al., |
| 甜味 | Zmsh2 | 编码AGPase酶 | 甜玉米 | Dong et al., |
| 糯性 | SH2和WX | 编码AGPase酶和GBSS酶 | 甜玉米和糯玉米 | Gao et al., |
| 抗倒伏 | ZmWx1 | 编码GBSS酶 | 纤维素和木质素含量增加 | Li et al., |
| stiff1 | 控制纤维素和木质素含量 | 秸秆强度增加 | Zhang et al., | |
| qpa1 | 半矮化 | 株高和穗位降低, 茎粗增加, 叶片更直立 | Wei et al., | |
| ZmPHYCs | 减弱避荫综合症 | 降低植株高度和穗位高度 | Li et al., | |
| 干旱 | ARGOS8 | 乙烯响应负调控因子 | 提高耐旱能力 | Shi et al., |
| ZmHDT103 | 编码乙酰化酶 | 提高耐旱能力 | Wang et al., | |
| ZmSRL5 | 编码CALSP蛋白 | 提高耐旱能力 | Pan et al., | |
| 抗除草剂 | ZmEPSPS | 抑制叶绿体中5-烯醇丙酮酰莽 草酸-3-磷酸合酶的作用 | 抗除草剂 | Kaul et al., |
| ALS2 | 编码乙酰乳酸合成酶 | 抗除草剂 | Svitashev et al., | |
| 病原侵染 | ZmLox3 | 编码脂肪氧化酶 | 抗黑粉菌 | Pathi et al., |
| ZmGDlα | 编码RabGDP解离抑制因子 | 抗粗缩病 | Liu et al., | |
| ZmCOI1a和ZmJAZ15 | 茉莉酸合成 | 抗茎腐病 | Ma et al., | |
| ZmFBL41 | E3泛素连接酶复合体的成员之一 | 抗纹枯病 | 李伟滔等, |
| [1] | Aman R, Ali Z, Butt H, Mahas A, Aljedaani F, Khan MZ, Ding SW, Mahfouz M (2018). RNA virus interference via CRISPR/Cas13a system in plants. Genome Biol 19, 1. |
| [2] | Anand A, Bass SH, Wu E, Wang N, Mcbride KE, Annaluru N, Miller M, Hua M, Jones TJ (2018). An improved ternary vector system for Agrobacterium-mediated rapid maize transformation. Plant Mol Biol 97, 187-200. |
| [3] | Bai MY, Lin WX, Peng CY, Song PZ, Kuang HQ, Lin JN, Zhang JP, Wang JY, Chen B, Li HR, Kong FJ, Jia GF, Guan YF (2024a). Expressing a human RNA demethylase as an assister improves gene-editing efficiency in plants. Mol Plant 17, 363-366. |
| [4] | Bai YH, Liu SN, Bai Y, Xu ZS, Zhao HN, Zhao HM, Lai JS, Liu Y, Song WB (2024b). Application of CRISPR/Cas12i.3 for targeted mutagenesis in broomcorn millet (Panicum miliaceum L.). J Integr Plant Biol 66, 1544-1547. |
| [5] | Bandyopadhyay A, Kancharla N, Javalkote VS, Dasgupta S, Brutnell TP (2020). CRISPR-Cas12a (Cpf1): a versatile tool in the plant genome editing tool box for agricultural advancement. Front Plant Sci 11, 584151. |
| [6] |
Bezrutczyk M, Hartwig T, Horschman M, Char SN, Yang JL, Yang B, Frommer WB, Sosso D (2018). Impaired phloem loading in zmsweet13a, b, c sucrose transporter triple knock-out mutants in Zea mays. New Phytol 218, 594-603.
DOI PMID |
| [7] | Butiuc-Keul A, Farkas A, Carpa R, Iordache D (2022). CRISPR-Cas system: the powerful modulator of accessory genomes in prokaryotes. Microb Physiol 32, 2-17. |
| [8] | Cao XS, Xie HT, Song ML, Zhao LH, Deng S, Tian YF, Li GF, Lang ZB, Zhu JK (2023). Extremely simplified cut- dip-budding method for genetic transformation and gene editing in Taraxacum kok-saghyz. Innovation Life 1, 100040. |
| [9] |
Cathomen T, Joung JK (2008). Zinc-finger nucleases: the next generation emerges. Mol Ther 16, 1200-1207.
DOI PMID |
| [10] | Chen F, Gu HY, Qi XQ, Lin RC, Qian Q, Xiao LT, Yang SH, Zuo JR, Bai YF, Chen ZD, Ding ZJ, Wang XJ, Jiang LW, Chong K, Wang L (2024). Achievements and advances of plant sciences research in China in 2023. Chin Bull Bot 59, 171-187. (in Chinese) |
|
陈凡, 顾红雅, 漆小泉, 林荣呈, 钱前, 萧浪涛, 杨淑华, 左建儒, 白永飞, 陈之端, 丁兆军, 王小菁, 姜里文, 种康, 王雷 (2024). 2023年中国植物科学重要研究进展. 植物学报 59, 171-187.
