

Chinese Bulletin of Botany ›› 2019, Vol. 54 ›› Issue (5): 606-619.DOI: 10.11983/CBB19053 cstr: 32102.14.CBB19053
• EXPERIMENTAL COMMUNICATIONS • Previous Articles Next Articles
Chun Zhou1,Ran Jiao1,Ping Hu2,Han Lin1,Juan Hu1,Na Xu1,Xianmei Wu2,Yuchun Rao1,*( ),Yuexing Wang2,*(
),Yuexing Wang2,*( )
)
Received:2019-03-20
Accepted:2019-06-20
Online:2019-09-01
Published:2020-03-10
Contact:
Yuchun Rao,Yuexing Wang
Chun Zhou,Ran Jiao,Ping Hu,Han Lin,Juan Hu,Na Xu,Xianmei Wu,Yuchun Rao,Yuexing Wang. Gene Mapping and Candidate Gene Analysis of Rice Early Senescence Mutant LS-es1[J]. Chinese Bulletin of Botany, 2019, 54(5): 606-619.
| Primer name | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| InDel-1 | AGCGGGGATGGAGATGATG | CTTGCCTCACACCAGATCTG |
| InDel-2 | GGCGCCTTTGTTCCATAGTT | GAGGAGCCAGTGGTAGCAG |
| InDel-3 | CGTTTTTACAACCAATTTTGGAA | CCATCTTCTACCTCCGGACA |
| InDel-4 | GATTGGATTGGTTGCTCGCT | AACAGCGAATCGAGATGCAC |
| InDel-5 | TTACTGCTGCCGTTGTTTCA | TTGTGGACCTCCAGGATCAG |
| SGR | AGGGGTGGTACAACAAGCTG | GCTCCTTGCGGAAGATGTAG |
| Osh36 | GCACGGAGGCGAACGA | TTGAGCGGTAGCACCCATT |
| Osl85 | GAGCAACGGCGTGGAGA | GCGGCGGTAGAGGAGATG |
| OsNAP | CAAGAAGCCGAACGGTTC | GTTAGAGTGGAGCAGCAT |
| Actin | CAGGCCGTCCTCTCTCTGTA | AAGGATAGCATGGGGGAGAG |
Table 1 Primers used for gene mapping and qRT-PCR
| Primer name | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| InDel-1 | AGCGGGGATGGAGATGATG | CTTGCCTCACACCAGATCTG |
| InDel-2 | GGCGCCTTTGTTCCATAGTT | GAGGAGCCAGTGGTAGCAG |
| InDel-3 | CGTTTTTACAACCAATTTTGGAA | CCATCTTCTACCTCCGGACA |
| InDel-4 | GATTGGATTGGTTGCTCGCT | AACAGCGAATCGAGATGCAC |
| InDel-5 | TTACTGCTGCCGTTGTTTCA | TTGTGGACCTCCAGGATCAG |
