

Chinese Bulletin of Botany ›› 2016, Vol. 51 ›› Issue (1): 89-97.DOI: 10.11983/CBB14207
Previous Articles Next Articles
Meiping Lü1, Yuanzhong Wang2, Hengyu Huang1*
Received:2014-12-08
Accepted:2015-05-30
Online:2016-01-01
Published:2016-02-01
Contact:
Huang Hengyu
About author:? These authors contributed equally to this paper
Meiping Lü, Yuanzhong Wang, Hengyu Huang. Callus Induction and High Efficiency Plant Regeneration System Establishment of Pararuellia delavayana[J]. Chinese Bulletin of Botany, 2016, 51(1): 89-97.
| Level | Factor | ||
|---|---|---|---|
| A (6-BA, mg·L -1) | B (NAA, mg·L -1) | C (KT, mg·L -1) | |
| 1 | 0.5 | 0.1 | 0.01 |
| 2 | 1.0 | 0.5 | 0.05 |
| 3 | 1.5 | 1.0 | 0.10 |
Table 1 L9 (34) orthogonal design for callus induction and bud induction of Pararuellia delavayana
| Level | Factor | ||
|---|---|---|---|
| A (6-BA, mg·L -1) | B (NAA, mg·L -1) | C (KT, mg·L -1) | |
| 1 | 0.5 | 0.1 | 0.01 |
| 2 | 1.0 | 0.5 | 0.05 |
| 3 | 1.5 | 1.0 | 0.10 |
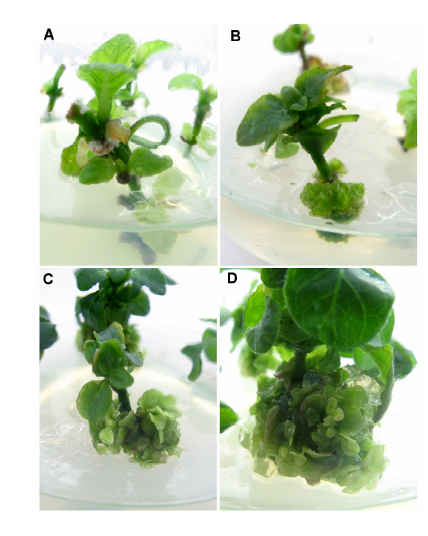
Figure 1 Callus induction and bud induction in Pararuellia delavayana(A) Axillary buds spring up quickly after 7 days; (B) Green callus deriving from the base of explants after 17 days; (C) Green buds from callus after 25 days; (D) Cluster buds from callus after 35 days
| No. | Hormone (mg·L-1) | Rate of callus induced (%) | Rate of buds induced (%) | ||||
|---|---|---|---|---|---|---|---|
| A (6-BA) | B (NAA) | C (KT) | D (Error) | ||||
| C1 | 0.5 | 0.1 | 0.01 | (1) | 25.12 | 53.03 | |
| C2 | 0.5 | 0.5 | 0.05 | (2) | 40.67 | 60.31 | |
| C3 | 0.5 | 1.0 | 0.10 | (3) | 49.23 | 64.80 | |
| C4 | 1.0 | 0.1 | 0.05 | (3) | 55.35 | 66.31 | |
| C5 | 1.0 | 0.5 | 0.10 | (1) | 85.38 | 97.55 | |
| C6 | 1.0 | 1.0 | 0.01 | (2) | 82.89 | 93.70 | |
| C7 | 1.5 | 0.1 | 0.10 | (2) | 58.51 | 89.51 | |
| C8 | 1.5 | 0.5 | 0.01 | (3) | 57.95 | 89.78 | |
| C9 | 1.5 | 1.0 | 0.05 | (1) | 50.97 | 63.62 | |
| Callus induced | K1 | 38.34 | 46.33 | 55.32 | 53.82 | ||
| K2 | 75.54 | 61.33 | 49.00 | 60.69 | |||
| K3 | 55.81 | 61.03 | 64.37 | 54.18 | |||
| R1 | 37.20 | 15.00 | 15.38 | 6.87 | |||
| Buds induced | K1 | 59.38 | 69.62 | 78.84 | 71.40 | ||
| K2 | 85.85 | 82.55 | 63.41 | 81.17 | |||
| K3 | 80.97 | 74.04 | 83.95 | 73.63 | |||
| R2 | 26.47 | 12.93 | 20.54 | 9.77 | |||
Table 2 Results of callus induction and bud induction by L9 (34) orthogonal test in Pararuellia delavayana
| No. | Hormone (mg·L-1) | Rate of callus induced (%) | Rate of buds induced (%) | ||||
|---|---|---|---|---|---|---|---|
| A (6-BA) | B (NAA) | C (KT) | D (Error) | ||||
| C1 | 0.5 | 0.1 | 0.01 | (1) | 25.12 | 53.03 | |
| C2 | 0.5 | 0.5 | 0.05 | (2) | 40.67 | 60.31 | |
