

植物学报 ›› 2021, Vol. 56 ›› Issue (5): 533-543.DOI: 10.11983/CBB21012 cstr: 32102.14.CBB21012
闫一皓1,2, 王頔1,*( ), 李静一1, 张文颖1, 郝渊鹏1, 夏菲1, 李慧1, 白红彤1, 石雷1,*(
), 李静一1, 张文颖1, 郝渊鹏1, 夏菲1, 李慧1, 白红彤1, 石雷1,*( )
)
收稿日期:2021-01-18
接受日期:2021-04-19
出版日期:2021-09-01
发布日期:2021-08-31
通讯作者:
王頔,石雷
作者简介:shilei@ibcas.ac.cn基金资助:
Yihao Yan1,2, Di Wang1,*( ), Jingyi Li1, Wenying Zhang1, Yuanpeng Hao1, Fei Xia1, Hui Li1, Hongtong Bai1, Lei Shi1,*(
), Jingyi Li1, Wenying Zhang1, Yuanpeng Hao1, Fei Xia1, Hui Li1, Hongtong Bai1, Lei Shi1,*( )
)
Received:2021-01-18
Accepted:2021-04-19
Online:2021-09-01
Published:2021-08-31
Contact:
Di Wang,Lei Shi
摘要: 牛至精油中的主要成分香芹酚和百里香酚具有较强的抑菌活性和替代抗生素的良好潜力。为了筛选有价值的遗传变异, 构建了牛至(Origanum vulgare)的60Co-γ射线育种体系, 利用固相微萃取联合气相色谱-质谱方法进行挥发性成分测定, 并通过主成分分析和层次聚类等多元分析法, 深入探讨60Co-γ射线对牛至M1代形态特征、腺毛密度和腺毛大小以及挥发性成分的影响。结果表明, 牛至种子的半致死剂量为16.39 Gy; 辐照M1代植株的株高、茎粗、分枝数、叶长和叶宽均发生变化, 筛选到多株形态突变体; 同时, 腺毛密度和腺毛大小发生多种变化, 从163株存活株中筛选出25株腺毛密度与大小均显著增大的单株;60Co-γ射线处理对牛至挥发性成分的种类影响相对较小, 主要影响挥发性成分的含量, 并可诱导5种化学型, 即香芹酚型、百里香酚型、γ-萜品烯型、β-石竹烯型和大根香叶烯型; 最终筛选获得6株香芹酚和百里香酚含量升高的突变单株。研究证实了60Co-γ射线辐照可作为牛至育种的一种有效诱变手段, 适宜辐照剂量为20 Gy。研究为牛至优良种质资源的选育提供了基础数据和新途径。
闫一皓, 王頔, 李静一, 张文颖, 郝渊鹏, 夏菲, 李慧, 白红彤, 石雷. 60Co-γ射线辐照种子对牛至形态与挥发性成分的影响. 植物学报, 2021, 56(5): 533-543.
Yihao Yan, Di Wang, Jingyi Li, Wenying Zhang, Yuanpeng Hao, Fei Xia, Hui Li, Hongtong Bai, Lei Shi. Effects of 60Co-γ Ray Radiation on Morphology and Volatile Components of Origanum vulgare. Chinese Bulletin of Botany, 2021, 56(5): 533-543.