| SGR | AGGGGTGGTACAACAAGCTG | GCTCCTTGCGGAAGATGTAG |
| Osh36 | GCACGGAGGCGAACGA | TTGAGCGGTAGCACCCATT |
| Osl85 | GAGCAACGGCGTGGAGA | GCGGCGGTAGAGGAGATG |
| OsNAP | CAAGAAGCCGAACGGTTC | GTTAGAGTGGAGCAGCAT |
| Actin | CAGGCCGTCCTCTCTCTGTA | AAGGATAGCATGGGGGAGAG |
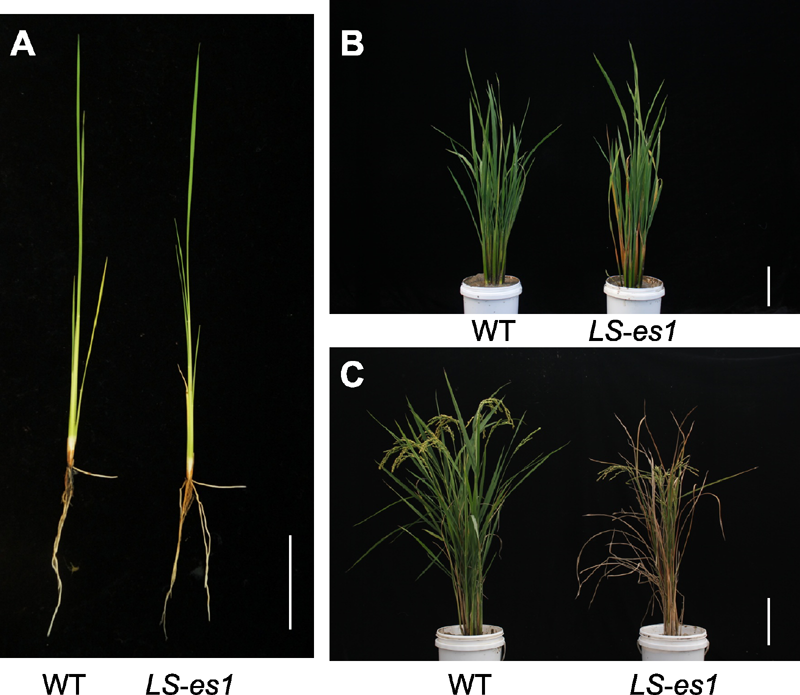
Figure 1 Phenotypes of rice wild type (WT) TP309 and mu- tant LS-es1 (A) Phenotypes at seedling stage; (B) Phenotypes at tillering stage; (C) Phenotypes at maturity stage. Bars=6 cm
| Agronomic traits | TP309 | LS-es1 |
|---|---|---|
| Effective number of panicle | 13.40±5.41 | 8.20±0.84 |
| Flag leaf length (cm) | 39.46±8.16 | 35.68±4.08 |
| Secondary branch number | 29.80±6.87 | 18.00±4.47* |
| Tiller number | 16.40±5.46 | 11.40±2.70 |
| Filled grain number per panicle | 169.60±8.08 | 125.60±24.83** |
| Seed-setting rate (%) | 81.01±6.98 | 64.76±17.23 |
Table 2 The comparison of agronomic traits between rice wild type TP309 and mutant LS-es1
| Agronomic traits | TP309 | LS-es1 |
|---|---|---|
| Effective number of panicle | 13.40±5.41 | 8.20±0.84 |
| Flag leaf length (cm) | 39.46±8.16 | 35.68±4.08 |
| Secondary branch number | 29.80±6.87 | 18.00±4.47* |
| Tiller number | 16.40±5.46 | 11.40±2.70 |
| Filled grain number per panicle | 169.60±8.08 | 125.60±24.83** |
| Seed-setting rate (%) | 81.01±6.98 | 64.76±17.23 |
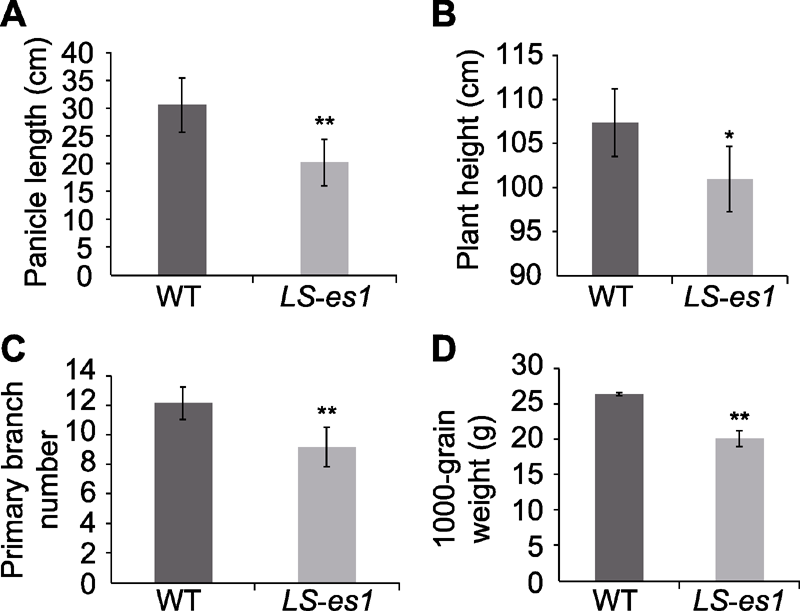
Figure 2 The comparison of agronomic traits between rice wild type (WT) TP309 and mutant LS-es1 (A) Panicle length; (B) Plant height; (C) Primary branch number; (D) 1000-grain weight. * and ** indicate significant differences between TP309 and LS-es1 at 0.05 and 0.01 level, respectively.