DOI |
|
| [11] | Chen L, Zhang S, Xue NN, Hong MJ, Zhang XH, Zhang D, Yang J, Bai SJ, Huang YF, Meng HW, Wu H, Luan CM, Zhu BY, Ru GM, Gao HY, Zhong LP, Liu MZ, Liu MY, Cheng YY, Yi CQ, Wang LR, Zhao YX, Song GJ, Li DL (2023). Engineering a precise adenine base editor with minimal bystander editing. Nat Chem Biol 19, 101-110. |
| [12] |
Chen PJ, Hussmann JA, Yan J, Knipping F, Ravisankar P, Chen PF, Chen CD, Nelson JW, Newby GA, Sahin M, Osborn MJ, Weissman JS, Adamson B, Liu DR (2021). Enhanced prime editing systems by manipulating cellular determinants of editing outcomes. Cell 184, 5635-5652.
DOI PMID |
| [13] |
Chen RR, Xu QL, Liu Y, Zhang JJ, Ren DT, Wang GY, Liu YJ (2018). Generation of transgene-free maize male sterile lines using the CRISPR/Cas9 system. Front Plant Sci 9, 1180.
DOI PMID |
| [14] |
Cong L, Ran FA, Cox D, Lin SL, Barretto R, Habib N, Hsu PD, Wu XB, Jiang WY, Marraffini LA, Zhang F (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819-823.
DOI PMID |
| [15] |
Cox DBT, Gootenberg JS, Abudayyeh OO, Franklin B, Kellner MJ, Joung J, Zhang F (2017). RNA editing with CRISPR-Cas13. Science 358, 1019-1027.
DOI PMID |
| [16] |
Doman JL, Pandey S, Neugebauer ME, An MR, Davis JR, Randolph PB, Mcelroy A, Gao XD, Raguram A, Richter MF, Everette KA, Banskota S, Tian K, Tao YA, Tolar J, Osborn MJ, Liu DR (2023). Phage-assisted evolution and protein engineering yield compact, efficient prime editors. Cell 186, 3983-4002.
DOI PMID |
| [17] |
Doman JL, Raguram A, Newby GA, Liu DR (2020). Evaluation and minimization of Cas9-independent off-target DNA editing by cytosine base editors. Nat Biotechnol 38, 620-628.
DOI PMID |
| [18] |
Dong L, Li LN, Liu CL, Liu CX, Geng SF, Li XH, Huang CL, Mao L, Chen SJ, Xie CX (2018). Genome editing and double-fluorescence proteins enable robust maternal haploid induction and identification in maize. Mol Plant 11, 1214-1217.
DOI PMID |
| [19] |
Dong L, Qi XT, Zhu JJ, Liu CL, Zhang X, Cheng BJ, Mao L, Xie CX (2019). Supersweet and waxy: meeting the diverse demands for specialty maize by genome editing. Plant Biotechnol J 17, 1853-1855.
DOI PMID |
| [20] |
Freije CA, Myhrvold C, Boehm CK, Lin AE, Welch NL, Carter A, Metsky HC, Luo CY, Abudayyeh OO, Gooten- berg JS, Yozwiak NL, Zhang F, Sabeti PC (2019). Programmable inhibition and detection of RNA viruses using Cas13. Mol Cell 76, 826-837.
DOI PMID |
| [21] | Gao HR, Gadlage MJ, Lafitte HR, Lenderts B, Yang MZ, Schroder M, Farrell J, Snopek K, Peterson D, Feigenbutz L, Jones S, St Clair G, Rahe M, Sanyour- Doyel N, Peng CN, Wang LJ, Young JK, Beatty M, Dahlke B, Hazebroek J, Greene TW, Cigan AM, Chilcoat ND, Meeley RB (2020). Superior field performance of waxy corn engineered using CRISPR-Cas9. Nat Biotechnol 38, 579-581. |
| [22] |
Gaudelli NM, Lam DK, Rees HA, Solá-Esteves NM, Barrera LA, Born DA, Edwards A, Gehrke JM, Lee SJ, Liquori AJ, Murray R, Packer MS, Rinaldi C, Slaymaker IM, Yen J, Young LE, Ciaramella G (2020). Directed evolution of adenine base editors with increased activity and therapeutic application. Nat Biotechnol 38, 892-900.
DOI PMID |
| [23] | Gong P, Bontinck M, Demuynck K, De Block J, Gevaert K, Eeckhout D, Persiau G, Aesaert S, Coussens G, Van Lijsebettens M, Pauwels L, De Jaeger G, Inzé D, Nelissen H (2022). SAMBA controls cell division rate during maize development. Plant Physiol 188, 411-424. |
| [24] |
Gootenberg JS, Abudayyeh OO, Lee JW, Essletzbichler P, Dy AJ, Joung J, Verdine V, Donghia N, Daringer NM, Freije CA, Myhrvold C, Bhattacharyya RP, Livny J, Regev A, Koonin EV, Hung DT, Sabeti PC, Collins JJ, Zhang F (2017). Nucleic acid detection with CRISPR- Cas13a/C2c2. Science 356, 438-442.
DOI PMID |
| [25] |
Grünewald J, Zhou RH, Iyer S, Lareau CA, Garcia SP, Aryee MJ, Joung JK (2019). CRISPR DNA base editors with reduced RNA off-target and self-editing activities. Nat Biotechnol 37, 1041-1048.
