

植物学报 ›› 2021, Vol. 56 ›› Issue (5): 544-558.DOI: 10.11983/CBB21014 cstr: 32102.14.CBB21014
吴丹丹1, 陈永坤1,2, 杨宇1, 孔春艳1, 龚明1,*( )
)
收稿日期:2021-01-18
接受日期:2021-05-07
出版日期:2021-09-01
发布日期:2021-08-31
通讯作者:
龚明
作者简介:* E-mail: gongming6307@163.com基金资助:
Dandan Wu1, Yongkun Chen1,2, Yu Yang1, Chunyan Kong1, Ming Gong1,*( )
)
Received:2021-01-18
Accepted:2021-05-07
Online:2021-09-01
Published:2021-08-31
Contact:
Ming Gong
摘要: 小桐子(Jatropha curcas)是一种极具潜力的能源植物及冷敏植物, 12°C低温锻炼可显著提高其耐冷性。在全基因组水平上对小桐子半胱氨酸蛋白酶家族及靶向其基因的miRNAs进行鉴定、生物信息学分析和表达特性分析, 并对该基因家族成员与miRNAs互作参与调控小桐子对低温锻炼的响应进行解析。结果表明, 在小桐子基因组中共鉴定到39个半胱氨酸蛋白酶基因, 定位于11条染色体上, 可分为6个亚家族(C1A、C2、C12、C13、C14和C15), 编码181-2 158个氨基酸残基的多肽, 均具有Cys和His活性位点。基于miRNA组和降解组测序结果, 发现有283个miRNAs靶向调控小桐子半胱氨酸蛋白酶基因家族的14个成员。对靶向JcDEK1、JcRD21B和JcXBCP3L的miRNAs在12°C低温锻炼过程中的共表达分析表明, 这些miRNAs参与半胱氨酸蛋白酶基因表达的调控, 且这种调控可能与低温锻炼诱导的小桐子耐冷性增强有关。研究结果有助于深入理解小桐子半胱氨酸蛋白酶基因家族功能及其与相应miRNAs的互作, 以及通过互作调控小桐子对低温的响应。
吴丹丹, 陈永坤, 杨宇, 孔春艳, 龚明. 小桐子半胱氨酸蛋白酶家族和相应miRNAs的鉴定及其对低温锻炼的响应. 植物学报, 2021, 56(5): 544-558.
Dandan Wu, Yongkun Chen, Yu Yang, Chunyan Kong, Ming Gong. Identification of the Cysteine Protease Family and Corresponding miRNAs in Jatropha curcas and Their Response to Chill-hardening. Chinese Bulletin of Botany, 2021, 56(5): 544-558.
| Primer name | Sequence (5′→ 3′) |
|---|---|
| miR1314-y-F | GCCGGTCTCCAATGTTAGG |
| miR1151-y-F | ATCTGGTTGTGGGACCCG |
| miR5156-x-F | GCGAGACTGTGAAACTGCAAA |
| miR6483-y-F | GCGTTGTAGAAATTTTCAGGATCA |
| JcXBCP3L-F | TCTGTGGGTATGGATGGTTCTGC |
| JcXBCP3L-R | ACCCCAGTCAGTACCCCACGA |
| JcRD21B-F | CAACGCTTTAGGAGAGAAGGAA |
| JcRD21B-R | ACGCAACTTGTTCCTCCTGAC |
| JcDEK1-F | TGCTGGGAAATTCTGGTG |
| JcDEK1-R | AGCCGTCAAACCCACCA |
| Reverse primer | GCTGTCAACGATACGCTACGTAACG |
| U6-F | GGAACGATACAGAGAAGATT |
| GAPDH-F | TGAAGGACTGGAGAGGTGGAAGAGC |
| GAPDH-R | ATCAACAGTTGGAACACGGAAAGCC |
表1 qRT-PCR相关引物
Table 1 The related primers for qRT-PCR
| Primer name | Sequence (5′→ 3′) |
|---|---|
| miR1314-y-F | GCCGGTCTCCAATGTTAGG |
| miR1151-y-F | ATCTGGTTGTGGGACCCG |
| miR5156-x-F | GCGAGACTGTGAAACTGCAAA |
| miR6483-y-F | GCGTTGTAGAAATTTTCAGGATCA |
| JcXBCP3L-F | TCTGTGGGTATGGATGGTTCTGC |
| JcXBCP3L-R | ACCCCAGTCAGTACCCCACGA |
| JcRD21B-F | CAACGCTTTAGGAGAGAAGGAA |
| JcRD21B-R | ACGCAACTTGTTCCTCCTGAC |
| JcDEK1-F | TGCTGGGAAATTCTGGTG |