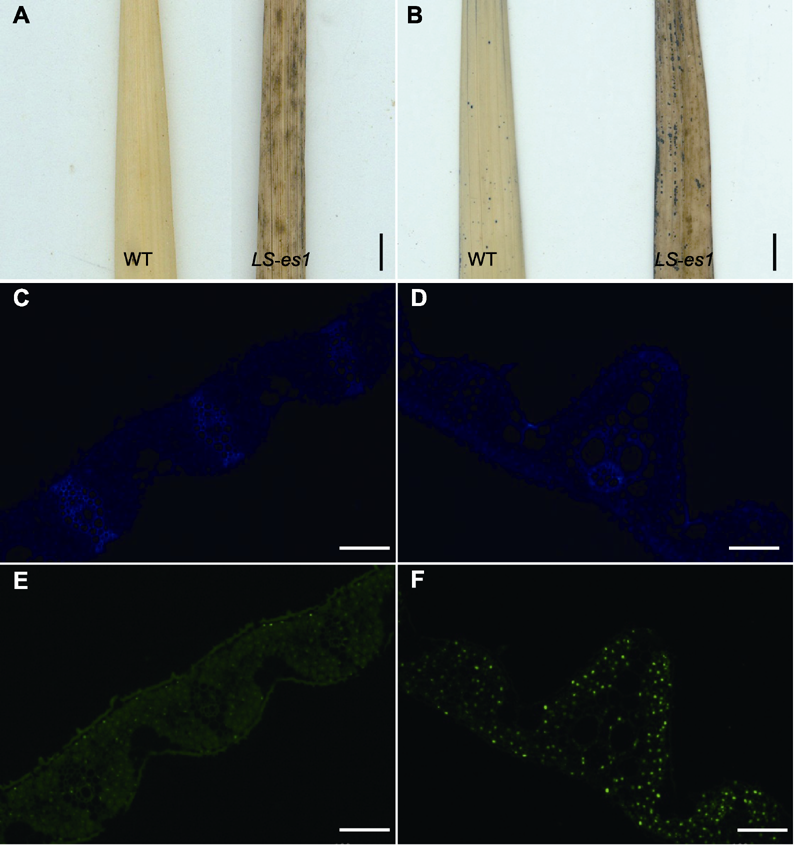
Figure 3 Histochemical analysis of rice wild type (WT) TP309 and mutant LS-es1 leaves DAB staining of wild-type and LS-es1 leaves (Bar=2 cm);(B) NBT staining of wild-type and LS-es1 leaves (Bar=2 cm); (C), (E) Tunel detection of wild-type leaves (Bars=100 μm); (D), (F) Tunel detection of LS-es1 leaves (Bars=100 μm).
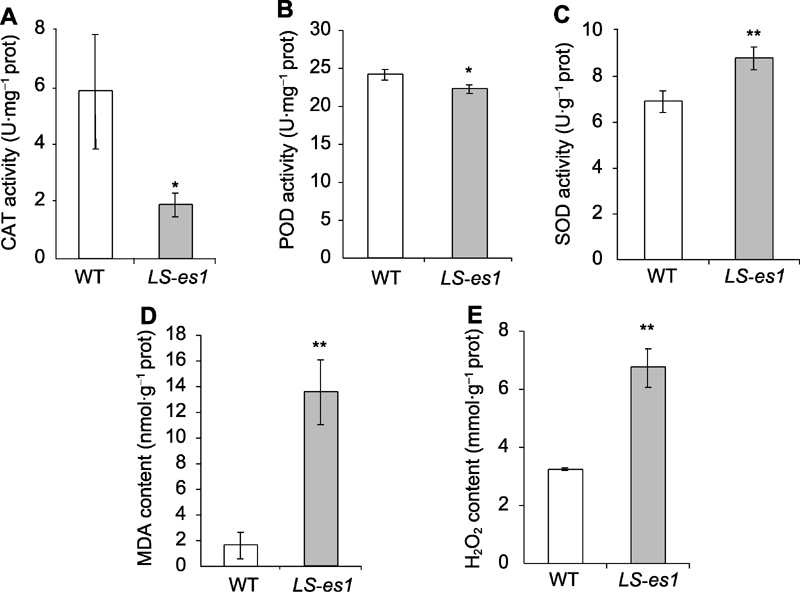
Figure 4 Catalase (CAT) (A), peroxisome (POD) (B), and superoxide dismutase (SOD) (C) activities and malondialdehyde (MDA) (D) and H2O2 (E) contents of LS-es1 and wild type (WT) at heading stage of rice * and ** indicate significant differences between TP309 and LS-es1 at 0.05 and 0.01 level, respectively.