| C3 | 0.5 | 1.0 | 0.10 | (3) | 49.23 | 64.80 | |
| C4 | 1.0 | 0.1 | 0.05 | (3) | 55.35 | 66.31 | |
| C5 | 1.0 | 0.5 | 0.10 | (1) | 85.38 | 97.55 | |
| C6 | 1.0 | 1.0 | 0.01 | (2) | 82.89 | 93.70 | |
| C7 | 1.5 | 0.1 | 0.10 | (2) | 58.51 | 89.51 | |
| C8 | 1.5 | 0.5 | 0.01 | (3) | 57.95 | 89.78 | |
| C9 | 1.5 | 1.0 | 0.05 | (1) | 50.97 | 63.62 | |
| Callus induced | K1 | 38.34 | 46.33 | 55.32 | 53.82 | ||
| K2 | 75.54 | 61.33 | 49.00 | 60.69 | |||
| K3 | 55.81 | 61.03 | 64.37 | 54.18 | |||
| R1 | 37.20 | 15.00 | 15.38 | 6.87 | |||
| Buds induced | K1 | 59.38 | 69.62 | 78.84 | 71.40 | ||
| K2 | 85.85 | 82.55 | 63.41 | 81.17 | |||
| K3 | 80.97 | 74.04 | 83.95 | 73.63 | |||
| R2 | 26.47 | 12.93 | 20.54 | 9.77 | |||
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1966.454 | 2 | 983.227 | 21.923 | P<0.05 |
| B (NAA) | 441.480 | 2 | 220.740 | 4.922 | P>0.05 |
| C (KT) | 358.389 | 2 | 179.195 | 3.995 | P>0.05 |
| Error | 89.699 | 2 | 44.850 |
Table 3 Variance analysis of callus induction in Pararuellia delavayana
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1966.454 | 2 | 983.227 | 21.923 | P<0.05 |
| B (NAA) | 441.480 | 2 | 220.740 | 4.922 | P>0.05 |
| C (KT) | 358.389 | 2 | 179.195 | 3.995 | P>0.05 |
| Error | 89.699 | 2 | 44.850 |
| Levels | Mean | 0.05 level |
|---|---|---|
| 2 | 75.54 | a |
| 3 | 55.81 | ab |
| 1 | 38.34 | b |
Table 4 Duncan’s test in three levels of 6-BA
| Levels | Mean | 0.05 level |
|---|---|---|
| 2 | 75.54 | a |
| 3 | 55.81 | ab |
| 1 | 38.34 | b |
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1190.812 | 2 | 595.406 | 7.566 | P>0.05 |
| B (NAA) | 259.114 | 2 | 129.557 | 1.646 | P>0.05 |
| C (KT) | 685.951 | 2 | 342.976 | 4.358 | P>0.05 |
| Error | 157.393 | 2 | 78.696 |
Table 5 Variance analysis of bud induction in Pararuellia delavayana
| Source | Type III sum of square | df | Mean square | F | Significance |
|---|---|---|---|---|---|
| A (6-BA) | 1190.812 | 2 | 595.406 | 7.566 | P>0.05 |
| B (NAA) | 259.114 | 2 | 129.557 | 1.646 | P>0.05 |
| C (KT) | 685.951 | 2 | 342.976 | 4.358 | P>0.05 |
| Error | 157.393 | 2 | 78.696 |

Figure 2 Proliferation and growth of adventitious shoots in Pararuellia delavayana(A) Small buds differentiation after 10 days; (B) Calluses blooming after 20 days; (C) Adventitious shoots after 30 days; (D) Adventitious seedlings after 40 days
| Media | Regeneration coefficient (%) | Growth condition |
|---|---|---|
| B5 | 11.05 | Adventitious shoots with lower induction rate were stronger, the vitrification was reduced obviously |
| N6 | 1.65 | Plantlets with no callus induction turned reddish, the vitrification was reduced obviously but the growth slowed |