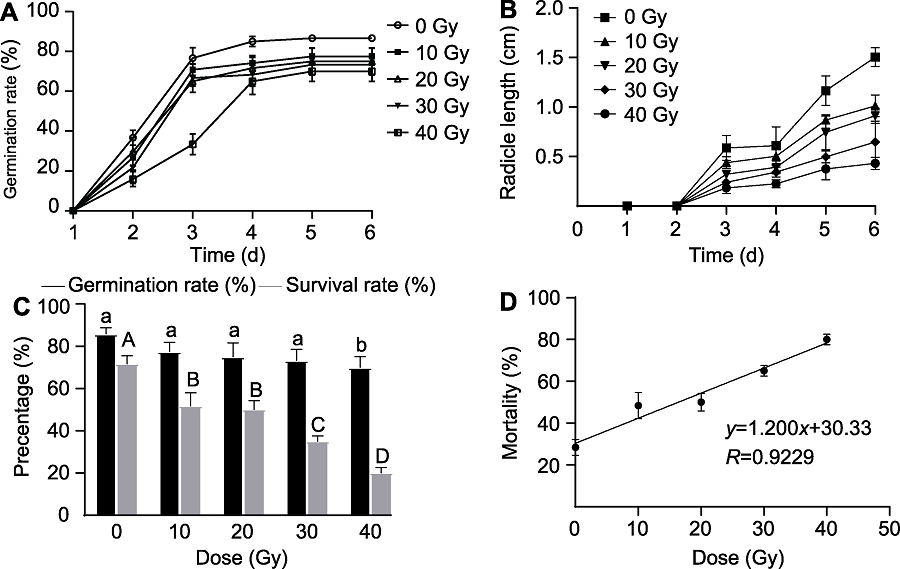
图1 不同剂量60Co-γ辐照后牛至种子的萌发与成苗情况 (A) 萌发率; (B) 胚根长度; (C) 萌发率和存活率; (D) 剂量效应曲线。不同小写字母表示各处理间萌发率差异显著(P<0.05)。不同大写字母表示各处理间存活率差异显著(P<0.05)。
Figure 1 Germination and seedling formation of oregano seeds treated with different doses of 60Co-γ radiation (A) Germination rate; (B) Radicle length; (C) Germination rate and survival rate; (D) Dose effect curve. Different lowercase letters indicate significant differences in germination rate among different treatments (P<0.05). Different capital letters indicate significant differences in survival rate among different treatments (P<0.05).
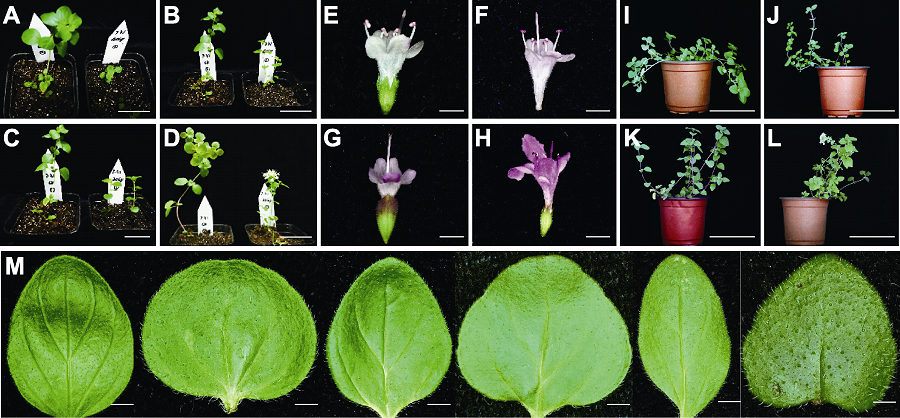
图2 60Co-γ射线辐照处理后牛至的形态变异 (A)-(D) 苗期突变: 矮化(A)、基部分枝(B)、节间距缩短(C)和花期提前(D); (E)-(H) 花色变异: 对照株(CK)花瓣粉白色(E)、变异株20-2花瓣粉色(F)、10-11花瓣白紫相间(G)和10-4花瓣紫色(H); (I)-(L) 株型变异: CK匍匐(I)、突变株20-13直立(J)、30-5直立(K)和10-19直立(L); (M) 叶片形态突变, 从左至右依次为CK、10-4、20-2、20-19、30-8和40-6。Bars=2 mm
Figure 2 Phenotypic variation of oregano individual plants after 60Co-γ radiation (A)-(D) Mutation at seedling stage: dwarfing (A), basal branching (B), shortening of internode spacing (C), and early flowering (D); (E)-(H) Variation of flower color: the petals were pink-white in control (CK) (E), pink in mutant 20-2 (F), white and violet in mutant 10-11 (G), and violet in mutant 10-4 (H); (I)-(L) Variation of plant shape: the plant shape changed from creepers of CK (I), to up straight of 20-13 (J), 30-5 (K), and 10-19 (L); (M) Variation of leaf morphology, from left to right were CK, 10-4, 20-2, 20-19, 30-8, and 40-6. Bars=2 mm
| Dose (Gy) | Height (cm) | Stem diameter (mm) | Number of branches | Leaf length (cm) | Leaf width (cm) | Leaf length and width ratio |
|---|---|---|---|---|---|---|
| 0 | 28.10±3.87 a | 0.87±0.84 b | 4.20±0.79 bc | 2.14±0.22 b | 1.49±0.20 b | 1.43±0.03 a |
| 10 | 18.60±2.99 b | 0.94±0.14 b | 4.51±1.36 b | 2.36±0.16 a | 1.58±0.18 b | 1.49±0.13 a |
| 20 | 20.00±2.83 b | 0.97±0.05 b | 6.02±1.25 a | 2.31±0.12 ab | 1.76±0.19 a | 1.31±0.06 b |
| 30 | 19.10±2.23 b | 1.06±0.09 b | 4.00±0.94 b | 2.03±0.17 ab | 1.49±0.12 b | 1.36±0.10 b |
| 40 | 15.50±2.23 c | 1.10±0.01 a | 3.02±0.82 c | 1.85±0.24 c | 1.29±0.19 c | 1.43±0.08 a |
表1 不同剂量60Co-γ射线辐照对牛至分枝数和叶形的影响(平均值±标准差)
Table 1 Effects of different doses of 60Co-γ radiation on branch number and leaf shape of Origanum vulgare (means±SD)
| Dose (Gy) | Height (cm) | Stem diameter (mm) | Number of branches | Leaf length (cm) | Leaf width (cm) | Leaf length and width ratio |