DOI PMID |
| [26] |
Haapaniemi E, Botla S, Persson J, Schmierer B, Taipale J (2018). CRISPR-Cas9 genome editing induces a p53- mediated DNA damage response. Nat Med 24, 927-930.
DOI PMID |
| [27] | He XL, Liu PC, Ma BJ, Chen XF (2022). Advance in gene- editing technology based on CRISPR/Cas9 and its application in plants. Chin Bull Bot 57, 508-531. (in Chinese) |
|
何晓玲, 刘鹏程, 马伯军, 陈析丰 (2022). 基于CRISPR/ Cas9的基因编辑技术研究进展及其在植物中的应用. 植物学报 57, 508-531.
DOI |
|
| [28] |
Hille F, Richter H, Wong SP, Bratovič M, Ressel S, Charpentier E (2018). The biology of CRISPR-Cas: backward and forward. Cell 172, 1239-1259.
DOI PMID |
| [29] | Hu JC, Sun Y, Li BS, Liu Z, Wang ZW, Gao Q, Guo MY, Liu GW, Zhao KT, Gao CX (2024). Strand-preferred base editing of organellar and nuclear genomes using CyDENT. Nat Biotechnol 42, 936-945. |
| [30] |
Huang J, Lu G, Liu L, Raihan MS, Xu JT, Jian LM, Zhao LX, Tran TM, Zhang QH, Liu J, Li WQ, Wei CX, Braun DM, Li Q, Fernie AR, Jackson D, Yan JB (2020). The Kernel size-related quantitative trait locus qKW9 encodes a pentatricopeptide repeat protein that affects photosynthesis and grain filling. Plant Physiol 183, 1696-1709.
DOI PMID |
| [31] | Huang JY, Lin QP, Fei HY, He ZX, Xu H, Li YJ, Qu KL, Han P, Gao Q, Li BS, Liu GW, Zhang LX, Hu JC, Zhang R, Zuo EW, Luo YL, Ran YD, Qiu JL, Zhao KT, Gao CX (2023). Discovery of deaminase functions by structure- based protein clustering. Cell 186, 3182-3195. |
| [32] | Jiang YL, Li ZW, Liu XZ, Zhu TT, Xie K, Hou QC, Yan TW, Niu CF, Zhang SW, Yang MB, Xie RR, Wang J, Li JP, An XL, Wan XY (2021). ZmFAR1 and ZmABCG26 regulated by microRNA are essential for lipid metabolism in maize anther. Int J Mol Sci 22, 7916. |
| [33] | Jiang YY, Chai YP, Lu MH, Han XL, Lin QP, Zhang Y, Zhang Q, Zhou Y, Wang XC, Gao CX, Chen QJ (2020). Prime editing efficiently generates W542L and S621I double mutations in two ALS genes in maize. Genome Biol 21, 257. |
| [34] | Jin S, Zong Y, Gao Q, Zhu ZX, Wang YP, Qin P, Liang CZ, Wang DW, Qiu JL, Zhang F, Gao CX (2019). Cytosine, but not adenine, base editors induce genome-wide off- target mutations in rice. Science 364, 292-295. |
| [35] |
Jinek M, Chylinski K, Fonfara I, Hauer M, Doudna JA, Charpentier E (2012). A programmable dual-RNA-guided DNA endonuclease in adaptive bacterial immunity. Science 337, 816-821.
DOI PMID |
| [36] | Jinek M, Jiang FG, Taylor DW, Sternberg SH, Kaya E, Ma EB, Anders C, Hauer M, Zhou KH, Lin S, Kaplan M, Iavarone AT, Charpentier E, Nogales E, Doudna JA (2014). Structures of Cas9 endonucleases reveal RNA- mediated conformational activation. Science 343, e1247997. |
| [37] | Kaul T, Thangaraj A, Jain R, Bharti J, Kaul R, Verma R, Sony SK, Abdel Motelb KF, Yadav P, Agrawal PK (2024). CRISPR/Cas9-mediated homology donor repair base editing system to confer herbicide resistance in maize (Zea mays L.). Plant Physiol Biochem 207, 108374. |
| [38] |
Kelliher T, Starr D, Su XJ, Tang GZ, Chen ZY, Carter J, Wittich PE, Dong SJ, Green J, Burch E, Mccuiston J, Gu WN, Sun YJ, Strebe T, Roberts J, Bate NJ, Que QD (2019). One-step genome editing of elite crop germplasm during haploid induction. Nat Biotechnol 37, 287-292.
DOI PMID |
| [39] |
Kim YB, Komor AC, Levy JM, Packer MS, Zhao KT, Liu DR (2017). Increasing the genome-targeting scope and precision of base editing with engineered Cas9-cytidine deaminase fusions. Nat Biotechnol 35, 371-376.
DOI PMID |
| [40] |
Koblan LW, Doman JL, Wilson C, Levy JM, Tay T, Newby GA, Maianti JP, Raguram A, Liu DR (2018). Improving cytidine and adenine base editors by expression optimization and ancestral reconstruction. Nat Biotechnol 36, 843-846.
