

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (1): 64-71.DOI: 10.11983/CBB18010 cstr: 32102.14.CBB18010
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Yang Xian,Xin Dong,Xiaoman Xie( ),Dan Wu,Biao Han,Yan Wang
),Dan Wu,Biao Han,Yan Wang
Received:2018-01-11
Accepted:2018-08-01
Online:2019-01-01
Published:2019-07-31
Contact:
Xiaoman Xie
Yang Xian,Xin Dong,Xiaoman Xie,Dan Wu,Biao Han,Yan Wang. Effect of Conservation Conditions on Restricting Conservation of Acer rubrum cv. ‘Somerset’[J]. Chinese Bulletin of Botany, 2019, 54(1): 64-71.
| Treatment | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photoperiod (h) | 24 | 16 | 12 | 8 | 24 | 16 | 12 | 8 | 16 | 16 | 16 | 16 |
| Light intensity (μmol·m-2·s-1) | 62.50 | 62.50 | 62.50 | 62.50 | 31.25 | 31.25 | 31.25 | 31.25 | 62.50 | 62.50 | 62.50 | 31.25 |
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 15 | 8 | 25/18* | 25/18* |
Table 1 Experimental design of in vitro conservation of Acer rubrum L. cv. ‘Somerset’
| Treatment | T1 | T2 | T3 | T4 | T5 | T6 | T7 | T8 | T9 | T10 | T11 | T12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Photoperiod (h) | 24 | 16 | 12 | 8 | 24 | 16 | 12 | 8 | 16 | 16 | 16 | 16 |
| Light intensity (μmol·m-2·s-1) | 62.50 | 62.50 | 62.50 | 62.50 | 31.25 | 31.25 | 31.25 | 31.25 | 62.50 | 62.50 | 62.50 | 31.25 |
| Temperature (°C) | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 25 | 15 | 8 | 25/18* | 25/18* |
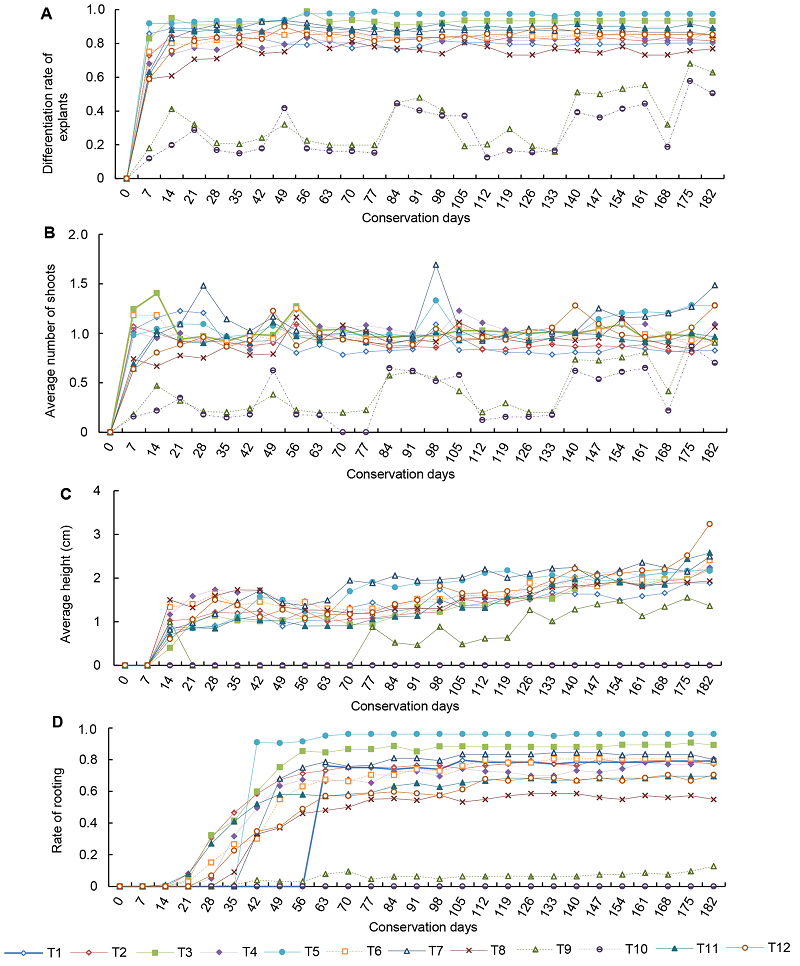
Figure 1 Growth curve of Acer rubrum cv. ‘Somerset’ (A) Differentiation rate of explants; (B) Average number of shoots; (C) Average height; (D) Rate of rooting. T1-T12 see Table 1.