|---|---|---|---|---|---|---|
| 0 | 28.10±3.87 a | 0.87±0.84 b | 4.20±0.79 bc | 2.14±0.22 b | 1.49±0.20 b | 1.43±0.03 a |
| 10 | 18.60±2.99 b | 0.94±0.14 b | 4.51±1.36 b | 2.36±0.16 a | 1.58±0.18 b | 1.49±0.13 a |
| 20 | 20.00±2.83 b | 0.97±0.05 b | 6.02±1.25 a | 2.31±0.12 ab | 1.76±0.19 a | 1.31±0.06 b |
| 30 | 19.10±2.23 b | 1.06±0.09 b | 4.00±0.94 b | 2.03±0.17 ab | 1.49±0.12 b | 1.36±0.10 b |
| 40 | 15.50±2.23 c | 1.10±0.01 a | 3.02±0.82 c | 1.85±0.24 c | 1.29±0.19 c | 1.43±0.08 a |
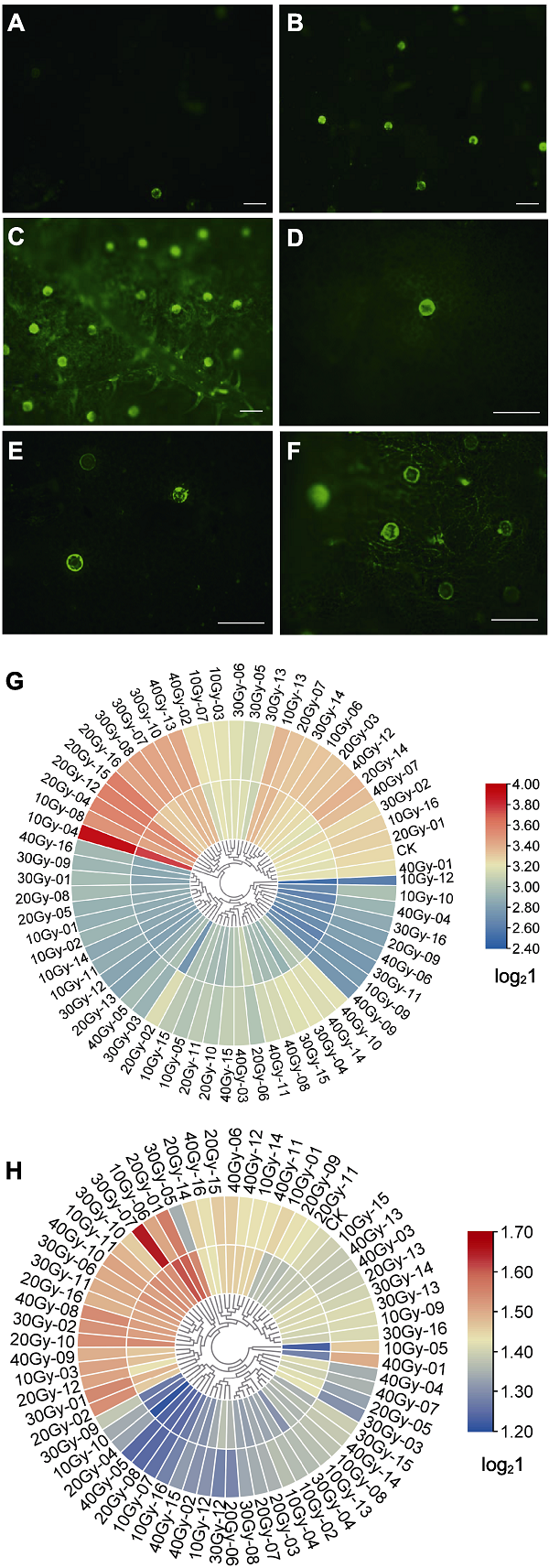
图3 不同剂量60Co-γ辐照对牛至存活单株腺毛密度和腺毛大小的影响 (A)-(F) 荧光显微镜下观察到的腺毛密度变异(bars=200 μm); (G) 腺毛密度聚类热图; (H) 腺毛大小聚类热图, 内环表示近轴端, 外环表示远轴端。
Figure 3 Effects of different doses of 60Co-γ radiation on the density and size of glandular hair of survivals (A)-(F) The varied density of glandular hairs under fluorescence microscopy (bars=200 μm); (G) Cluster thermography of glandular hair density; (H) Cluster thermography of glandular hair size, the inner ring represents the adaxial end, and the outer ring represents the abaxial end.
| Dose (Gy) | Glandular hair density (·cm-2) | Glandular hair size (·mm-2) | ||
|---|---|---|---|---|
| Adaxial end | Abaxial end | Adaxial end | Abaxial end | |