| JcDEK1-R | AGCCGTCAAACCCACCA |
| Reverse primer | GCTGTCAACGATACGCTACGTAACG |
| U6-F | GGAACGATACAGAGAAGATT |
| GAPDH-F | TGAAGGACTGGAGAGGTGGAAGAGC |
| GAPDH-R | ATCAACAGTTGGAACACGGAAAGCC |
| Locus ID | Gene name | Subgroup | Chr. | Size (aa) | Mw (Da) | pI |
|---|---|---|---|---|---|---|
| XM_012209792.1 | JcRD21A | C1A | Chr. 10 | 475 | 52682.16 | 5.39 |
| XM_012221410.1 | JcSAG12H4 | C1A | Chr. 4 | 340 | 37417.89 | 5.13 |
| XM_012226309.1 | JcSAG12H1 | C1A | Chr. 3 | 311 | 34256.46 | 6.89 |
| XM_012216029.1 | JcSAG12H2 | C1A | Chr. 7 | 268 | 28610.75 | 4.66 |
| XM_012216557.1 | JcXBCP3 | C1A | Chr. 4 | 441 | 48817.81 | 6.06 |
| XM_012218796.1 | JcRD21A1 | C1A | Chr. 10 | 358 | 40417.25 | 5.10 |
| XM_012223641.1 | JcALP1 | C1A | Chr. 7 | 347 | 38935.92 | 8.43 |
| XM_012225588.1 | JcRD19B | C1A | Chr. 10 | 409 | 44955.43 | 6.71 |
| XM_012230158.1 | JcCEP2 | C1A | Chr. 7 | 358 | 39943.84 | 6.19 |
| XM_012233098.1 | JcSAG12H5 | C1A | Chr. 9 | 340 | 37592.12 | 5.01 |
| XM_012236390.1 | JcXBCP3L | C1A | Chr. 3 | 524 | 58042.85 | 5.24 |
| XM_012219020.1 | JcXCP1 | C1A | Chr. 5 | 349 | 39054.13 | 5.59 |
| XM_012211812.1 | JcRD19A | C1A | Chr. 2 | 370 | 40564.71 | 5.95 |
| XM_012212907.1 | JcRD21B | C1A | Chr. 4 | 466 | 51785.03 | 5.28 |
| XM_012213199.1 | JcSAG12H3 | C1A | Chr. 2 | 339 | 37598.38 | 5.94 |
| XM_012216569.1 | JcRD19C | C1A | Chr. 4 | 368 | 40923.12 | 5.81 |
| XM_012218200.1 | JcXCP2 | C1A | Chr. 4 | 350 | 39176.15 | 5.40 |
| XM_012221663.1 | JcTHI1 | C1A | Chr. 11 | 358 | 39435.49 | 5.88 |
| XM_012223907.1 | JcRD21C | C1A | Chr. 7 | 366 | 41119.35 | 5.40 |
| XM_012224546.1 | JcCEP1 | C1A | Chr. 5 | 360 | 40142.92 | 5.69 |
| XM_020685390.1 | JcSAG12H6 | C1A | Chr. 3 | 181 | 20779.04 | 9.49 |
| XM_020685389.1 | JcSAG12H7 | C1A | Chr. 3 | 181 | 20779.04 | 9.49 |
| XM_012227664.1 | JcCTB1 | C1A | Chr. 8 | 358 | 39709.10 | 6.07 |
| XM_012225478.1 | JcDEK1 | C2 | Chr. 1 | 2158 | 239010.06 | 6.03 |
| XM_012224060.1 | JcUCH2 | C12 | Chr. 4 | 337 | 38597.28 | 6.43 |
| XM_012214672.1 | JcUCH3 | C12 | Chr. 9 | 236 | 26006.51 | 4.66 |
| XM_012231737.1 | JcVPE2 | C13 | Chr. 9 | 495 | 55104.34 | 5.58 |
| XM_012221936.1 | JcVPE1 | C13 | Chr. 11 | 493 | 54253.23 | 5.93 |
| XM_012232060.1 | JcVPE3 | C13 | Chr. 5 | 485 | 54983.84 | 6.22 |