DOI PMID |
| [41] | Komor AC, Kim YB, Packer MS, Zuris JA, Liu DR (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420-424. |
| [42] | Komor AC, Zhao KT, Packer MS, Gaudelli NM, Waterbury AL, Koblan LW, Kim YB, Badran AH, Liu DR (2017). Improved base excision repair inhibition and bacteriophage Mu Gam protein yields C:G-to-T:A base editors with higher efficiency and product purity. Sci Adv 3, eaao4774. |
| [43] | Kurt IC, Zhou RH, Iyer S, Garcia SP, Miller BR, Langner LM, Grünewald J, Joung JK (2021). CRISPR C-to-G base editors for inducing targeted DNA transversions in human cells. Nat Biotechnol 39, 41-46. |
| [44] |
Lee HK, Smith HE, Liu CY, Willi M, Hennighausen L (2020). Cytosine base editor 4 but not adenine base editor generates off-target mutations in mouse embryos. Commun Biol 3, 19.
DOI PMID |
| [45] | Li CX, Liu CL, Qi XT, Wu YC, Fei XH, Mao L, Cheng BJ, Li XH, Xie CX (2017a). RNA-guided Cas9 as an in vivo desired-target mutator in maize. Plant Biotechnol J 15, 1566-1576. |
| [46] | Li J, Zhang HW, Si XM, Tian YH, Chen KL, Liu JX, Chen HB, Gao CX (2017b). Generation of thermosensitive male-sterile maize by targeted knockout of the ZmTMS5 gene. J Genet Genomics 44, 465-468. |
| [47] | Li JF, Norville JE, Aach J, Mccormack M, Zhang DD, Bush J, Church GM, Sheen J (2013). Multiplex and homologous recombination-mediated genome editing in Arabidopsis and Nicotiana benthamiana using guide RNA and Cas9. Nat Biotechnol 31, 688-691. |
| [48] | Li N, Sun Y, Yang LY (2013). Cytological study of pollen-mediated plant transformation method based on GFP observation. Chin Bull Bot 48, 616-622. (in Chinese) |
|
李娜, 孙毅, 杨利艳 (2013). 基于GFP观察的花粉介导植物转基因方法的细胞学研究. 植物学报 48, 616-622.
DOI |
|
| [49] | Li QQ, Wu GX, Zhao YP, Wang BB, Zhao BB, Kong DX, Wei HB, Chen CX, Wang HY (2020). CRISPR/Cas9- mediated knockout and overexpression studies reveal a role of maize phytochrome C in regulating flowering time and plant height. Plant Biotechnol J 18, 2520-2532. |
| [50] | Li WT, He M, Chen XW (2019). Discovery of ZmFBL41Chang7-2 as a key weapon against banded leaf and sheath blight resistance in maize. Chin Bull Bot 54, 547-549. (in Chinese) |
|
李伟滔, 贺闽, 陈学伟 (2019). ZmFBL41Chang7-2: 玉米抗纹枯病的关键利器. 植物学报 54, 547-549.
DOI |
|
| [51] | Li X (2018). Optimization of the Genetic Transformation System of Caladium bicolor ‘Hongtao K’ and the Introduction of AtPAP1 Gene and Maize Lc Gene. Master’s thesis. Chongqing: Southwest University. (in Chinese) |
| 李想 (2018). 彩叶芋‘红桃K’遗传转化体系的优化及AtPAP1基因与玉米Lc基因的导入. 硕士论文. 重庆: 西南大学. | |
| [52] | Liang RH, He ZX, Zhao KT, Zhu HC, Hu JC, Liu GW, Gao Q, Liu MY, Zhang R, Qiu JL, Gao CX (2024). Prime editing using CRISPR-Cas12a and circular RNAs in human cells. Nat Biotechnol doi: 10.1038/s41587-023-02095-x. |
| [53] | Liu C, Li Y, Dai PH, Yao ZP, Liu XD (2020). Construction of genome editing vectors targeting CKXs in maize. Mol Plant Breed 18, 7051-7055. (in Chinese) |
| 刘超, 李月, 代培红, 姚正培, 刘晓东 (2020). 玉米CKXs基因组编辑载体的构建. 分子植物育种 18, 7051-7055. | |
| [54] | Liu CL, Kong M, Yang F, Zhu JJ, Qi XT, Weng JF, Di DP, Xie CX (2022). Targeted generation of null mutants in ZmGDIα confers resistance against maize rough dwarf disease without agronomic penalty. Plant Biotechnol J 20, 803-805. |
| [55] | Liu CX, Li X, Meng DX, Zhong Y, Chen C, Dong X, Xu XW, Chen BJ, Li W, Li L, Tian XL, Zhao HM, Song WB, Luo HS, Zhang QH, Lai JS, Jin WW, Yan JB, Chen SJ (2017). A 4bp insertion at ZmPLA1 encoding a putative phospholipase A generates haploid induction in maize. Mol Plant 10, 520-522. |
| [56] | Liu J, Yan JB (2019). A teosinte rare allele increases maize plant density and yield. Chin Bull Bot 54, 554-557. (in Chinese) |
|
刘杰, 严建兵 (2019). 大刍草稀有等位基因促进玉米密植高产. 植物学报 54, 554-557.