| MS (1/2NH4NO3) | 7.93 | Weak adventitious shoots, the vitrification was reduced obviously |
Table 6 Results of proliferation and growth in Pararuellia delavayana in different basic medium
| Media | Regeneration coefficient (%) | Growth condition |
|---|---|---|
| B5 | 11.05 | Adventitious shoots with lower induction rate were stronger, the vitrification was reduced obviously |
| N6 | 1.65 | Plantlets with no callus induction turned reddish, the vitrification was reduced obviously but the growth slowed |
| MS (1/2NH4NO3) | 7.93 | Weak adventitious shoots, the vitrification was reduced obviously |
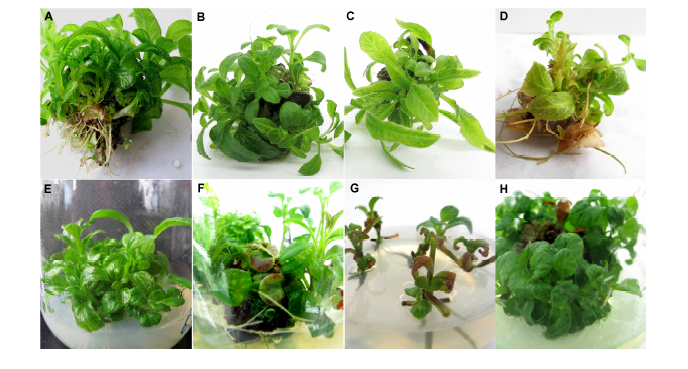
Figure 3 Phenomenon and improving results of vitrification in Pararuellia delavayana(A) Plantlets of the fourth generation after 40 days; (B) Plantlets of the fourth generation; (C) Plantlets of the fifth generation; (D) Vitrification plantlets of the sixth generation; (E) Proliferous plantlets in B5 medium after 40 days; (F) Proliferous plantlets in MS (NH4NO3 halved) medium after 40 days; (G) Proliferous plantlets in N6 medium after 30 days; (H) Proliferous plantlets of B5 and MS medium used interchangeably twice after 40 days
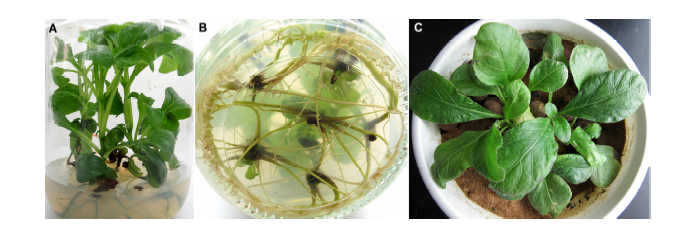
Figure 4 Rooting culture and transplanting of Pararuellia delavayana(A) Rooting plantlets in MS medium after 45 days; (B) Root system in MS medium after 45 days; (C) Transplanting plantlets
| 1 | 蔡能, 易自力, 李祥 (2003). 改善植物大规模组织培养条件的研究进展. 植物学通报 20, 745-751. |
| 2 | 蔡祖国, 徐小彪, 周会萍 (2005). 植物组织培养中的玻璃化现象及其预防. 生物技术通讯 16, 353-355. |
| 3 | 陈兵先, 黄宝灵, 吕成群, 杨来安, 陈文军, 任玲, 王劲松 (2011). 植物组织培养试管苗玻璃化现象研究进展. 林业科技开发 25, 1-5. |
| 4 | 陈雪, 张金柱, 潘兵兵, 桑成瑾, 马雪, 杨涛, 车代弟 (2011). 月季愈伤组织的诱导及植株再生. 植物学报 46, 569-574. |
| 5 | 冯欢, 易姝利, 谢佳恒, 雷梦琦, 黄萱 (2014). 微型月季愈伤组织诱导及植株再生研究. 植物学报 49, 595-602. |
| 6 | 国家中药管理局《中华本草》编委会 (1998). 中华本草(第7卷). 上海: 上海科学技术出版社. pp. 6478. |
| 7 | 胡峰, 施琼, 黄烈健 (2014). 黑木相思愈伤组织诱导及植株再生. 植物学报 49, 603-610. |
| 8 | 黄格 (2010). 药用植物穿心莲组培快繁及其影响因子的研究. 硕士论文. 南宁: 广西大学. pp. 6-9. |
| 9 | 姜北 (2001). 五种药用植物化学成分与生物活性研究. 硕士论文. 昆明: 中国科学院昆明植物研究所. pp. 9-16. |
| 10 | 江苏新医学院 (1977). 中药大词典(上册). 上海: 上海科学技术出版社. pp. 813. |
| 11 | 李红, 李永文 (2007). 金脉单药花栽培管理技术. 河北农业科技 6, 37. |
| 12 | 李林轩, 凌征柱, 李翠, 彭凌, 韦坤华 (2013). 珐菲亚组织培养条件的优化研究. 中草药 44, 1334-1337. |
| 13 | 李胜, 李唯, 杨德龙, 曹孜义 (2003). 植物试管苗玻璃化现象研究进展. 甘肃农业大学学报 38, 1-16. |
| 14 | 吕春 (2009). 红斑枪刀药组织培养体系研究. 硕士论文. 成都: 四川农业大学. pp. 8-12. |
| 15 | 苏钛, 黄宁珍, 付传明 (2009). 匙羹藤组织培养条件优化研究. 广西植物 29, 87-91. |
| 16 | 孙清荣, 孙洪雁, 张力思, 周广方, 刘庆忠 (2010). 酸枣叶片不定梢形成及玻璃化的影响因素. 西北植物学报 30, 1039-1044. |
| 17 | 吴征镒, 李锡文 (2010). 中国植物志(第70卷). 北京: 科学出版社. pp. 55. |