| Net photosynthetic rate | Stomatal conductance | Intercellular CO2 concentration | Transpiration rate | SPAD | |
|---|---|---|---|---|---|
| TP309 | 9.4±0.961 | 0.110±0.01 | 263±3 | 4.63±0.289 | 40.633±1.206 |
| LS-es1 | 2.225±1.407** | 0.0403±0.007** | 312.5±10.606** | 2.3±0.283** | 27.65±2.333** |
Table 3 The comparison of SPAD value and photosynthetic rate between rice TP309 and LS-es1
| Net photosynthetic rate | Stomatal conductance | Intercellular CO2 concentration | Transpiration rate | SPAD | |
|---|---|---|---|---|---|
| TP309 | 9.4±0.961 | 0.110±0.01 | 263±3 | 4.63±0.289 | 40.633±1.206 |
| LS-es1 | 2.225±1.407** | 0.0403±0.007** | 312.5±10.606** | 2.3±0.283** | 27.65±2.333** |
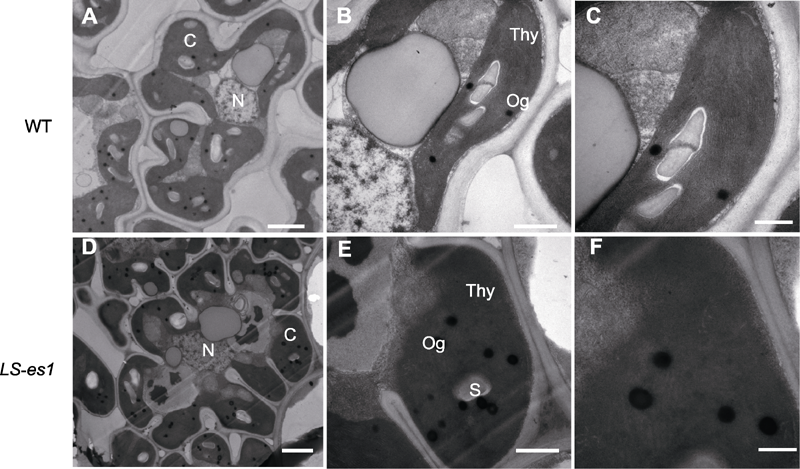
Figure 5 Transmission electron microscopy (TEM) analysis of chloroplast in rice wild type (WT) TP309 and mutant LS-es1 (A) TP309 leaf cells in 6000X; (B) TP309 leaf cells in 25000X; (C) TP309 leaf cells in 40000X; (D) LS-es1 leaf cells in 6000X; (E) LS-es1 leaf cells in 25000X; (F) LS-es1 leaf cells in 40000X. N: Cell nucleus; C: Chloroplast; Thy: Thylakoid; S: Starch granule; Og: Eosinophil. Bars=1 μm
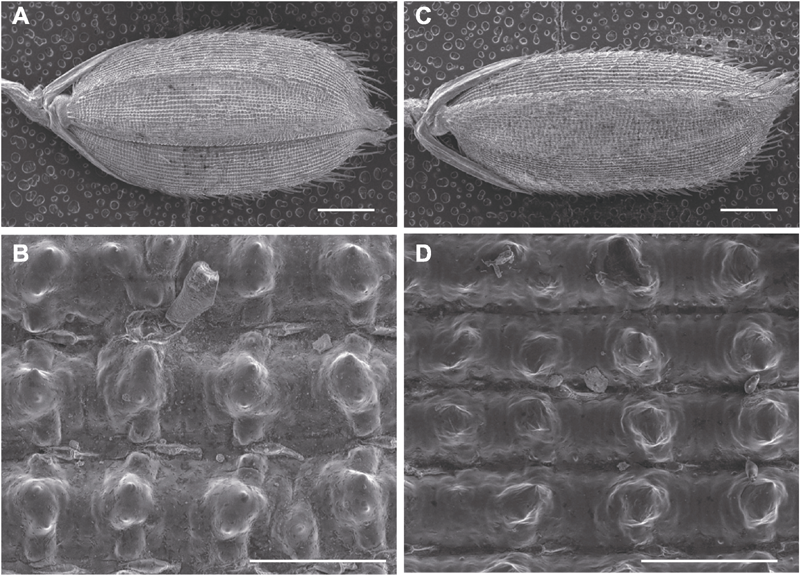
Figure 6 Scanning electron microscopy (SEM) analysis of seeds in rice wild type TP309 and mutant LS-es1 (A), (B) TP309 seed; (C), (D) LS-es1 seed. (A), (C) Bars=1 mm; (B), (D) Bars=100 μm
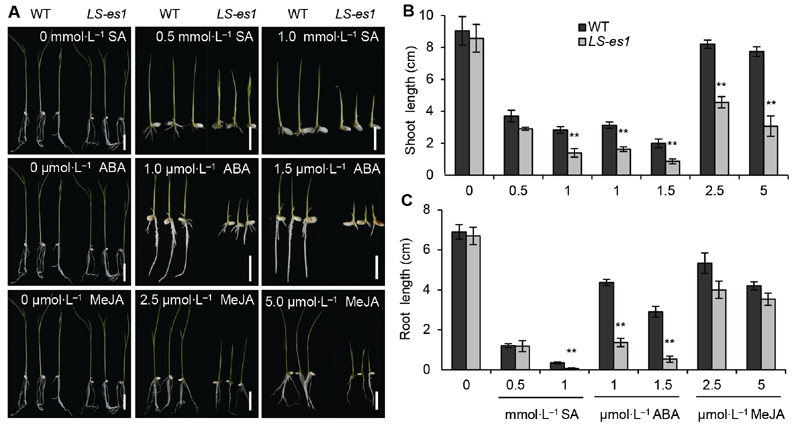
Figure 7 Inhibition of exogenous hormone treatment on the growth of seedlings in rice wild type (WT) TP309 and mutant LS-es1 (A) Salicylic acid (SA) (top), abscisic acid (ABA) (middle), methyl jasmonate (MeJA) (bottom) (Bars=2 cm); (B) Comparison of shoot length of TP309 and LS-es1 seedlings treated with hormones; (C) Comparison of root length of TP309 and LS-es1 seedlings treated with hormones. ** indicate significant differences at 0.01 level between TP309 and LS-es1.