| XM_012229170.1 | JcAMC4G | C14 | Chr. 5 | 418 | 45953.22 | 5.18 |
| Locus ID | Gene name | Subgroup | Chr. | Size (aa) | Mw (Da) | pI |
| XM_012227353.1 | JcAMC9A | C14 | Chr. 3 | 317 | 35221.20 | 5.84 |
| XM_012213747.1 | JcAMC3B | C14 | Chr. 2 | 374 | 41063.04 | 5.71 |
| XM_012213748.1 | JcAMC1C | C14 | Chr. 2 | 330 | 37646.13 | 8.05 |
| XM_012213889.1 | JcAMC2D | C14 | Chr. 2 | 406 | 45165.20 | 8.65 |
| XM_012213890.1 | JcAMC1E | C14 | Chr. 2 | 335 | 37746.43 | 8.10 |
| XM_012228350.1 | JcAMC1F | C14 | Chr. 8 | 362 | 39681.00 | 6.41 |
| XM_012235144.1 | JcAMC1H | C14 | Chr. 5 | 370 | 40503.80 | 6.34 |
| XM_012230023.1 | JcPCP1 | C15 | Chr. 7 | 217 | 23577.96 | 6.07 |
| XM_012233456.1 | JcPCP2 | C15 | Chr. 6 | 219 | 23969.43 | 6.08 |
表2 小桐子中已鉴定的半胱氨酸蛋白酶的理化性质
Table 2 Physicochemical properties of identified cysteine proteins in Jatropha curcas
| Locus ID | Gene name | Subgroup | Chr. | Size (aa) | Mw (Da) | pI |
|---|---|---|---|---|---|---|
| XM_012209792.1 | JcRD21A | C1A | Chr. 10 | 475 | 52682.16 | 5.39 |
| XM_012221410.1 | JcSAG12H4 | C1A | Chr. 4 | 340 | 37417.89 | 5.13 |
| XM_012226309.1 | JcSAG12H1 | C1A | Chr. 3 | 311 | 34256.46 | 6.89 |
| XM_012216029.1 | JcSAG12H2 | C1A | Chr. 7 | 268 | 28610.75 | 4.66 |
| XM_012216557.1 | JcXBCP3 | C1A | Chr. 4 | 441 | 48817.81 | 6.06 |
| XM_012218796.1 | JcRD21A1 | C1A | Chr. 10 | 358 | 40417.25 | 5.10 |
| XM_012223641.1 | JcALP1 | C1A | Chr. 7 | 347 | 38935.92 | 8.43 |
| XM_012225588.1 | JcRD19B | C1A | Chr. 10 | 409 | 44955.43 | 6.71 |
| XM_012230158.1 | JcCEP2 | C1A | Chr. 7 | 358 | 39943.84 | 6.19 |
| XM_012233098.1 | JcSAG12H5 | C1A | Chr. 9 | 340 | 37592.12 | 5.01 |
| XM_012236390.1 | JcXBCP3L | C1A | Chr. 3 | 524 | 58042.85 | 5.24 |
| XM_012219020.1 | JcXCP1 | C1A | Chr. 5 | 349 | 39054.13 | 5.59 |
| XM_012211812.1 | JcRD19A | C1A | Chr. 2 | 370 | 40564.71 | 5.95 |
| XM_012212907.1 | JcRD21B | C1A | Chr. 4 | 466 | 51785.03 | 5.28 |
| XM_012213199.1 | JcSAG12H3 | C1A | Chr. 2 | 339 | 37598.38 | 5.94 |
| XM_012216569.1 | JcRD19C | C1A | Chr. 4 | 368 | 40923.12 | 5.81 |
| XM_012218200.1 | JcXCP2 | C1A | Chr. 4 | 350 | 39176.15 | 5.40 |
| XM_012221663.1 | JcTHI1 | C1A | Chr. 11 | 358 | 39435.49 | 5.88 |
| XM_012223907.1 | JcRD21C | C1A | Chr. 7 | 366 | 41119.35 | 5.40 |