| 18 | 郗浩江, 葛素因, 王芳 (2013). 新疆紫草组织培养中试管苗玻璃化的控制与修复. 新疆师范大学学报 32, 21-25. |
| 19 | 张爱莲 (2005). 爵床,锥头麻和木姜冬青的化学成分研究. 硕士论文. 成都: 中国科学院成都有机化学研究所. pp. 6-18. |
| 20 | 张翠玉, 廖晴 (1991). 月季试管苗玻璃化原因及控制方法研究. 新疆农业科学 2, 76-78. |
| 21 | 张庆红, 汤丽云, 司徒少金, 王莎, 何国振 (2014). 药用植物栀子的组织培养. 植物学报 49, 331-336. |
| 22 | 周菊花, 林证明, 梁海曼 (1990). 控制瑞香试管苗玻璃化的研究. 园艺学报 17, 229-232. |
| 23 | Chakrabarty D, Park SY, Ali MB, Shin KS, Paek KY (2006). Hyperhydricity in apple: ultrastructure and physico- logical aspects.Tree Physiol 26, 377-388. |
| 24 | Ivanova M, Van SJ (2008). Effect of ammonium ions and cytokinins on hyperhydricity and multiplication rate of in vitro regenerated shoots of Aloe polyphylla.Plant Cell Tiss Org Cult 92, 227-231. |
| 25 | Kevers C, Fanck T, Strasser RJ, Dommes J, Gaspar T (2004). Hyperhydricity of micropropagated shoots: a typically stress-induced change of physiological state.Plant Cell Tiss Org Cult 77, 181-191. |
| 26 | Mayor ML, Nestares G, Zorzoli R, Picardi LA (2003). Reduction of hyperhydricity in sunfllower tissue culture.Plant Cell Tiss Org Cult 72, 99-103. |
| 27 | Piqueras A, Cortina M, Serna MD, Casas JL (2002). Polyamines and hyperhydricity in micropropagated carnation plants.Plant Sci 162, 671-678. |
| 28 | Saher S, Piqueras A, Hellin E, Olmos E (2004). Hyperhydricity in micro-propagated carnation shoot: the role of oxidative stress.Physiol Plant 120, 152-161. |
| 29 | Sreedhar RV, Venkatachalam L, Neelwarne B (2009). Hyperhydricity related morphologic and biochemical ch- anges in vanilla (Vanilla planifolia).J Plant Growth Regul 28, 46-57. |
| 30 | Wang YL, Wang XD, Zhao B, Wang YC (2007). Reduction of hyperhydricity in the culture of Lepidium meyenii shoots by the addition of rare earth elements.J Plant Growth Regul 52, 151-159. |
| 31 | Wu Z, Chen LJ, Long YJ (2009). Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoot.In Vitro Cell Dev Biol-Plant 45, 483-490. |
| [1] | . Establishment of Tissue Culture and Rapid System of Erythropalum scandens Based on Orthogonal Test [J]. Chinese Bulletin of Botany, 2024, 59(1): 0-0. |
| [2] | Yefei Liu, Haixia Zhao, Xiping Jiang, Rui Qiu, Xinyue Zhou, Yan Zhao, Chunxiang Fu. Establishment of Highly Efficient Tissue Culture and Agrobacterium-mediated Callus Infection Systems for Hordeum brevisubulatum [J]. Chinese Bulletin of Botany, 2023, 58(3): 440-448. |
| [3] | Jinchun Lu, Lina Cao, Guanjie Tong, Xinying Wang, Liying Zhang, Xin Yu, Huifang Li, Yanhui Li. Establishment of Callus Induction and Regeneration System of Anemone silvestris [J]. Chinese Bulletin of Botany, 2022, 57(2): 217-226. |