| Hybrid combination (male/female) | F1 pheno- type | Namber of F2 normal phenotype | Namber of F2 mutant phenotype | Number of F2 population | χ2 (3:1) |
|---|---|---|---|---|---|
| LS-es1/TN1 | Normal | 1381 | 471 | 1852 | 0.1842 |
| LS-es1/ZF802 | Normal | 526 | 178 | 704 | 0.0303 |
Table 4 Genetic analysis of rice early senescence phenotypes of LS-es1
| Hybrid combination (male/female) | F1 pheno- type | Namber of F2 normal phenotype | Namber of F2 mutant phenotype | Number of F2 population | χ2 (3:1) |
|---|---|---|---|---|---|
| LS-es1/TN1 | Normal | 1381 | 471 | 1852 | 0.1842 |
| LS-es1/ZF802 | Normal | 526 | 178 | 704 | 0.0303 |
| 1 | 陈昆松, 李方, 徐昌杰, 张上隆, 傅承新 (2004). 改良CTAB法用于多年生植物组织基因组DNA的大量提取. 遗传 26, 529-531. |
| 2 | 邓接楼, 王艾平, 何长水, 王爱斌, 徐芬芬 (2011). 硅肥对水稻生长发育及产量品质的影响. 广东农业科学 38(12), 58-61. |
| 3 | 段俊, 梁承邺, 黄毓文 (1997). 杂交水稻开花结实期间叶片衰老. 植物生理学报 23, 139-144. |
| 4 | 华春, 王仁雷 (2003). 杂交稻及其三系叶片衰老过程中SOD、CAT活性和MDA含量的变化. 西北植物学报 23, 406-409. |
| 5 | 冷语佳 (2013). 水稻早衰基因ES10的遗传分析与基因定位. 硕士论文. 北京: 中国农业科学院. pp. 16-17. |
| 6 | 刘翔 (2014). EMS诱变技术在植物育种中的研究进展. 激光生物学报 23, 197-201. |
| 7 | 孙玉莹 (2013). 水稻叶片早衰基因PSL2的图位克隆及功能初步分析. 硕士论文. 北京: 中国农业科学院. pp. 17-65. |
| 8 | 徐娜, 徐江民, 蒋玲欢, 饶玉春 (2017). 水稻叶片早衰成因及分子机理研究进展. 植物学报 52, 102-112. |
| 9 | 张丽霞 (2000). 水稻叶片衰老相关基因的分离. 硕士论文. 福州: 福建农林大学. pp. 20-54. |
| 10 | Ansari MI, Lee RH, Chen SCG (2005). A novel senescence-associated gene encoding γ-aminobutyric acid (GABA): pyruvate transaminase is upregulated during rice leaf senescence. Physiol Plant 123, 1-8. |
| 11 | Chen HL, Li CR, Liu LP, Zhao JY, Cheng XZ, Jiang GH, Zhai WX (2016). The Fd-GOGAT1 mutant gene lc7 confers resistance to Xanthomonas oryzae pv. oryzae in rice. Sci Rep 6, 26411. |
| 12 | Chen LJ, Wuriyanghan H, Zhang YQ, Duan KX, Chen HW, Li QT, Lu X, He SJ, Ma B, Zhang WK, Lin Q, Chen SY, Zhang JS (2013a). An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark- induced leaf senescence in rice. Plant Physiol 163, 1752-1765. |
| 13 | Chen Y, Xu YY, Luo W, Li WX, Chen N, Zhang DJ, Chong K (2013b). The F-box protein OsFBK12 targets OsSAMS1 for degradation and affects pleiotropic phenotypes including leaf senescence in rice. Plant Physiol 163, 1673-1685. |
| 14 | Fanata WID, Lee KH, Son BH, Yoo JY, Harmoko R, Ko KS, Ramasamy NK, Kim KH, Oh DB, Jung HS, Kim JY, Lee SY, Lee KO (2013). N-glycan maturation is crucial for cytokinin-mediated development and cellulose synthesis in Oryza sativa. Plant J 73, 966-979. |
| 15 | Gan S, Amasino RM (1997). Making sense of senescence: molecular genetic regulation and manipulation of leaf senescence. Plant Physiol 113, 313-319. |
| 16 | Gan SS, Hörtensteiner S (2013). Frontiers in plant senescence research: from bench to bank. Plant Mol Biol 82, 503-504. |
| 17 | Hideg E, Kálai T, Kós PB, Asada K, Hideg K (2006). Singlet oxygen in plants—its significance and possible detection with double (fluorescent and spin) indicator reagents. Photochem Photobiol 82, 1211-1218. |
| 18 | Huang LM, Sun QW, Qin FJ, Li C, Zhao Y, Zhou DX (2007). Down-regulation of a SILENT INFORMATION REGULATOR 2-related histone deacetylase gene, OsS- RT1, induces DNA fragmentation and cell death in rice. Plant Physiol 144, 1508-1519. |
| 19 | Huang QN, Shi YF, Zhang XB, Song LX, Feng BH, Wang HM, Xu X, Li XH, Guo D, Wu JL (2016). Single base substitution in OsCDC48 is responsible for premature senescence and death phenotype in rice. J Integr Plant Biol 58, 12-28. |
| 20 | Jiang HW, Li MR, Liang NT, Yan HB, Wei YB, Xu XL, Liu J, Xu ZF, Chen F, Wu GJ (2007). Molecular cloning and function analysis of the stay green gene in rice. Plant J 52, 197-209. |