DOI |
|
| [57] | Liu L, Gallagher J, Arevalo ED, Chen R, Skopelitis T, Wu QY, Bartlett M, Jackson D (2021). Enhancing grain- yield-related traits by CRISPR-Cas9 promoter editing of maize CLE genes. Nat Plants 7, 287-294. |
| [58] | Liu WY, Lin HH, Yu CP, Chang CK, Chen HJ, Lin JJ, Lu MYJ, Tu SL, Shiu SH, Wu SH, Ku MSB, Li WH (2020). Maize ANT1 modulates vascular development, chloroplast development, photosynthesis, and plant growth. Proc Natl Acad Sci USA 117, 21747-21756. |
| [59] | Liu XS, Gu DF, Zhang YR, Jiang YL, Xiao Z, Xu RF, Qin RY, Li J, Wei PC (2024). Conditional knockdown of OsMLH1 to improve plant prime editing systems without disturbing fertility in rice. Genome Biol 25, 131. |
| [60] | Lowe K, La Rota M, Hoerster G, Hastings C, Wang N, Chamberlin M, Wu E, Jones T, Gordon-Kamm W (2018). Rapid genotype "independent" Zea mays L. (maize) trans- formation via direct somatic embryogenesis. In Vitro Cell Dev Biol Plant 54, 240-252. |
| [61] | Lowe K, Wu E, Wang N, Hoerster G, Hastings C, Cho MJ, Scelonge C, Lenderts B, Chamberlin M, Cushatt J, Wang LJ, Ryan L, Khan T, Chow-Yiu J, Hua W, Yu M, Banh J, Bao ZM, Brink K, Igo E, Rudrappa B, Shamseer PM, Bruce W, Newman L, Shen B, Zheng PZ, Bidney D, Falco C, Register J, Zhao ZY, Xu DP, Jones T, Gordon-Kamm W (2016). Morphogenic regulators Baby boom and Wuschel improve monocot transformation. Plant Cell 28, 1998-2015. |
| [62] | Luo Y, Zhang ML, Liu Y, Liu J, Li WQ, Chen GS, Peng Y, Jin M, Wei WJ, Jian LM, Yan J, Fernie AR, Yan JB (2022). Genetic variation in YIGE1 contributes to ear length and grain yield in maize. New Phytol 234, 513-526. |
| [63] | Lv P, Su F, Chen FY, Yan CX, Xia DD, Sun H, Li SS, Duan ZQ, Ma CL, Zhang H, Wang MG, Niu XM, Zhu JK, Zhang JS (2024). Genome editing in rice using CRISPR/ Cas12i3. Plant Biotechnol J 22, 379-385. |
| [64] | Ma L, Sun YL, Ruan XS, Huang PC, Wang S, Li SF, Zhou Y, Wang F, Cao Y, Wang Q, Wang ZH, Kolomiets MV, Gao XQ (2021). Genome-wide characterization of jasmonates signaling components reveals the essential role of ZmCOI1a-ZmJAZ15 action module in regulating maize immunity to gibberella stalk rot. Int J Mol Sci 22, 870. |
| [65] | Mahmud S, Ahmed J, Aziz MA, Hasan MR, Shaon SM, Bhuiyan MNAH, Rahman MF, Rakib HH, Islam MS (2017). Clustered regularly interspaced short palindromic repeats Cas systems: a comprehensive review. Int J Basic Clin Pharmacol 4, 613-622. |
| [66] |
Mali P, Yang LH, Esvelt KM, Aach J, Guell M, Dicarlo JE, Norville JE, Church GM (2013). RNA-guided human genome engineering via Cas9. Science 339, 823-826.
DOI PMID |
| [67] |
Mishra R, Joshi RK, Zhao KJ (2020). Base editing in crops: current advances, limitations and future implications. Plant Biotechnol J 18, 20-31.
DOI PMID |
| [68] | Mladenov E, Iliakis G (2011). Induction and repair of DNA double strand breaks: the increasing spectrum of non- homologous end joining pathways. Mutat Res 711, 61-72. |
| [69] | Mu LY (2022). Creation of Gene Editing Materials for Three Maize 100-Kernel Weight Candidate Genes. Master’s thesis. Wuhan: Huazhong Agricultural University. (in Chinese). |
| 穆路遥 (2022). 三个玉米百粒重候选基因基因编辑材料的创制. 硕士论文. 武汉: 华中农业大学. | |
| [70] | Nekrasov V, Staskawicz B, Weigel D, Jones JDG, Kamoun S (2013). Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat Biotechnol 31, 691-693. |
| [71] | Nelson JW, Randolph PB, Shen SP, Everette KA, Chen PJ, Anzalone AV, An MR, Newby GA, Chen JC, Hsu A, Liu DR (2022). Engineered pegRNAs improve prime editing efficiency. Nat Biotechnol 40, 402-410. |
| [72] |
Ning Q, Jian YN, Du YF, Li YF, Shen XM, Jia HT, Zhao R, Zhan JM, Yang F, Jackson D, Liu L, Zhang ZX (2021). An ethylene biosynthesis enzyme controls quantitative variation in maize ear length and kernel yield. Nat Commun 12, 5832.