| 0 | 163.53±11.43 a | 182.22±6.07 a | 0.33±0.00 c | 0.37±0.01 b |
| 10 | 138.56±15.89 b | 155.04±14.09 b | 0.34±0.02 bc | 0.37±0.01 b |
| 20 | 160.15±8.23 a | 182.18±12.68 a | 0.42±0.11 a | 0.44±0.15 a |
| 30 | 137.49±5.47 b | 156.79±20.84 b | 0.39±0.01 ab | 0.39±0.01 b |
| 40 | 123.11±2.89 b | 147.89±6.73 b | 0.37±0.00 b | 0.38±0.01 b |
表2 不同剂量60Co-γ射线辐照对牛至腺毛密度和大小的影响(平均值±标准差)
Table 2 Effects of different doses of 60Co-γ radiation on density and size of glandular hair of oregano (means±SD)
| Dose (Gy) | Glandular hair density (·cm-2) | Glandular hair size (·mm-2) | ||
|---|---|---|---|---|
| Adaxial end | Abaxial end | Adaxial end | Abaxial end | |
| 0 | 163.53±11.43 a | 182.22±6.07 a | 0.33±0.00 c | 0.37±0.01 b |
| 10 | 138.56±15.89 b | 155.04±14.09 b | 0.34±0.02 bc | 0.37±0.01 b |
| 20 | 160.15±8.23 a | 182.18±12.68 a | 0.42±0.11 a | 0.44±0.15 a |
| 30 | 137.49±5.47 b | 156.79±20.84 b | 0.39±0.01 ab | 0.39±0.01 b |
| 40 | 123.11±2.89 b | 147.89±6.73 b | 0.37±0.00 b | 0.38±0.01 b |
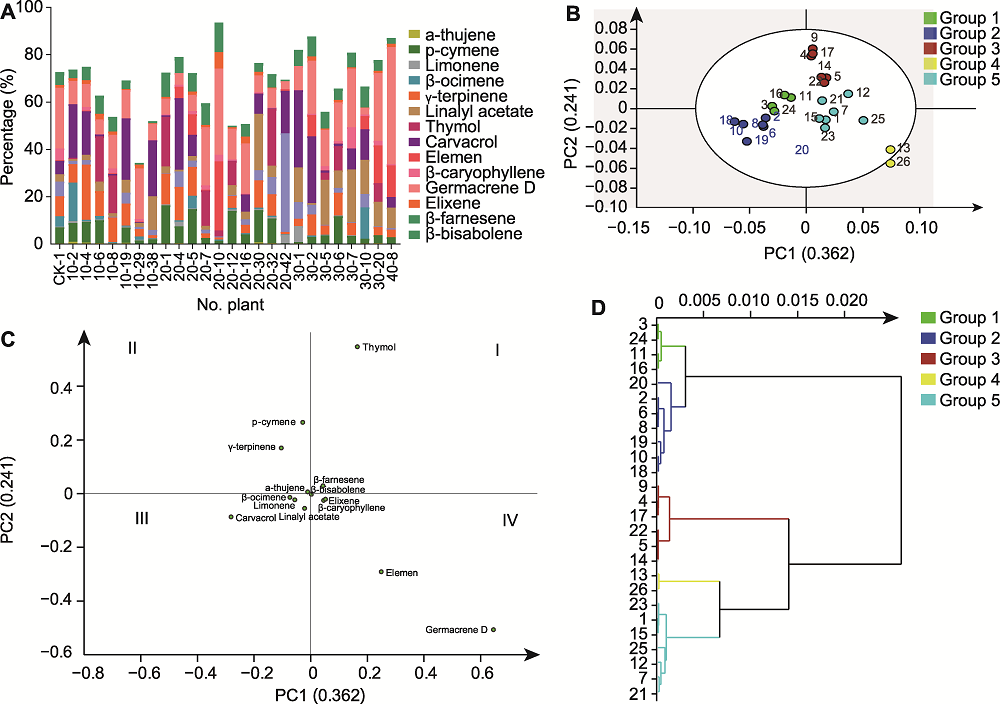
图4 25株60Co-γ辐照变异株的挥发性成分分析 (A) 成分占比分析; (B) 主成分得分图; (C) 主成分因子载荷图; (D) 层次聚类分析
Figure 4 Analysis of volatile components of 25 mutants after 60Co-γ irradiation (A) Analysis of proportion of components; (B) PCA score chart; (C) PCA factor load chart; (D) Hierarchical cluster analysis