| 21 | Jiao BB, Wang JJ, Zhu XD, Zeng LJ, Li Q, He ZH (2012). A novel protein RLS1 with NB-ARM domains is involved in chloroplast degradation during leaf senescence in rice. Mol Plant 5, 205-217. |
| 22 | Kariola T, Brader G, Li J, Palva ET (2005). Chlorophyllase 1, a damage control enzyme, affects the balance between defense pathways in plants. Plant Cell 17, 282-294. |
| 23 | Kong ZS, Li MN, Yang WY, Xu WY, Xue YB (2006). A novel nuclear-localized CCCH-type zinc finger protein, OsDOS, is involved in delaying leaf senescence in rice. Plant Physiol 141, 1376-1388. |
| 24 | Kusaba M, Ito H, Morita R, Iida S, Sato Y, Fujimoto M, Kawasaki S, Tanaka R, Hirochika H, Nishimura M, Tanaka A (2007). Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. Plant Cell 19, 1362-1375. |
| 25 | Lee RH, Lin MC, Chen SC (2004). A novel alkaline α-galactosidase gene is involved in rice leaf senescence. Plant Mol Biol 55, 281-295. |
| 26 | Lee RH, Wang CH, Huang LT, Chen SCG (2001). Leaf senescence in rice plants: cloning and characterization of senescence up-regulated genes. J Exp Bot 52, 1117-1121. |
| 27 | Leng YJ, Yang YL, Ren DY, Huang LC, Dai LP, Wang YQ, Chen L, Tu ZJ, Gao YH, Li XY, Zhu L, Hu J, Zhang GH, Gao ZY, Guo LB, Kong ZS, Lin YJ, Qian Q, Zeng DL (2017). A rice PECTATE LYASE-LIKE gene is required for plant growth and leaf senescence. Plant Physiol 174, 1151-1166. |
| 28 | Liang CZ, Wang YQ, Zhu YN, Tang JY, Hu B, Liu LC, Ou SJ, Wu HK, Sun XH, Chu JF, Chu CC (2014). OsNAP connects abscisic acid and leaf senescence by fine-tuning abscisic acid biosynthesis and directly targeting senescence-associated genes in rice. Proc Natl Acad Sci USA 111, 10013-10018. |
| 29 | Lim PO, Kim HJ, Nam HG (2007). Leaf senescence. Annu Rev Plant Biol 58, 115-136. |
| 30 | Lin AH, Wang YQ, Tang JY, Xue P, Li CL, Liu LC, Hu B, Yang FQ, Loake GJ, Chu CC (2012). Nitric oxide and protein S-nitrosylation are integral to hydrogen peroxide-induced leaf cell death in rice. Plant Physiol 158, 451-464. |
| 31 | Lin YH, Tan LB, Zhao L, Sun XY, Sun CQ (2016). RLS3, a protein with AAA+ domain localized in chloroplast, sustains leaf longevity in rice. J Integr Plant Biol 58, 971-982. |
| 32 | Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2 -ΔΔCt method . Methods 25, 402-408. |
| 33 | Luan WJ, Shen A, Jin ZP, Song SS, Li ZL, Sha AH (2013). Knockdown of OsHox33, a member of the class III homeodomain-leucine zipper gene family, accelerates leaf senescence in rice. Sci China Life Sci 56, 1113-1123. |
| 34 | Mahalingam R, Jambunathan N, Gunjan SK, Faustin E, Weng H, Ayoubi P (2006). Analysis of oxidative signaling induced by ozone in Arabidopsis thaliana. Plant Cell Environ 29, 1357-1371. |
| 35 | McCabe MS, Garratt LC, Schepers F, Jordi WJRM, Stoopen GM, Davelaar E, van Rhijn JHA, Power JB, Davey MR (2001). Effects of PSAG12- IPT gene expression on development and senescence in transgenic lettuce. Plant Physiol 127, 505-516. |
| 36 | Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M (2010). Defect in non-yellow coloring 3, an α/β hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. Plant J 59, 940-952. |
| 37 | Navabpour S, Morris K, Allen R, Harrison E, A-H- Mackerness S, Buchanan-Wollaston V (2003). Expression of senescence-enhanced genes in response to oxidative stress. J Exp Bot 54, 2285-2292. |