| XM_012224546.1 | JcCEP1 | C1A | Chr. 5 | 360 | 40142.92 | 5.69 |
| XM_020685390.1 | JcSAG12H6 | C1A | Chr. 3 | 181 | 20779.04 | 9.49 |
| XM_020685389.1 | JcSAG12H7 | C1A | Chr. 3 | 181 | 20779.04 | 9.49 |
| XM_012227664.1 | JcCTB1 | C1A | Chr. 8 | 358 | 39709.10 | 6.07 |
| XM_012225478.1 | JcDEK1 | C2 | Chr. 1 | 2158 | 239010.06 | 6.03 |
| XM_012224060.1 | JcUCH2 | C12 | Chr. 4 | 337 | 38597.28 | 6.43 |
| XM_012214672.1 | JcUCH3 | C12 | Chr. 9 | 236 | 26006.51 | 4.66 |
| XM_012231737.1 | JcVPE2 | C13 | Chr. 9 | 495 | 55104.34 | 5.58 |
| XM_012221936.1 | JcVPE1 | C13 | Chr. 11 | 493 | 54253.23 | 5.93 |
| XM_012232060.1 | JcVPE3 | C13 | Chr. 5 | 485 | 54983.84 | 6.22 |
| XM_012229170.1 | JcAMC4G | C14 | Chr. 5 | 418 | 45953.22 | 5.18 |
| Locus ID | Gene name | Subgroup | Chr. | Size (aa) | Mw (Da) | pI |
| XM_012227353.1 | JcAMC9A | C14 | Chr. 3 | 317 | 35221.20 | 5.84 |
| XM_012213747.1 | JcAMC3B | C14 | Chr. 2 | 374 | 41063.04 | 5.71 |
| XM_012213748.1 | JcAMC1C | C14 | Chr. 2 | 330 | 37646.13 | 8.05 |
| XM_012213889.1 | JcAMC2D | C14 | Chr. 2 | 406 | 45165.20 | 8.65 |
| XM_012213890.1 | JcAMC1E | C14 | Chr. 2 | 335 | 37746.43 | 8.10 |
| XM_012228350.1 | JcAMC1F | C14 | Chr. 8 | 362 | 39681.00 | 6.41 |
| XM_012235144.1 | JcAMC1H | C14 | Chr. 5 | 370 | 40503.80 | 6.34 |
| XM_012230023.1 | JcPCP1 | C15 | Chr. 7 | 217 | 23577.96 | 6.07 |
| XM_012233456.1 | JcPCP2 | C15 | Chr. 6 | 219 | 23969.43 | 6.08 |
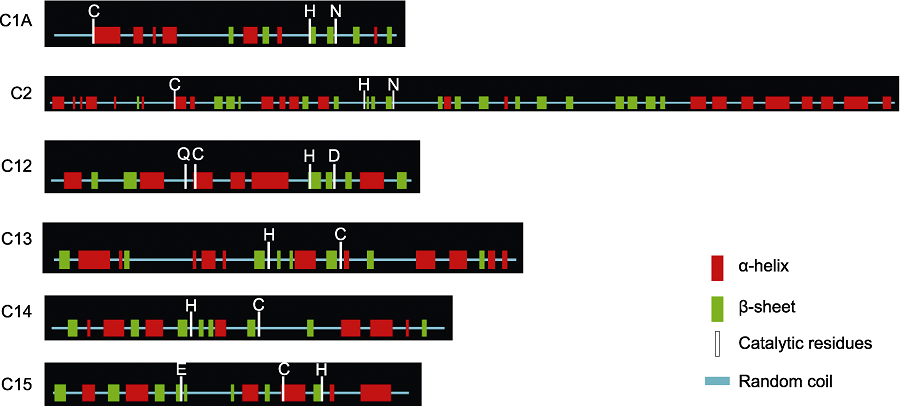
图2 小桐子6个半胱氨酸蛋白酶亚家族的蛋白结构 C: 半胱氨酸; D: 天冬氨酸; E: 谷氨酸; H: 组氨酸; N: 天冬酰胺; Q: 谷氨酰胺
Figure 2 Protein structure of the six cysteine protease subfamilies in Jatropha curcas C: Cys; D: Asp; E: Glu; H: His; N: Asn; Q: Gln
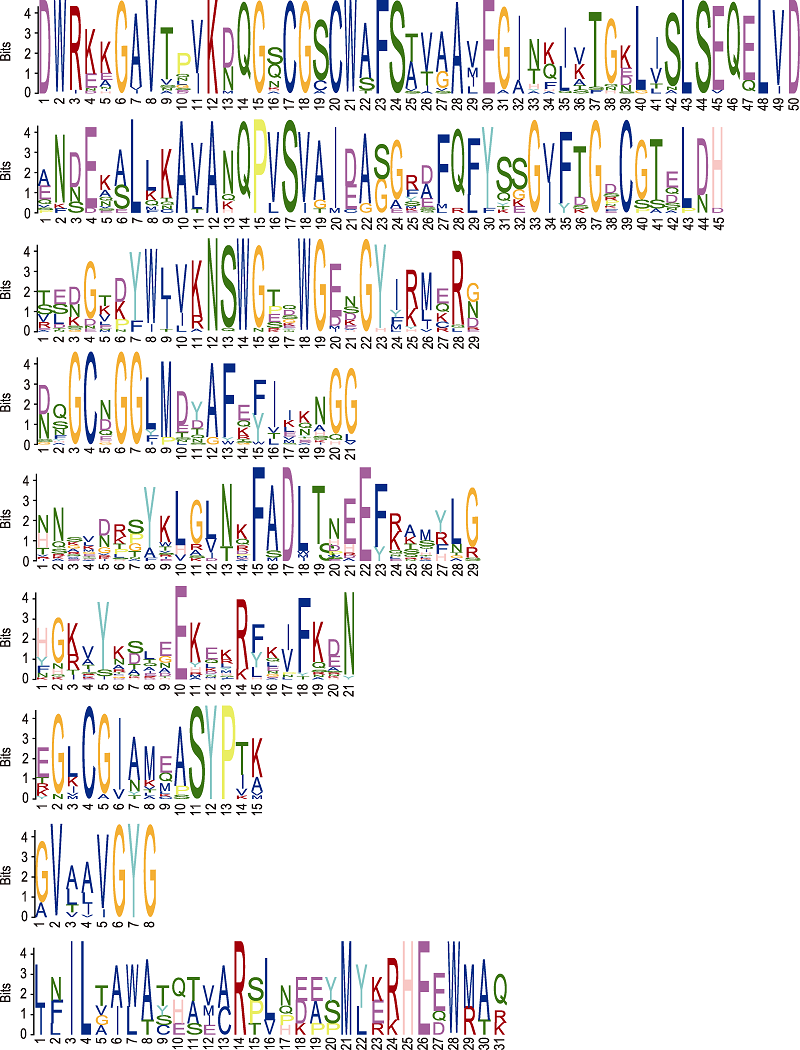
图3 使用MEME软件确定的小桐子半胱氨酸蛋白酶家族9个重要的基序 横坐标表示基序的长度; 纵坐标表示基序的保守程度。
Figure 3 The top nine motifs of cysteine protease family in Jatropha curcas identified using the MEME software The abscissa represents the length of the motif; the ordinate indicates the conservatism of the motif.
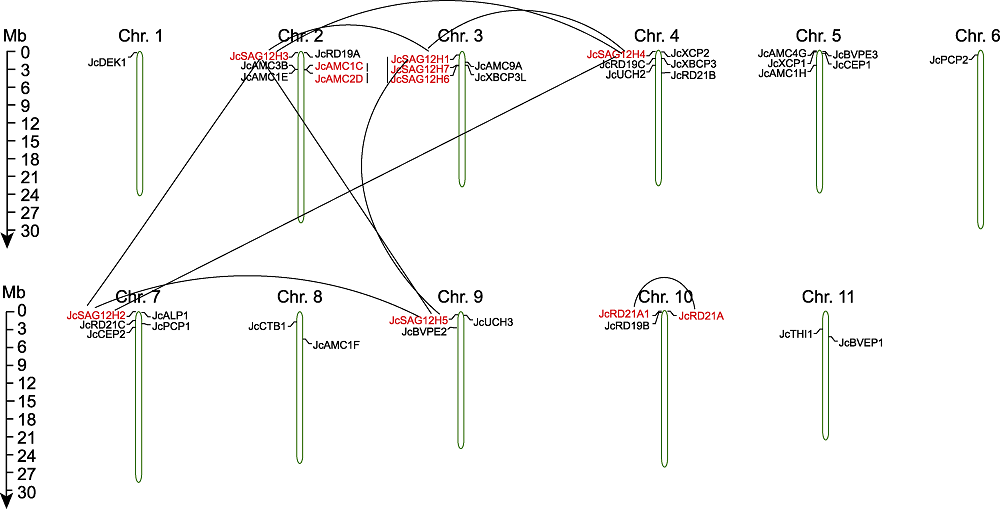
图4 小桐子半胱氨酸蛋白酶基因染色体定位及成对发生的重复事件 重复基因对用线显示和连接。
Figure 4 Chromosome location and repetitive events of cysteine protease gene pairs in Jatropha curcas Repeated gene pairs are shown and linked by the lines.