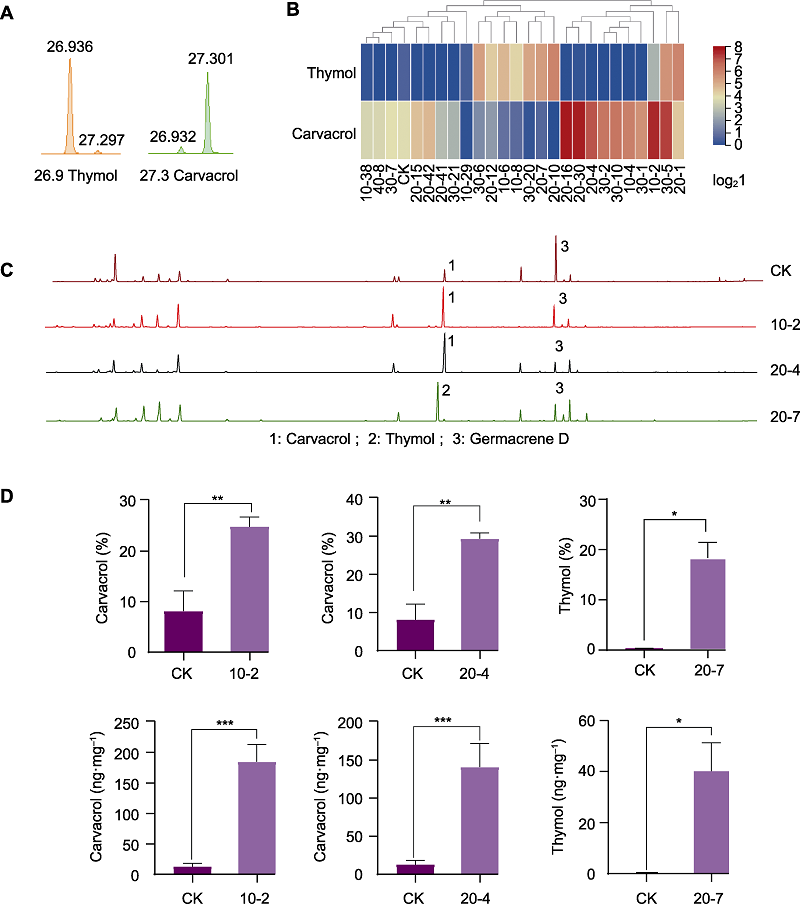
图5 60Co-γ辐照后筛选成分优株的香芹酚和百里香酚含量分析 (A) 香芹酚与百里香酚的合成竞争; (B) 25株辐照变异株香芹酚与百里香酚含量热图分析, 红色方向代表数值增大, 蓝色方向代表数值减小, 所有数据均经过对数转换; (C) 对照与筛选出的成分优株色谱图; (D) 对照与筛选出的成分优株香芹酚及百里香酚含量对比。每株叶片混样, 重复测量3次取平均值。CK: 对照。* 0.05水平差异显著, ** 0.01水平差异极显著, *** 0.001水平差异极显著
Figure 5 Content analysis of carvacrol and thymol in selected superior plants after 60Co-γ radiation (A) Carvacrol competes with thymol in synthesis; (B) Thermogram analysis of carvacrol and thymol content in 25 irradiated plants, the red direction represents an increase in the value and the blue direction represents a decrease in the value, all the data were processed by logarithm; (C) Chromatogram of components between control and treated individuals; (D) Content comparison of carvacrol and thymol between control and treated individuals. The leaves of each sample were mixed and analyzed three times, where the average was taken. CK: Control. * significant differences at the level of 0.05, ** extremely significant differences at the level of 0.01, *** extremely significant differences at the level of 0.001
| [1] |
董燕梅, 张文颖, 凌正一, 李靖锐, 白红彤, 李慧, 石雷 (2020). 转录因子调控植物萜类化合物生物合成研究进展. 植物学报 55, 340-350.