DOI PMID |
| [73] | Nishida K, Arazoe T, Yachie N, Banno S, Kakimoto M, Tabata M, Mochizuki M, Miyabe A, Araki M, Hara KY, Shimatani Z, Kondo A (2016). Targeted nucleotide editing using hybrid prokaryotic and vertebrate adaptive immune systems. Science 353, 1248. |
| [74] | Pan ZY, Liu M, Zhao HL, Tan ZD, Liang K, Sun Q, Gong DM, He HJ, Zhou WQ, Qiu FZ (2020). ZmSRL5 is involved in drought tolerance by maintaining cuticular wax structure in maize. J Integr Plant Biol 62, 1895-1909. |
| [75] | Pathi KM, Rink P, Budhagatapalli N, Betz R, Saado I, Hiekel S, Becker M, Djamei A, Kumlehn J (2020). Engineering smut resistance in maize by site-directed mutagenesis of LIPOXYGENASE 3. Front Plant Sci 11, 543895. |
| [76] | Qi XT (2019). Engineering Liguleless, Super-Sweet and Waxy Compound Mazie Varieties via CRISPR-Cas9. PhD disseration. Hefei: Anhui Agricultural University. (in Chinese) |
| 祁显涛 (2019). 基于CRISPR-Cas9的玉米紧凑株型与甜糯复合型性状定向遗传改良. 博士论文. 合肥: 安徽农业大学. (in Chinese) | |
| [77] | Qi XT, Wu H, Jiang HY, Zhu JJ, Huang CL, Zhang X, Liu CL, Cheng BJ (2020). Conversion of a normal maize hybrid into a waxy version using in vivo CRISPR/Cas9 targeted mutation activity. Crop J 8, 440-448. |
| [78] |
Qiao DX, Wang JY, Lu MH, Xin CP, Chai YP, Jiang YY, Sun W, Cao ZH, Guo SY, Wang XC, Chen QJ (2023). Optimized prime editing efficiently generates heritable mutations in maize. J Integr Plant Biol 65, 900-906.
DOI |
| [79] |
Ren JJ, Wu PH, Trampe B, Tian XL, Lübberstedt T, Chen SJ (2017). Novel technologies in doubled haploid line development. Plant Biotechnol J 15, 1361-1370.
DOI PMID |
| [80] |
Richter MF, Zhao KT, Eton E, Lapinaite A, Newby GA, Thuronyi BW, Wilson C, Koblan LW, Zeng J, Bauer DE, Doudna JA, Liu DR (2020). Phage-assisted evolution of an adenine base editor with improved Cas domain compatibility and activity. Nat Biotechnol 38, 883-891.
DOI PMID |
| [81] |
Schoof H, Lenhard M, Haecker A, Mayer KFX, Jürgens G, Laux T (2000). The stem cell population of Arabidopsis shoot meristems in maintained by a regulatory loop between the CLAVATA and WUSCHEL genes. Cell 100, 635-644.
DOI PMID |
| [82] |
Shan QW, Wang YP, Li J, Zhang Y, Chen KL, Liang Z, Zhang K, Liu JX, Xi JJ, Qiu JL, Gao CX (2013). Targeted genome modification of crop plants using a CRISPR-Cas system. Nat Biotechnol 31, 686-688.
DOI PMID |
| [83] |
Shi JR, Gao HR, Wang HY, Lafitte HR, Archibald RL, Yang MZ, Hakimi SM, Mo H, Habben JE (2017). ARGOS8 variants generated by CRISPR-Cas9 improve maize grain yield under field drought stress conditions. Plant Biotechnol J 15, 207-216.
DOI PMID |
| [84] |
Shmakov S, Abudayyeh OO, Makarova KS, Wolf YI, Gootenberg JS, Semenova E, Minakhin L, Joung J, Konermann S, Severinov K, Zhang F, Koonin EV (2015). Discovery and functional characterization of diverse class 2 CRISPR-Cas systems. Mol Cell 60, 385-397.
DOI PMID |
| [85] |
Siebert R, Puchta H (2002). Efficient repair of genomic double-strand breaks by homologous recombination between directly repeated sequences in the plant genome. Plant Cell 14, 1121-1131.
DOI PMID |
| [86] | Sun C, Lei Y, Li BS, Gao Q, Li YJ, Cao W, Yang C, Li HC, Wang ZW, Li Y, Wang YP, Liu J, Zhao KT, Gao CX (2024). Precise integration of large DNA sequences in plant genomes using prime root editors. Nat Biotechnol 42, 316-327. |
| [87] |
Svitashev S, Schwartz C, Lenderts B, Young JK, Mark Cigan A (2016). Genome editing in maize directed by CRISPR-Cas9 ribonucleoprotein complexes. Nat Commun 7, 13274.
DOI PMID |
| [88] |
Svitashev S, Young JK, Schwartz C, Gao HR, Falco SC, Cigan AM (2015). Targeted mutagenesis, precise gene editing, and site-specific gene insertion in maize using Cas9 and guide RNA. Plant Physiol 169, 931-945.