| [4] | Churan Li, Ling Fu, Yun Liu, Xiaoqin Yang, Guolei Zhu, Sida Xie, Huancheng Ma, Ping Zhao. Optimization of Cell Suspension Culture Conditions of Vaccinium dunalianum [J]. Chinese Bulletin of Botany, 2022, 57(2): 227-235. |
| [5] | Yanmin Li, Hui Jiang, Zhenzhu Fu, Jing Zhang, Xin Yuan, Huijuan Wang, Jie Gao, Xiaoyu Dong, Limin Wang, Hechen Zhang. Callus Induction and Somatic Embryogenesis in Anther Culture of Paeonia lactiflora [J]. Chinese Bulletin of Botany, 2021, 56(4): 443-450. |
| [6] | Qian Luo, Yansha Zhang, Jing Ou. Callus Induction and Plant Regeneration of Cerasus serrulata var. lannesiana cv. ‘Grandiflora’ [J]. Chinese Bulletin of Botany, 2021, 56(4): 451-461. |
| [7] | Pengfei Du, Yu Wang, Yingping Cao, Song Yang, Zhichao Sun, Decai Mao, Jiajun Yan, Daxu Li, Meizhen Sun, Chunxiang Fu, Shiqie Bai. Establishment of Biolistic Mediated Transformation System for Elymus sibiricus [J]. Chinese Bulletin of Botany, 2021, 56(1): 62-70. |
| [8] | Dongrui Zhang, Zhigang Bu, Lingling Chen, Ying Chang. Establishment of a Tissue Culture and Rapid Propagation System of Dryopteris fragrans [J]. Chinese Bulletin of Botany, 2020, 55(6): 760-767. |
| [9] | Yaqin Wang, Ludan Wei, Wenjing Wang, Baojun Liu, Chunling Zhang, Junwei Zhang, Yanhong He. The Establishment and Optimization of a Regeneration System for Marigold (Tagetes erecta) [J]. Chinese Bulletin of Botany, 2020, 55(6): 749-759. |
| [10] | Jianfei Liu, Yan Liu, Kejian Liu, Yang Chi, Zhifa Huo, Yonghong Huo, Xiangling You. Optimization of the Regeneration System from Somatic Embryogenesis in Larix olgensis [J]. Chinese Bulletin of Botany, 2020, 55(5): 605-612. |
| [11] | Yan Xiao,Zhenxing Wang,Dongming Li,Yanhua Qi, Enhebayaer. Optimization of Tissue Culture and Plant Regeneration System of Mature Embryo of Leymus chinensis [J]. Chinese Bulletin of Botany, 2020, 55(2): 192-198. |
| [12] | Ying Feng,Lianwen Qian,Qingliang Lin. The Effect of Different Hormones on Explant Browning and Callus Browning in Cyclocarya paliurus [J]. Chinese Bulletin of Botany, 2019, 54(5): 634-641. |
| [13] | Xiaomei Liu,Lili Sun,Xiangdong Fu,Hong Liao. An Effective Method for the Rooting of Tea Cuttings [J]. Chinese Bulletin of Botany, 2019, 54(4): 531-538. |
| [14] | Zhang Xuhong, Wang Di, Liang Zhenxu, Sun Meiyu, Zhang Jinzheng, Shi Lei. Callus Induction and Establishment of a Plant Regeneration System with Lilium martagon [J]. Chinese Bulletin of Botany, 2018, 53(6): 840-847. |
| [15] | Fang Liu, Yinghong Tang, Youmei Yuan, Qingquan Guo, Fan Shen, Jianrong Chen. Tissue Culture of the Succulent Plant Sedum clavatum [J]. Chinese Bulletin of Botany, 2016, 51(2): 251-256. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||