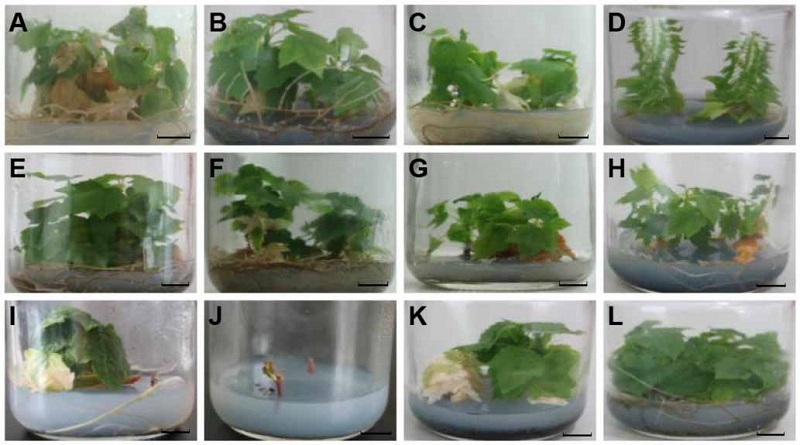
Figure 2 In vitro conservation for 182 days of Acer rubrum cv. ‘Somerset’ (A) T1; (B) T2; (C) T3; (D) T4; (E) T5; (F) T6; (G) T7; (H) T8; (I) T9; (J) T10; (K) T11; (L) T12. T1-T12 see Table 1. Bars=1 cm
| Conservation days | Significance testing of P and LI | Significance testing of Tm | Significance testing of DTV and LI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | LI | P×LI | Tm | DTV | LI | DTV×LI | ||||
| Differentiation rate of explants | 7 | 0** | 0.018* | 0.002** | 0** | 0.05 | 0.84 | 0.589 | ||
| 14 | 0** | 0.004** | 0.036* | 0.005** | 0.914 | 0.009** | 0.115 | |||
| 56 | 0.002** | 0.075 | 0.001** | 0** | 0.771 | 0.283 | 0.302 | |||
| 182 | 0.297 | 0.907 | 0.01* | 0** | 0.322 | 0.86 | 0.564 | |||
| Average number of shoots | 7 | 0** | 0** | 0** | 0** | 0** | 0.695 | 0.248 | ||
| 14 | 0** | 0.003** | 0.002** | 0.009** | 0.013* | 0.772 | 0.008** | |||
| 28 | 0** | 0.236 | 0** | 0** | 0.8 | 0.488 | 0.479 | |||
| 49 | 0.017* | 0.248 | 0.033* | 0** | 0.003** | 0.244 | 0.563 | |||
| 98 | 0.129 | 0.113 | 0.237 | 0.001** | 0.036* | 0.209 | 0.574 | |||
| 147 | 0.167 | 0.011* | 0.193 | 0.006** | 0.383 | 0.217 | 0.992 | |||
| 182 | 0.057 | 0.001** | 0.008** | 0.131 | 0.016* | 0.03* | 0.063 | |||
| Average height | 14 | 0.589 | 0.516 | 0.751 | 0.081 | 0.071 | 0.641 | 0.680 | ||
| 35 | 0.113 | 0.322 | 0.942 | 0** | 0.845 | 0.077 | 0.744 | |||
| 91 | 0.021* | 0** | 0.004** | 0.002** | 0.464 | 0** | 0.670 | |||
| 126 | 0.733 | 0.002** | 0.039* | 0.004** | 0.636 | 0.016* | 0.422 | |||
| 154 | 0.207 | 0.291 | 0.093 | 0.003** | 0.646 | 0.549 | 0.501 | |||
| 182 | 0.187 | 0.294 | 0.182 | 0** | 0.009** | 0.034* | 0.185 | |||
| Rate of rooting | 21 | 0.004** | 0.041* | 0.213 | 0.037* | 0.541 | 0.025* | 0.504 | ||
| 28 | 0** | 0** | 0.002** | 0.001** | 0.225 | 0.004** | 0.658 | |||