DOI PMID |
| [89] | Teng C, Zhang H, Hammond R, Huang K, Meyers BC, Walbot V (2020). Dicer-like 5 deficiency confers temperature-sensitive male sterility in maize. Nat Commun 11, 2912. |
| [90] | Wang BB, Zhu L, Zhao BB, Zhao YP, Xie YR, Zheng ZG, Li YY, Sun J, Wang HY (2019a). Development of a haploid-inducer mediated genome editing system for accelerating maize breeding. Mol Plant 12, 597-602. |
| [91] | Wang H, Yan SJ, Xin HJ, Huang WJ, Zhang H, Teng SZ, Yu YC, Fernie AR, Lu XD, Li PC, Li SY, Zhang CY, Ruan YL, Chen LQ, Lang ZH (2019b). A subsidiary cell-localized glucose transporter promotes stomatal conductance and photosynthesis. Plant Cell 31, 1328-1343. |
| [92] |
Wang N, Arling M, Hoerster G, Ryan L, Wu E, Lowe K, Gordon-Kamm W, Jones TJ, Chilcoat ND, Anand A (2020). An efficient gene excision system in maize. Front Plant Sci 11, 1298.
DOI PMID |
| [93] | Wang N, Gent JI, Dawe RK (2021a). Haploid induction by a maize cenh3 null mutant. Sci Adv 7, eabe2299. |
| [94] |
Wang W, Pan QL, He F, Akhunova A, Chao S, Trick H, Akhunov E (2018). Transgenerational CRISPR-Cas9 activity facilitates multiplex gene editing in allopolyploid wheat. Crispr J 1, 65-74.
DOI PMID |
| [95] | Wang XD, Guo YH, Wang YR, Peng YL, Zhang HW, Zheng J (2024). ZmHDT103 negatively regulates drought stress tolerance in maize seedlings. Agronomy 14, 134. |
| [96] | Wang YX (2021). Using CRISPR/Cas Gene Editing Technology to Create Scented Corn. Master’s thesis. Jinan: Shandong Normal University. (in Chinese) |
| 王彦晓 (2021). 利用CRISPR/Cas基因编辑技术创建香味玉米. 硕士论文. 济南: 山东师范大学. (in Chinese) | |
| [97] | Wang YX, Liu XQ, Zheng XX, Wang WX, Yin XQ, Liu HF, Ma CL, Niu XM, Zhu JK, Wang F (2021b). Creation of aromatic maize by CRISPR/Cas. J Integr Plant Biol 63, 1664-1670. |
| [98] | Wei L, Zhang X, Zhang ZH, Liu HH, Lin ZW (2018). A new allele of the Brachytic2 gene in maize can efficiently modify plant architecture. Heredity 121, 75-86. |
| [99] |
Wu GX, Zhao YP, Shen RX, Wang BB, Xie YR, Ma XJ, Zheng ZG, Wang HY (2019). Characterization of maize phytochrome-interacting factors in light signaling and photomorphogenesis. Plant Physiol 181, 789-803.
DOI PMID |
| [100] | Xu JT, Liu XG, Jin ML, Pan H, Han BZ, Li MJ, Yan S, Hu GQ, Yan JB (2022). Establishment of genotype-independent high-efficiency transformation system in maize. Acta Agron Sin 48, 2987-2993. (in Chinese) |
|
许洁婷, 刘相国, 金敏亮, 潘弘, 韩宝柱, 李梦娇, 岩说, 胡国庆, 严建兵 (2022). 不依赖基因型的高效玉米遗传转化体系的建立. 作物学报 48, 2987-2993.
DOI |
|
| [101] | Xu RB, Li YF, Sui Z, Lan TY, Song WJ, Zhang M, Zhang YR, Xing JW (2021). A C-terminal encoded peptide, ZmCEP1, is essential for kernel development in maize. J Exp Bot 72, 5390-5406. |
| [102] |
Yan F, Kuang YJ, Ren B, Wang JW, Zhang DW, Lin HH, Yang B, Zhou XP, Zhou HB (2018). Highly efficient A·T to G·C base editing by Cas9n-guided tRNA adenosine deaminase in rice. Mol Plant 11, 631-634.
DOI PMID |
| [103] | Yan J, Oyler-Castrillo P, Ravisankar P, Ward CC, Levesque S, Jing Y, Simpson D, Zhao AQ, Li H, Yan WH, Goudy L, Schmidt R, Solley SC, Gilbert LA, Chan MM, Bauer DE, Marson A, Parsons LR, Adamson B (2024). Improving prime editing with an endogenous small RNA- binding protein. Nature 628, 639-647. |
| [104] | Yang F, Li Y (2017). The new generation tool for CRISPR genome editing: CRISPR/Cpf1. Chin J Biotechnol 33, 361-371. (in Chinese) |
| 杨帆, 李寅 (2017). 新一代基因组编辑系统CRISPR/Cpf1. 生物工程学报 33, 361-371. | |
| [105] | Zhang JJ, Zhang XF, Chen RR, Yang L, Fan KJ, Liu Y, Wang GY, Ren ZJ, Liu YJ (2020a). Generation of transgene-free semidwarf maize plants by gene editing of Gibberellin-Oxidase20-3 using CRISPR/Cas9. Front Plant Sci 11, 1048. |
| [106] | Zhang X, Shi YX, Lu BS, Wu Y, Liu Y, Wang YD, Yang JX, Zhao JR (2021). Creation of new maize variety with fragrant rice like flavor by editing BADH2-1 and BADH2-2 using CRISPR/Cas9. Sci Agric Sin 54, 2064-2072. (in Chinese) |
| 张翔, 史亚兴, 卢柏山, 武莹, 刘亚, 王元东, 杨进孝, 赵久然 (2021). 利用CRISPR/Cas9技术编辑BADH2-1/BADH2-2创制香米味道玉米新种质. 中国农业科学 54, 2064-2072. | |
| [107] |
Zhang Y, Zhang F, Li XH, Baller JA, Qi YP, Starker CG, Bogdanove AJ, Voytas DF (2013). Transcription activator-like effector nucleases enable efficient plant genome engineering. Plant Physiol 161, 20-27.