| 38 | Qiao YL, Jiang WZ, Lee J, Park BS, Choi MS, Piao RH, Woo MO, Roh JH, Han LZ, Paek NC, Seo HS, Koh HJ (2010). SPL28 encodes a clathrin-associated adaptor protein complex 1, medium subunit μ1 (AP1M1) and is responsible for spotted leaf and early senescence in rice (Oryza sativa). New Phytol 185, 258-274. |
| 39 | Rao YC, Yang YL, Xu J, Li XJ, Leng YJ, Dai LP, Huang LC, Shao GS, Ren DY, Hu J, Guo LB, Pan JW, Zeng DL (2015). EARLY SENESCENCE1 encodes a SCAR-LIKE PROTEIN2 that affects water loss in rice. Plant Physiol 169, 1225-1239. |
| 40 | Schippers JH, Schmidt R, Wagstaff C, Jing HC (2015). Living to die and dying to live: the survival strategy behind leaf senescence. Plant Physiol 169, 914-930. |
| 41 | Singh S, Giri MK, Singh PK, Siddiqui A, Nandi AK (2013). Down-regulation of OsSAG12-1 results in enhanced senescence and pathogen-induced cell death in transgenic rice plants. J Biosci 38, 583-592. |
| 42 | Sun LT, Wang YH, Liu LL, Wang CM, Gan T, Zhang ZY, Wang YL, Wang D, Niu M, Long WH, Li XH, Zheng M, Jiang L, Wan JM (2017). Isolation and characterization of a spotted leaf 32 mutant with early leaf senescence and enhanced defense response in rice. Sci Rep 7, 41846. |
| 43 | Tamiru M, Takagi H, Abe A, Yokota T, Kanzaki H, Okamoto H, Saitoh H, Takahashi H, Fujisaki K, Oikawa K, Uemura A, Natsume S, Jikumaru Y, Matsuura H, Umemura K, Terry MJ, Terauchi R (2016). A chloroplast-localized protein LESION AND LAMINA BENDING affects defence and growth responses in rice. New Phytol 210, 1282-1297. |
| 44 | Tang YY, Li MR, Chen YP, Wu PZ, Wu GJ, Jiang HW (2011). Knockdown of Os PAO and OsRCCR1 cause different plant death phenotypes in rice. J Plant Physiol 168, 1952-1959. |
| 45 | Undan JR, Tamiru M, Abe A, Yoshida K, Kosugi S, Takagi H, Yoshida K, Kanzaki H, Saitoh H, Fekih R, Sharma S, Undan J, Yano M, Terauchi R (2012). Mutation in OsLMS, a gene encoding a protein with two double-stranded RNA binding motifs, causes lesion mimic phenotype and early senescence in rice(Oryza sativa L.). Genes Genet Syst 87, 169-179. |
| 46 | Wang S, Lei CL, Wang JL, Ma J, Tang S, Wang CL, Zhao KJ, Tian P, Zhang H, Qi CY, Cheng ZJ, Zhang X, Guo XP, Liu LL, Wu CY, Wan JM (2017). SPL33, encoding an eEF1A-like protein, negatively regulates cell death and defense responses in rice. J Exp Bot 68, 899-913. |
| 47 | Wu HB, Wang B, Chen YL, Liu YG, Chen LT (2013). Characterization and fine mapping of the rice premature senescence mutant ospse1. Theor Appl Genet 126, 1897-1907. |
| 48 | Wu ZM, Zhang X, He B, Diao LP, Sheng SL, Wang JL, Guo XP, Su N, Wang LF, Jiang L, Wang CM, Zhai HQ, Wan JM (2007). A chlorophyll-deficient rice mutant with impaired chlorophyllide esterification in chlorophyll biosynthesis. Plant Physiol 145, 29-40. |
| 49 | Yamatani H, Sato Y, Masuda Y, Kato Y, Morita R, Fukunaga K, Nagamura Y, Nishimura M, Sakamoto W, Tanaka A, Kusaba M (2013). Nyc4, the rice ortholog of Arabidopsis THF1, is involved in the degradation of chlorophyll-protein complexes during leaf senescence. Plant J 74, 652-662. |
| 50 | Yoshida S (2003). Molecular regulation of leaf senescence. Curr Opin Plant Biol 6, 79-84. |
| 51 | Zhou Y, Huang WF, Liu L, Chen TY, Zhou F, Lin YJ (2013). Identification and functional characterization of a rice NAC gene involved in the regulation of leaf senescence. BMC Plant Biol 13, 132. |
| 52 | Zhou Y, Liu L, Huang WF, Yuan M, Zhou F, Li XH, Lin YJ (2014). Overexpression of OsSWEET5 in rice causes growth retardation and precocious senescence. PLoS One 9, e94210. |
| [1] |
Juan Cui, Xiaoyu Yu, Yuejiao Yu, Chengwei Liang, Jian Sun, Wenfu Chen.