| 35 | 0** | 0** | 0.012* | 0** | 0.409 | 0.008** | 0.885 | |||
| 42 | 0.885 | 0.35 | 0** | 0** | 0.896 | 0.003** | 0.336 | |||
| 49 | 0** | 0.002** | 0** | 0** | 0.018* | 0.006** | 0.415 | |||
| 56 | 0** | 0.001** | 0** | 0** | 0.005** | 0.036* | 0.905 | |||
| 63 | 0** | 0.329 | 0.004** | 0** | 0.01* | 0.49 | 0.432 | |||
| 70 | 0** | 0.156 | 0.001** | 0** | 0.054 | 0.448 | 0.423 | |||
| 182 | 0.015* | 0.284 | 0.007** | 0** | 0.09 | 0.713 | 0.901 | |||
Table 2 The influence of light and temperature on the in vitro conservation of Acer rubrum L. cv. ‘Somerset’
| Conservation days | Significance testing of P and LI | Significance testing of Tm | Significance testing of DTV and LI | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| P | LI | P×LI | Tm | DTV | LI | DTV×LI | ||||
| Differentiation rate of explants | 7 | 0** | 0.018* | 0.002** | 0** | 0.05 | 0.84 | 0.589 | ||
| 14 | 0** | 0.004** | 0.036* | 0.005** | 0.914 | 0.009** | 0.115 | |||
| 56 | 0.002** | 0.075 | 0.001** | 0** | 0.771 | 0.283 | 0.302 | |||
| 182 | 0.297 | 0.907 | 0.01* | 0** | 0.322 | 0.86 | 0.564 | |||
| Average number of shoots | 7 | 0** | 0** | 0** | 0** | 0** | 0.695 | 0.248 | ||
| 14 | 0** | 0.003** | 0.002** | 0.009** | 0.013* | 0.772 | 0.008** | |||
| 28 | 0** | 0.236 | 0** | 0** | 0.8 | 0.488 | 0.479 | |||
| 49 | 0.017* | 0.248 | 0.033* | 0** | 0.003** | 0.244 | 0.563 | |||
| 98 | 0.129 | 0.113 | 0.237 | 0.001** | 0.036* | 0.209 | 0.574 | |||
| 147 | 0.167 | 0.011* | 0.193 | 0.006** | 0.383 | 0.217 | 0.992 | |||
| 182 | 0.057 | 0.001** | 0.008** | 0.131 | 0.016* | 0.03* | 0.063 | |||
| Average height | 14 | 0.589 | 0.516 | 0.751 | 0.081 | 0.071 | 0.641 | 0.680 | ||
| 35 | 0.113 | 0.322 | 0.942 | 0** | 0.845 | 0.077 | 0.744 | |||
| 91 | 0.021* | 0** | 0.004** | 0.002** | 0.464 | 0** | 0.670 | |||