DOI PMID |
| [108] | Zhang ZH, Zhang X, Lin ZL, Wang J, Liu HQ, Zhou LN, Zhong SY, Li Y, Zhu C, Lai JS, Li XR, Yu JM, Lin ZW (2020b). A large transposon insertion in the stiff1 promoter increases stalk strength in maize. Plant Cell 32, 152-165. |
| [109] | Zhou CY, Sun YD, Yan R, Liu YJ, Zuo EW, Gu C, Han LX, Wei Y, Hu XD, Zeng R, Li YX, Zhou HB, Guo F, Yang H (2019). Off-target RNA mutation induced by DNA base editing and its elimination by mutagenesis. Nature 571, 275-278. |
| [110] |
Zong Y, Liu YJ, Xue CX, Li BS, Li XY, Wang YP, Li J, Liu GW, Huang XX, Cao XF, Gao CX (2022). An engineered prime editor with enhanced editing efficiency in plants. Nat Biotechnol 40, 1394-1402.
DOI PMID |
| [111] |
Zuo EW, Sun YD, Wei W, Yuan TL, Ying WQ, Sun H, Yuan LY, Steinmetz LM, Li YX, Yang H (2019). Cytosine base editor generates substantial off-target single-nucleotide variants in mouse embryos. Science 364, 289-292.
DOI PMID |
| [1] | 李园, 范开建, 安泰, 李聪, 蒋俊霞, 牛皓, 曾伟伟, 衡燕芳, 李虎, 付俊杰, 李慧慧, 黎亮. 玉米自然群体自交系农艺性状的多环境全基因组预测初探[J]. 植物学报, 2024, 59(6): 1041-1053. |
| [2] | 吴锁伟, 安学丽, 万向元. 玉米雄性不育机理及其在工程核不育制种中的应用[J]. 植物学报, 2024, 59(6): 932-949. |
| [3] | 郑名敏, 黄强, 张鹏, 刘孝伟, 赵卓凡, 易洪杨, 荣廷昭, 曹墨菊. 玉米细胞质雄性不育及育性恢复研究进展[J]. 植物学报, 2024, 59(6): 999-1006. |
| [4] | 杜庆国, 李文学. lncRNA调控玉米生长发育和非生物胁迫研究进展[J]. 植物学报, 2024, 59(6): 950-962. |
| [5] | 王子阳, 刘升学, 杨志蕊, 秦峰. 玉米抗旱性的遗传解析[J]. 植物学报, 2024, 59(6): 883-902. |
| [6] | 杨娟, 赵月磊, 陈晓远, 王宝宝, 王海洋. 玉米开花期调控机理及育种应用[J]. 植物学报, 2024, 59(6): 912-931. |
| [7] | 闫恒宇, 李朝霞, 李玉斌. 高温对玉米生长的影响及中国耐高温玉米筛选研究进展[J]. 植物学报, 2024, 59(6): 1007-1023. |
| [8] | 杨文丽, 李钊, 刘志铭, 张志华, 杨今胜, 吕艳杰, 王永军. 不同熟期玉米叶片衰老特性及其对叶际细菌的影响[J]. 植物学报, 2024, 59(6): 1024-1040. |
| [9] | 王涛, 冯敬磊, 张翠. 高温胁迫影响玉米生长发育的分子机制研究进展[J]. 植物学报, 2024, 59(6): 963-977. |
| [10] | 胡丹玲, 孙永伟. 病毒介导的植物基因组编辑技术研究进展[J]. 植物学报, 2024, 59(3): 452-462. |
| [11] | 程可心, 杜尧, 李凯航, 王浩臣, 杨艳, 金一, 何晓青. 玉米与叶际微生物组的互作遗传机制[J]. 植物生态学报, 2024, 48(2): 215-228. |
| [12] | 周文期, 周玉乾, 李永生, 何海军, 杨彦忠, 王晓娟, 连晓荣, 刘忠祥, 胡筑兵. 玉米ZmICE2基因调控气孔发育[J]. 植物学报, 2023, 58(6): 866-881. |
| [13] | 于熙婷, 黄学辉. 现代玉米起源新见解——两类大刍草的混血[J]. 植物学报, 2023, 58(6): 857-860. |
| [14] | 郭彦君, 陈枫, 罗敬文, 曾为, 许文亮. 植物细胞壁木聚糖的生物合成及其应用[J]. 植物学报, 2023, 58(2): 316-334. |
| [15] | 郭丽, 王雪涵, 田丰. 多组学整合网络: 一把精准解码玉米功能基因组的钥匙[J]. 植物学报, 2023, 58(1): 1-5. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||