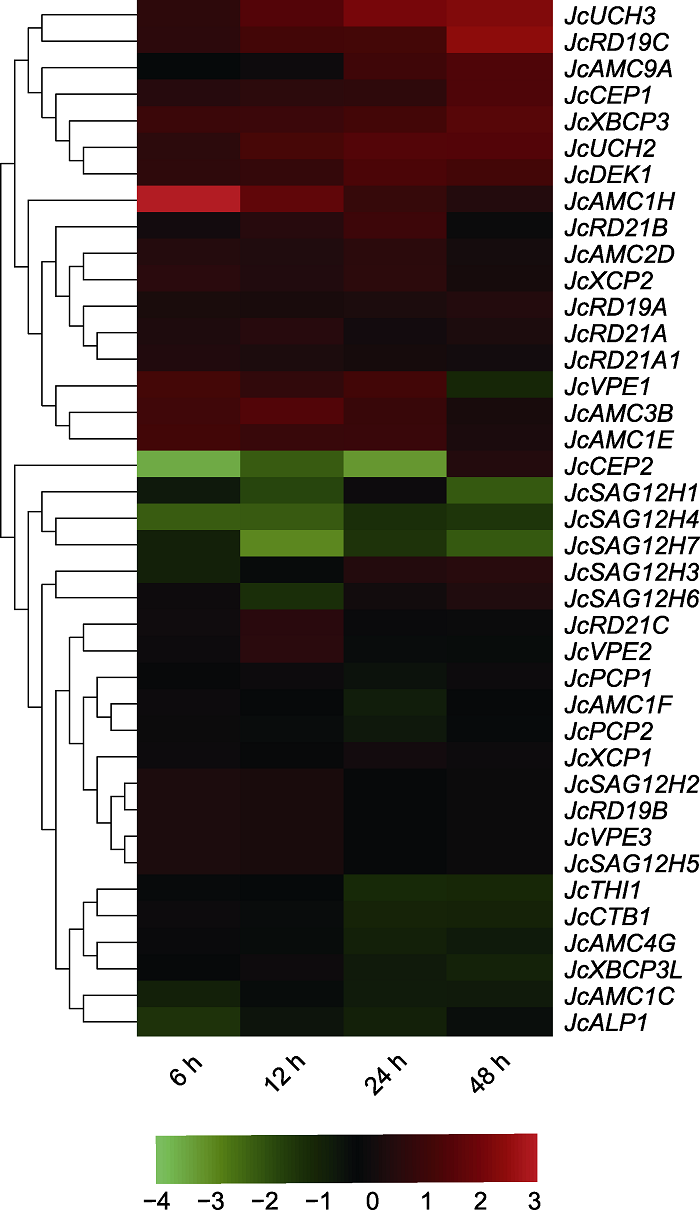
图6 小桐子幼苗叶片半胱氨酸蛋白酶家族基因在12°C低温锻炼期间基因表达的热图
Figure 6 Heatmap of gene expression of cysteine protease family in leaves of Jatropha curcas seedlings during chill-hardening at 12°C
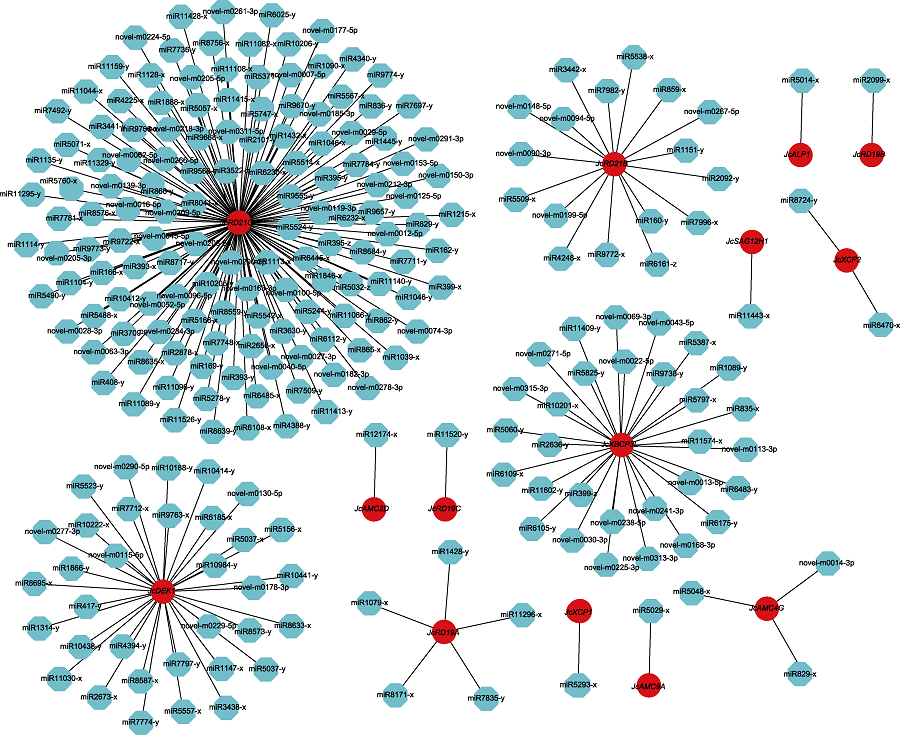
图7 miRNAs靶向调控小桐子半胱氨酸蛋白酶家族基因的网络图 红色代表半胱氨酸蛋白酶基因, 蓝色代表miRNAs。
Figure 7 Networks of targeted regulation among miRNAs and their corresponding cysteine protease family genes in Jatropha curcas Red represents cysteine protease gene and blue represents miRNAs.