| 126 | 0.733 | 0.002** | 0.039* | 0.004** | 0.636 | 0.016* | 0.422 | |||
| 154 | 0.207 | 0.291 | 0.093 | 0.003** | 0.646 | 0.549 | 0.501 | |||
| 182 | 0.187 | 0.294 | 0.182 | 0** | 0.009** | 0.034* | 0.185 | |||
| Rate of rooting | 21 | 0.004** | 0.041* | 0.213 | 0.037* | 0.541 | 0.025* | 0.504 | ||
| 28 | 0** | 0** | 0.002** | 0.001** | 0.225 | 0.004** | 0.658 | |||
| 35 | 0** | 0** | 0.012* | 0** | 0.409 | 0.008** | 0.885 | |||
| 42 | 0.885 | 0.35 | 0** | 0** | 0.896 | 0.003** | 0.336 | |||
| 49 | 0** | 0.002** | 0** | 0** | 0.018* | 0.006** | 0.415 | |||
| 56 | 0** | 0.001** | 0** | 0** | 0.005** | 0.036* | 0.905 | |||
| 63 | 0** | 0.329 | 0.004** | 0** | 0.01* | 0.49 | 0.432 | |||
| 70 | 0** | 0.156 | 0.001** | 0** | 0.054 | 0.448 | 0.423 | |||
| 182 | 0.015* | 0.284 | 0.007** | 0** | 0.09 | 0.713 | 0.901 | |||
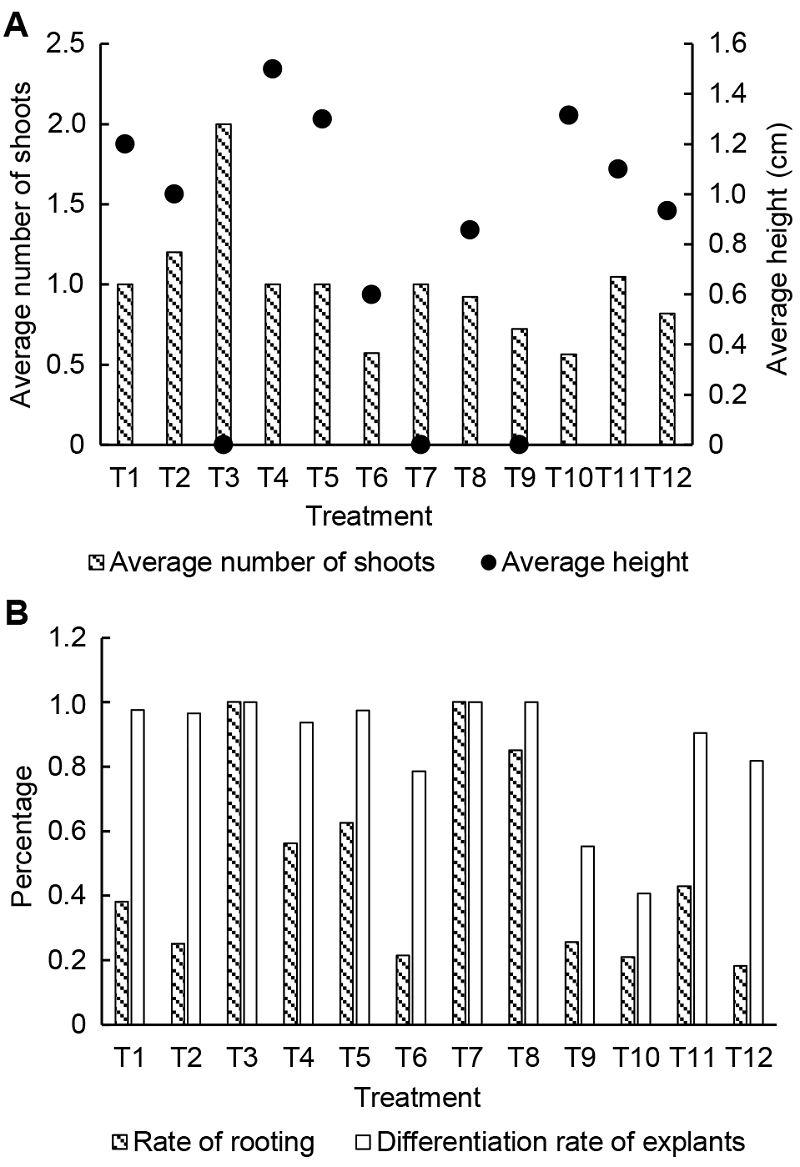
Figure 3 Recovering culture for 28 days of Acer rubrum cv. ‘Somerset’ (A) Average number of shoots and average height; (B) Differentiation rate of explants and rate of rooting. T1-T12 see Table 1.
| 1 |
兰伟, 陈发棣 ( 2010). 植物种质资源缓慢生长法保存研究进展. 阜阳师范学院学报(自然科学版) 27(2), 68-72.
DOI URL |
| 2 |
刘静波, 林士杰, 张忠辉, 谢朋, 蔡群, 张大伟, 勾天兵, 赵珊珊, 赵洪伟, 王志强 ( 2012). 槭属植物种质资源研究进展. 中国农学通报 28(25), 1-5.
DOI URL |
| 3 |
任桂萍, 王小菁, 朱根发 ( 2016). 不同光质的LED对蝴蝶兰组织培养增殖及生根的影响. 植物学报 51, 81-88.
DOI URL |
| 4 | 咸洋, 吴丹, 解孝满, 刘德深, 韩彪, 田莎莎, 刘丹 ( 2014). 一种红花槭的组培快繁方法. 中国专利, 201310493738.1. 2014-02-05. |
| 5 |
徐呈祥 ( 2012). 提高植物抗寒性的机理研究进展. 生态学报 32, 7966-7980.
DOI URL |
| 6 |
徐刚标 ( 2000). 植物种质资源离体保存研究进展. 中南林学院学报 20(4), 81-87.
DOI URL |
| 7 |
燕丽萍, 李丽, 刘翠兰, 吴德军, 王因花, 任飞, 赵梁军 ( 2016). 绒毛白蜡体胚诱导和植株再生. 植物学报 51, 807-816.
DOI URL |
| 8 |
杨博, 曹秀婷, 王政, 何松林 ( 2012). 昼夜温差对非洲菊试管苗生长的影响. 西北林学院学报 27(2), 88-92.
DOI URL |
| 9 |
张红岩, 吴小芹, 朱丽华, 谈家金 ( 2010). 抗病赤松组培丛生芽低温保存条件研究. 南京林业大学学报(自然科学版) 34(5), 7-11.