DOI |
| [2] | 郝渊鹏, 李静一, 杨瑞, 李慧, 白红彤, 石雷 (2020). 芳香植物精油的抗菌性及在动物生产中的应用. 植物学报 55, 644-657. |
| [3] | 黄桂丹 (2016). 60Co-γ射线辐射育种研究进展. 林业与环境科学 32(2), 107-111. |
| [4] | 李好勋, 王国栋 (2015). 植物腺毛次生代谢产物生物合成的研究进展. 中国科学: 生命科学 45, 557-568. |
| [5] | 刘录祥, 郭会君, 赵林姝, 李军辉, 古佳玉, 赵世荣, 王晶 (2009). 植物诱发突变技术育种研究现状与发展前景. 核农学报 23, 1001-1007. |
| [6] | 王晶, 刘录祥, 赵世荣, 杨俊诚, 郭会君, 赵林姝, 陈文华 (2003). 7Li离子束诱变紫松果菊的生物效应研究初报. 核农学报 17, 405-408. |
| [7] | 余蓉培, 李杨, 李东, 詹选怀, 石雷 (2015). 荚果蕨绿色球状体对60Coγ射线的辐射敏感性. 植物学报 50, 565-572. |
| [8] | 昝俊峰, 陈艳霞, 陈平, 刘焱文, 刘军锋 (2013). 不同产地牛至挥发性成分的SPME/GC/MS法分析. 时珍国医国药 24, 37-39. |
| [9] | 张婷婷, 马嘉伟, 王路尧, 唐克轩, 李杉, 赵静雅 (2018). 过量表达黄烷酮3-羟化酶基因(AaF3H)提高青蒿中青蒿素的含量. 生物技术进展 8, 55-62. |
| [10] | Arabaci T, Çelenk S, Özcan T, Martin E, Yazici T, Açar M, Üzel D, Dirmenci T (2021). Homoploid hybrids of Origanum (Lamiaceae) in Turkey: morphological and molecular evidence for a new hybrid. Plant Biosyst Int J Deal Asp Plant Biol 155, 470-482. |
| [11] | Bouyahya A, Zengin G, Belmehdi O, Bourais I, Chamkhi I, Taha D, Benali T, Dakka N, Bakri Y (2020). Origanum compactum Benth., from traditional use to biotechnological applications. J Food Biochem 44, e13251. |
| [12] | Eilert U (1987). Elicitation: methodology and aspects of application. In: Constabel F, Vasil IK, eds. Cell Culture in Phytochemistry. Amsterdam: Elsevier Science. pp. 153-196. |
| [13] |
Elshafie HS, Armentano MF, Carmosino M, Bufo SA, De Feo V, Camele I (2017). Cytotoxic activity of Origanum vulgare L. on hepatocellular carcinoma cell line HepG2 and evaluation of its biological activity. Molecules 22, 1435.
DOI URL |
| [14] |
Esnault MA, Legue F, Chenal C (2010). Ionizing radiation: advances in plant response. Environ Exp Bot 68, 231-237.
DOI URL |
| [15] |
Fan XT, Thayer DW, Sokora KJB (2004). Changes in growth and antioxidant status of alfalfa sprouts during sprouting as affected by gamma irradiation of seeds. J Food Prot 67, 561-566.
DOI URL |
| [16] |
Fikry S, Khalil N, Salama O (2019). Chemical profiling, biostatic and biocidal dynamics of Origanum vulgare L. essential oil. AMB Express 9, 41.
DOI URL |
| [17] |
Gavalas NP, Kalburtji KL, Kokkini S, Mamolos AP, Veresoglou DS (2011). Ecotypic variation in plant characteristics for Origanum vulgare subsp. hirtum populations. Biochem Syst Ecol 39, 562-569.
DOI URL |
| [18] |
Jan S, Parween T, Siddiqi TO, Mahmooduzzafar (2012). Effect of gamma radiation on morphological, biochemical, and physiological aspects of plants and plant products. Environ Rev 20, 17-39.
DOI URL |
| [19] |
Kazama Y, Ishii K, Hirano T, Wakana T, Yamada M, Ohbu S, Abe T (2017). Different mutational function of low- and high-linear energy transfer heavy-ion irradiation demonstrated by whole-genome resequencing of Arabidopsis mutants. Plant J 92, 1020-1030.
DOI URL |
| [20] |
Koseki PM, Villavicencio ALCH, Brito MS, Nahme LC, Sebastião KI, Rela PR, Almeida-Muradian LB, Mancini- Filho J, Freitas PCD (2002). Effects of irradiation in medicinal and eatable herbs. Radiat Phys Chem 63, 681-684.
DOI URL |
| [21] |
Kowalczyk A, Przychodna M, Sopata S, Bodalska A, Fecka I (2020). Thymol and thyme essential oil-new insights into selected therapeutic applications. Molecules 25, 4125.