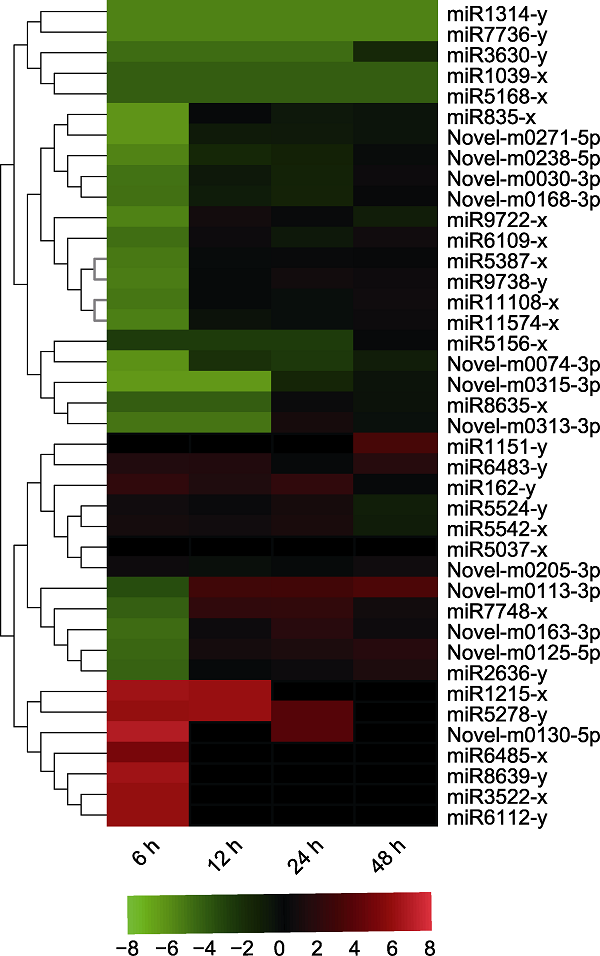
图8 靶向调控小桐子半胱氨酸蛋白酶家族基因的miRNAs在12°C低温锻炼期间的热图
Figure 8 Heatmap of miRNAs targeting cysteine protease family genes in Jatropha curcas during 12°C chill-hardening
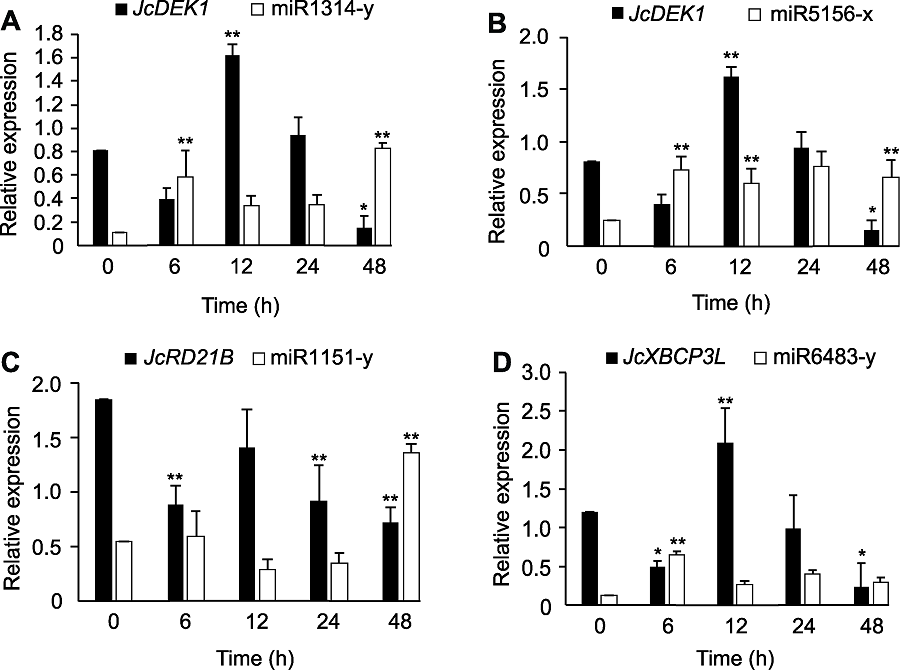
图9 小桐子幼苗在12°C低温锻炼期间半胱氨酸蛋白酶基因JcDEK1、JcRD21B、JcXBCP3L以及靶向这些基因miRNAs的qRT-PCR共表达分析 *与**分别表示不同时间的表达量与对照0小时在P<0.05与P<0.01水平差异显著。
Figure 9 Co-expression analysis by qRT-PCR of the cysteine protease genes JcDEK1, JcRD21B and JcXBCP3L as well as the miRNAs targeting these genes in Jatropha curcas seedlings during chill-hardening at 12°C * and ** indicated the significant differences between the expression level at different time and control 0 h at P<0.05 and P<0.01 level, respectively.
| [1] | 郝大海, 龚明 (2020). miRNA作用机制研究进展. 基因组学与应用生物学 39, 3647-3657. |
| [2] | 孔春艳, 陈永坤, 王莎莎, 郝大海, 杨宇, 龚明 (2019). 小桐子低温胁迫下microRNA实时荧光定量PCR内参的筛选与比较. 生物技术通报 35(7), 25-31. |
| [3] | 李忠光, 龚明 (2011). 不同化学消毒剂对小桐子种子萌发和幼苗生长的影响. 种子 30(2), 4-7, 12. |
| [4] | 宋敏, 张瑶, 王丽莹, 彭向永 (2020). 甘蓝型油菜ZF-HD基因家族的鉴定与系统进化分析. 植物学报 54, 699-710. |
| [5] | 王海波, 郭俊云, 唐利洲 (2019). 小桐子MAPKKKK基因家族的全基因组鉴定及表达分析. 植物生理学报 55, 367-377. |
| [6] |
王劲东, 周豫, 余佳雯, 范晓磊, 张昌泉, 李钱峰, 刘巧泉 (2020). miR172-AP2模块调控植物生长发育及逆境响应的研究进展. 植物学报 55, 205-215.
DOI |
| [7] | 吴丹丹, 陈永坤, 杨宇, 孔春艳, 龚明 (2021). 小桐子cystatin家族基因和相应miRNAs的鉴定及其在低温响应中可能的作用. 植物生理学报 57, 347-361. |
| [8] |
闫晨阳, 陈赢男 (2020). 4种模式植物LRR VIII-2亚家族基因的鉴定和进化历史分析. 植物学报 55, 442-456.
DOI |
| [9] | 张翠桔, 莫蓓莘, 陈雪梅, 崔洁 (2020). 植物miRNA作用方式的分子机制研究进展. 生物技术通报 36(7), 1-14. |
| [10] |
Ahmad R, Zuily-Fodil Y, Passaquet C, Bethenod O, Roche R, Repellin A (2014). Identification and characterization of MOR-CP, a cysteine protease induced by ozone and developmental senescence in maize ( Zea mays L.) leaves. Chemosphere 108, 245-250.
DOI URL |
| [11] |
Ao PX, Li ZG, Fan DM, Gong M (2013a). Involvement of antioxidant defense system in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35, 153-160.
DOI URL |
| [12] |
Ao PX, Li ZG, Gong M (2013b). Involvement of compatible solutes in chill hardening-induced chilling tolerance in Jatropha curcas seedlings. Acta Physiol Plant 35, 3457-3464.