DOI URL |
| 10 | 郑卫杰, 郭子霞, 王政, 何松林 ( 2011). 昼夜温差对文心兰试管苗生长的影响. 西北林学院学报 26(4), 137-141. |
| 11 | 周逊, 向长萍 ( 2008). 植物种质资源缓慢生长离体保存研究进展. 中国蔬菜 ( 11), 39-42. |
| 12 | Ahmad N, Abbasi BH, Fazal H, Khan MA, Afridi MS ( 2014). Effect of reverse photoperiod on in vitro regene- ration and piperine production in Piper nigrum L.C R Biologies 337, 19-28. |
| 13 |
Jouve L, Franck T, Gaspars T, Cattivelli L, Hausman JF ( 2000). Poplar acclimation to cold during in vitro conservation at low non-freezing temperature: metabolic and proteic changes.J Plant Physiol 157, 117-123.
DOI URL |
| 14 |
Kozai T, Watanabe K, Jeong BR ( 1995). Stem elongation and growth of Solanum tuberosum L. in vitro in response to photosynthetic photon flux, photoperiod and difference in photoperiod and dark period temperatures.Sci Hortic 64, 1-9.
DOI URL |
| 15 | Salcedo-Morales G, Rosas-Romero G, Nabor-Correa N, Bermúdez-Torres K, López-Laredo AR, Trejo-Tapia G ( 2009). Propagation and conservation of Castilleja tenu- iflora Benth.Polibotánica ( 28), 119-137. |
| 16 |
Sarasan V, Cripps R, Ramsay MM, Atherton C, Mcmi- chen M, Prendergast G, Rowntree JK ( 2006). Conservation in vitro of threatened plants—progress in the past decade.In Vitro Cell Dev Biol Plant 42, 206-214.
DOI URL |
| 17 | Silveira DG, Souza FVD, Pelacani CR, da Silva Souza A, da Silva Ledo CA, de Santana JRF ( 2009). Micropropagation and in vitro conservation of Neoglaziovia variegata (Arr. Cam.) Mez, a fiber producing bromeliad from Brazil. Braz Arch Biol Technol 52, 923-932. |
| [1] |
Tong Li, Churan Li, Zhiyu Zhang, Xiaoman Fu, Yun Liu, Yingjun Zhang, Liying Yang, Ping Zhao.
A Preliminary Study on Tissue Culture and Rapid Propagation Technology of Phyllanthus acidus [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’ [J]. Chinese Bulletin of Botany, 2024, 59(5): 800-809. |
| [3] | Wen Feng, Yuguo Wang. Establishment of an In Vitro Regeneration System for Stem Segments of Cultivated Dioscorea polystachya [J]. Chinese Bulletin of Botany, 2024, 59(5): 792-799. |
| [4] | Hao Zeng, Peifang Li, Zhihui Guo, Chunlin Liu, Ying Ruan. Establishment of a Regeneration System for Lunaria annua [J]. Chinese Bulletin of Botany, 2024, 59(3): 433-440. |
| [5] | Shangwen Zhang, Shiyu Huang, Tianwei Yang, Ting Li, Xiangjun Zhang, Manrong Gao. Establishment of a Tissue Culture and Rapid Propagation System for Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 99-109. |
| [6] | Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica [J]. Chinese Bulletin of Botany, 2023, 58(6): 926-934. |
| [7] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [8] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [9] | Mengyue Li, Liu Liu, Yan Liu, Xiaoman Zhang. Establishment of Tissue Culture System for Axillary Bud Regeneration of Primula × pubescens [J]. Chinese Bulletin of Botany, 2021, 56(6): 732-739. |
| [10] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [11] | Hong Luo, Xiaohui Wen, Yuanyuan Zhou, Silan Dai. Establishment of In Vitro Regeneration System of Helenium aromaticum [J]. Chinese Bulletin of Botany, 2020, 55(3): 318-328. |
| [12] | Sha Deng, Yanni Wu, Kunlin Wu, Lin Fang, Lin Li, Songjun Zeng. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China [J]. Biodiv Sci, 2020, 28(3): 385-400. |
| [13] | Wenting Zhang,Yanhong He,Ning Shu,Jingjing Xing,Baojun Liu,Manzhu Bao,Guofeng Liu. Plant Regeneration and Rapid Propagation System of Lilium bakerianum var. aureum [J]. Chinese Bulletin of Botany, 2019, 54(6): 773-778. |
| [14] | Fengluan Tang,Jian Zhao,Zhiguo Zhao,Ke Xia,Shuo Qiu. Tissue Culture and Rapid Propagation of Ardisia gigantifolia [J]. Chinese Bulletin of Botany, 2019, 54(3): 378-384. |
| [15] | An Baiyi, Guo Cainan, Bao Wenhui, Li Fengfei, Zhao He, Chen Li, An Fengyun. Rapid Propagation of Symplocos paniculata In Vitro [J]. Chinese Bulletin of Botany, 2018, 53(5): 693-699. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||