DOI URL |
| [22] |
Leyva-López N, Gutiérrez-Grijalva EP, Vazquez-Olivo G, Heredia JB (2017). Essential oils of oregano: biological activity beyond their antimicrobial properties. Molecules 22, 989.
DOI URL |
| [23] |
Lukas B, Schmiderer C, Novak J (2015). Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 119, 32-40.
DOI URL |
| [24] |
Majdi M, Malekzadeh-Mashhady A, Maroufi A, Crocoll C (2017). Tissue-specific gene-expression patterns of genes associated with thymol/carvacrol biosynthesis in thyme ( Thymus vulgaris L.) and their differential changes upon treatment with abiotic elicitors. Plant Physiol Bioch 115, 152-162.
DOI URL |
| [25] |
Morshedloo MR, Mumivand H, Craker LE, Maggi F (2018). Chemical composition and antioxidant activity of essential oils in Origanum vulgare subsp. gracile at different phenological stages and plant parts. J Food Process Preserv 42, e13516.
DOI URL |
| [26] |
Oladosu Y, Rafii MY, Abdullah N, Hussin G, Ramli A, Rahim HA, Miah G, Usman M (2016). Principle and application of plant mutagenesis in crop improvement: a review. Biotechnol Biotechnol Equip 30, 1-16.
DOI URL |
| [27] |
Oniga I, Puscas C, Silaghi-Dumitrescu R, Olah NK, Sevastre B, Marica R, Marcus I, Sevastre-Berghian AC, Benedec D, Pop CE, Hanganu D (2018). Origanum vulgare ssp. vulgare: chemical composition and biological studies. Molecules 23, 2077.
DOI URL |
| [28] |
Pan QF, Mustafa NR, Tang KX, Choi YH, Verpoorte R (2016). Monoterpenoid indole alkaloids biosynthesis and its regulation in Catharanthus roseus: a literature review from genes to metabolites. Phytochem Rev 15, 221-250.
DOI URL |
| [29] |
Rajput JD, Bagul SD, Pete UD, Zade CM, Padhye SB, Bendre RS (2018). Perspectives on medicinal properties of natural phenolic monoterpenoids and their hybrids. Mol Divers 22, 225-245.
DOI URL |
| [30] |
Sarrou E, Tsivelika N, Chatzopoulou P, Tsakalidis G, Menexes G, Mavromatis A (2017). Conventional breeding of Greek oregano ( Origanum vulgare ssp. hirtum) and development of improved cultivars for yield potential and essential oil quality. Euphytica 213, 104.
DOI URL |
| [31] |
Seo HY, Kim JH, Song HP, Kim DH, Byun MW, Kwon JH, Kim KS (2007). Effects of gamma irradiation on the yields of volatile extracts of Angelica gigas Nakai. Radiat Phys Chem 76, 1869-1874.
DOI URL |
| [32] |
Sharifi-Rad M, Varoni EM, Iriti M, Martorell M, Setzer WN, del Mar Contreras M, Salehi B, Soltani-Nejad A, Rajabi S, Tajbakhsh M, Sharifi-Rad J (2018). Carvacrol and human health: a comprehensive review. Phytother Res 32, 1675-1687.
DOI PMID |
| [33] |
Tang KX, Shen Q, Yan TX, Fu XQ (2014). Transgenic approach to increase artemisinin content in Artemisia annua L. Plant Cell Rep 33, 605-615.
DOI URL |
| [34] |
Topuz A, Ozdemir F (2003). Influences of γ-irradiation and storage on the carotenoids of sun-dried and dehydrated paprika. J Agric Food Chem 51, 4972-4977.
DOI URL |
| [35] |
Vardhan PV, Shukla LI (2017). Gamma irradiation of medicinally important plants and the enhancement of secondary metabolite production. Int J Radiat Biol 93, 967-979.
DOI PMID |
| [1] | 秦波 尤进茂 汪汉卿 鲁润华 王敏. 长叶水麻挥发性化学成分研究[J]. 植物学报, 2000, 17(05): 435-438. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||