DOI URL |
| [13] |
Chen C, Yu Y, Ding XD, Liu BD, Duanmu H, Zhu D, Sun XL, Cao L, Nisa ZU, Li Q, Zhu YM (2018). Genome-wide analysis and expression profiling of PP2C clade D under saline and alkali stresses in wild soybean and Arabidopsis. Protoplasma 255, 643-654.
DOI URL |
| [14] |
Chen CJ, Chen H, Zhang Y, Thomas HR, Frank MH, He YH, Xia R (2020). TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13, 1194-1202.
DOI URL |
| [15] |
Clark K, Franco JY, Schwizer S, Pang Z, Hawara E, Liebrand T (2018). An effector from the huanglongbing-associated pathogen targets citrus proteases. Nat Commun 9, 1718.
DOI URL |
| [16] |
Dando PM, Fortunato M, Strand GB, Smith TS, Barrett AJ (2003). Pyroglutamyl-peptidase I: cloning, sequencing, and characterisation of the recombinant human enzyme. Protein Expr Purif 28, 111-119.
DOI URL |
| [17] |
Earnshaw WC, Martins LM, Kaufmann SH (1999). Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem 68, 383-424.
PMID |
| [18] |
Finn RD, Penelope C, Eberhardt RY, Eddy SR, Jaina M, Mitchell AL, Potter SC, Marco P, Matloob Q, Amaia SV (2016). The Pfam protein families database: towards a more sustainable future. Nucleic Acids Res 44, D279-D285.
DOI URL |
| [19] |
Grudkowska M, Zagdanska B (2004). Multifunctional role of plant cysteine proteinases. Acta Biochim Pol 51, 609-624.
DOI URL |
| [20] |
Hara-Nishimura I (1995). Vacuolar processing enzyme responsible for maturation of vacuolar proteins. Seikagaku 67, 372-377.
PMID |
| [21] |
Hatsugai N, Kuroyanagi M, Nishimura M, Hara-Nishimura I (2006). A cellular suicide strategy of plants: vacuole- mediated cell death. Apoptosis 11, 905-911.
PMID |
| [22] |
Kuroyanagi M, Yamada K, Hatsugai N, Kondo M, Nishimura M, Hara-Nishimura I (2005). Vacuolar processing enzyme is essential for mycotoxin-induced cell death in Arabidopsis thaliana. J Biol Chem 280, 32914-32920.
PMID |
| [23] |
Li ZG, Zeng HZ, Ao PX, Gong M (2014). Lipid response to short-term chilling shock and long-term chill hardening in Jatropha curcas L. seedlings. Acta Physiol Plant 36, 2803-2814.
DOI URL |
| [24] |
Liu HJ, Hu MH, Wang Q, Cheng L, Zhang ZB (2018). Role of papain-like cysteine proteases in plant development. Front Plant Sci 9, 1717.
DOI URL |
| [25] |
Megha S, Basu U, Kav NNV (2018). Regulation of low temperature stress in plants by microRNAs. Plant Cell Environ 41, 1-15.
DOI URL |
| [26] |
Montes JM, Melchinger AE (2016). Domestication and breeding of Jatropha curcas L. Trend Plant Sci 21, 1045-1057.
DOI URL |
| [27] |
Niño MC, Kim MS, Kang KK, Cho YG (2020). Genome- wide identification and molecular characterization of cysteine protease genes in rice. Plant Biotechnol Rep 14, 69-87.
DOI URL |
| [28] |
Rawlings ND, Barrett AJ, Bateman A (2010). MEROPS: the peptidase database. Nucl Acids Res 38, D227-D233.
DOI URL |
| [29] | Rawlings ND, Salvesen G (2013). Handbook of Proteolytic Enzymes. Salt Lake City: Academic Press. pp. 1253-1257. |
| [30] |
Richau KH, Kaschani F, Verdoes M, Pansuriya TC, Niessen S, Stüber K, Colby T, Overkleeft HS, Bogyo M, Van der Hoorn RAL (2012). Subclassification and biochemical analysis of plant papain-like cysteine proteases displays subfamily-specific characteristics. Plant Physiol 158, 1583-1599.
DOI URL |
| [31] |
Shimada T, Yamada K, Kataoka M, Nakaune S, Koumoto Y, Kuroyanagi M, Tabata S, Kato T, Shinozaki K, Seki M, Kobayashi M, Kondo M, Nishimura M, Hara-Nishimura I (2003). Vacuolar processing enzymes are essential for proper processing of seed storage proteins in Arabidopsis thaliana. J Biol Chem 278, 32292-32299.
PMID |
| [32] |
Stroeher VL, Maclagan JL, Good AG (1997). Molecular cloning of a Brassica napus cysteine protease gene inducible by drought and low temperature stress. Physiol Plant 101, 389-397.
DOI URL |
| [33] |
Vorster BJ, Cullis CA, Kunert KJ (2019). Plant vacuolar processing enzymes. Front Plant Sci 10, 479.
DOI PMID |
| [34] |
Wang HB, Gong M, Guo JY, Xin H, Gao Y, Liu C, Dai DQ, Tang LZ (2018a). Genome-wide Identification of Jatropha curcas MAPK, MAPKK, and MAPKKK gene families and their expression profile under cold stress. Sci Rep 8, 16163.
DOI URL |
| [35] |
Wang HB, Zou ZR, Wang SS, Gong M (2013a). Global analysis of transcriptome responses and gene expression profiles to cold stress of Jatropha curcas L. PLoS One 8, e82817.
DOI URL |
| [36] | Wang SS, Wang HB, Gong M (2013b). Identification of microRNAs involved in chilling response by deep sequencing of Jatropha curcas L. small RNAs at the global genome level. In: Proceedings of the 21st European Biomass Conference and Exhibition. Copenhagen: European Union. pp. 356-363. |
| [37] |
Wang W, Zhou XM, Xiong HX, Mao WY, Zhao P, Sun MX (2018b). Papain-like and legumain-like proteases in rice: genome-wide identification, comprehensive gene feature characterization and expression analysis. BMC Plant Biol 18, 87.
DOI PMID |
| [38] |
Wani SH, Kumar V, Khare T, Tripathi P, Shah T, Ramakrishna C, Aglawe S, Mangrauthia SK (2020). miRNA applications for engineering abiotic stress tolerance in plants. Biologia 75, 1063-1081.
DOI URL |
| [39] |
Wilkinson KD, Laleli-Sahin E, Urbauer J, Larsen CN, Shih GH, Haas AL, Walsh STR, Wand AJ (1999). The binding site for UCH-L3 on ubiquitin: mutagenesis and NMR studies on the complex between ubiquitin and UCH- L3. J Mol Biol 291, 1067-1077.
PMID |
| [40] |
Wu PZ, Zhou CP, Cheng SF, Wu ZY, Lu WJ, Han JL, Chen YB, Chen Y, Ni PX, Wang Y, Xu X, Huang Y, Song C, Wang ZW, Shi N, Zhang XD, Fang XH, Yang Q, Jiang HW, Chen YP, Li MR, Wang Y, Chen F, Wang J, Wu GJ (2015). Integrated genome sequence and linkage map of physic nut ( Jatropha curcas L), a biodiesel plant. Plant J 81, 810-821.
DOI URL |
| [41] |
Zang QW, Wang CX, Li XY, Guo ZA, Jing RL, Zhao J, Chang XP (2010). Isolation and characterization of a gene encoding a polyethylene glycol-induced cysteine protease in common wheat. J Biosci 35, 379-388.
DOI URL |
| [42] |
Zhang X, Pan BZ, Chen MS, Chen W, Li J, Xu ZF, Liu CN (2019). JCDB: a comprehensive knowledge base for Jatropha curcas, an emerging model for woody energy plants. BMC Genomics 20, 958.
DOI PMID |
| [43] |
Zheng L, Chen SS, Xie LH, Lu ZC, Liu MY, Han XJ, Qiao GR, Jiang J, Zhuo RY, Qiu WM, He ZQ (2018). Overexpression of cysteine protease gene from Salix matsudana enhances salt tolerance in transgenic Arabidopsis. Environ Exp Bot 147, 53-62.
DOI URL |
| [44] |
Zou Z, Huang QX, Xie GS, Yang LF (2018). Genome-wide comparative analysis of papain-like cysteine protease family genes in castor bean and physic nut. Sci Rep 8, 331.
DOI URL |
| [1] | 杨莉, 曲茜彤, 陈子航, 邹婷婷, 王全华, 王小丽. 菠菜AT-hook基因家族鉴定与表达谱分析[J]. 植物学报, 2025, 60(3): 377-392. |
| [2] | 顾家琦, 朱福慧, 谢沛豪, 孟庆营, 郑颖, 张献龙, 袁道军. 棉属光敏色素PHY基因家族的全基因组鉴定与驯化选择分析[J]. 植物学报, 2024, 59(1): 34-53. |
| [3] | 孙福辉, 方慧仪, 温小蕙, 张亮生. 马银花MADS-box基因家族系统进化与表达分析[J]. 植物学报, 2023, 58(3): 404-416. |
| [4] | 张慧, 梁红凯, 智慧, 张林林, 刁现民, 贾冠清. 谷子β-胡萝卜素异构酶家族基因的表达与变异分析[J]. 植物学报, 2023, 58(1): 34-50. |
| [5] | 王菲菲, 周振祥, 洪益, 谷洋洋, 吕超, 郭宝健, 朱娟, 许如根. 大麦NF-YC基因鉴定及在盐胁迫下的表达分析[J]. 植物学报, 2023, 58(1): 140-149. |
| [6] | 王小龙,刘凤之,史祥宾,王孝娣,冀晓昊,王志强,王宝亮,郑晓翠,王海波. 葡萄NCED基因家族进化及表达分析[J]. 植物学报, 2019, 54(4): 474-485. |
| [7] | 唐康,杨若林. 大豆蛋白编码基因起源与进化[J]. 植物学报, 2019, 54(3): 316-327. |
| [8] | 郑军, 乔玲, 赵佳佳, 乔麟轶, 张世昌, 常建忠, 汤才国, 杨三维. 粗山羊草CCT家族基因序列分析及激素响应[J]. 植物学报, 2017, 52(2): 188-201. |
| [9] | 吴家富, 杨博文, 向珣朝, 许亮, 颜李梅. 不同水稻种质在不同生育期耐盐鉴定的差异[J]. 植物学报, 2017, 52(1): 77-88. |
| [10] | 包颖, 郭昌锋, 陈少华, 刘梅. 植物查尔酮合成酶超基因家族的分子进化[J]. 植物学报, 2015, 50(1): 55-71. |
| [11] | 李方正, 杨素欣, 吴春霞, 魏海超, 曲瑞莲, 冯献忠. 大豆KNOX基因家族的结构和表达分析[J]. 植物学报, 2012, 47(3): 236-247. |
| [12] | 黎颖;左开井;唐克轩. 植物GH3 基因家族的功能研究概况[J]. 植物学报, 2008, 25(05): 507-515. |
| [13] | 张剑 徐桂霞 薛皓月 胡瑾. 植物进化发育生物学的形成与研究进展[J]. 植物学报, 2007, 24(01): 1-30. |
| [14] | 郭尚敬 陈娜 孟庆伟. 叶绿体小分子量热激蛋白介绍[J]. 植物学报, 2005, 22(02): 223-230. |
| [15] | 王昕 种康. 植物小G 蛋白功能的研究进展[J]. 植物学报, 2005, 22(01): 1-10. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||