Analysis of Texture Factors and Genetic Basis Influencing the Differences in Eating Quality between Northeast China and Japanese Japonica Rice [J]. Chinese Bulletin of Botany, 2025, 60(4): 1-0. |
| [2] | Zhao Ling, Guan Ju, Liang Wenhua, Zhang Yong, Lu Kai, Zhao Chunfang, Li Yusheng, Zhang Yadong. Mapping of QTLs for Heat Tolerance at the Seedling Stage in Rice Based on a High-density Bin Map [J]. Chinese Bulletin of Botany, 2025, 60(3): 342-353. |
| [3] | Xinyu Li, Yue Gu, Feifei Xu, Jinsong Bao. Research Progress on Post-translational Modifications of Starch Biosynthesis-related Proteins in Rice Endosperm [J]. Chinese Bulletin of Botany, 2025, 60(2): 256-270. |
| [4] | Jianguo Li, Yi Zhang, Wenjun Zhang. Iron Plaque Formation and Its Effects on Phosphorus Absorption in Rice Roots [J]. Chinese Bulletin of Botany, 2025, 60(1): 132-143. |
| [5] | Ruifeng Yao, Daoxin Xie. Activation and Termination of Strigolactone Signal Perception in Rice [J]. Chinese Bulletin of Botany, 2024, 59(6): 873-877. |
| [6] | Jinjin Lian, Luyao Tang, Yinuo Zhang, Jiaxing Zheng, Chaoyu Zhu, Yuhan Ye, Yuexing Wang, Wennan Shang, Zhenghao Fu, Xinxuan Xu, Richeng Wu, Mei Lu, Changchun Wang, Yuchun Rao. Genetic Locus Mining and Candidate Gene Analysis of Antioxidant Traits in Rice [J]. Chinese Bulletin of Botany, 2024, 59(5): 738-751. |
| [7] | Jiahui Huang, Huimin Yang, Xinyu Chen, Chaoyu Zhu, Yanan Jiang, Chengxiang Hu, Jinjin Lian, Tao Lu, Mei Lu, Weilin Zhang, Yuchun Rao. Response Mechanism of Rice Mutant pe-1 to Low Light Stress [J]. Chinese Bulletin of Botany, 2024, 59(4): 574-584. |
| [8] | Jianmin Zhou. A Combat Vehicle with a Smart Brake [J]. Chinese Bulletin of Botany, 2024, 59(3): 343-346. |
| [9] | Chaoyu Zhu, Chengxiang Hu, Zhenan Zhu, Zhining Zhang, Lihai Wang, Jun Chen, Sanfeng Li, Jinjin Lian, Luyao Tang, Qianqian Zhong, Wenjing Yin, Yuexing Wang, Yuchun Rao. Mapping of QTLs Associated with Rice Panicle Traits and Candidate Gene Analysis [J]. Chinese Bulletin of Botany, 2024, 59(2): 217-230. |
| [10] | Bao Zhu, Jiangzhe Zhao, Kewei Zhang, Peng Huang. OsCKX9 is Involved in Regulating the Rice Lamina Joint Development and Leaf Angle [J]. Chinese Bulletin of Botany, 2024, 59(1): 10-21. |
| [11] | Yanli Fang, Chuanyu Tian, Ruyi Su, Yapei Liu, Chunlian Wang, Xifeng Chen, Wei Guo, Zhiyuan Ji. Mining and Preliminary Mapping of Rice Resistance Genes Against Bacterial Leaf Streak [J]. Chinese Bulletin of Botany, 2024, 59(1): 1-9. |
| [12] | Qiwei Jia, Qianqian Zhong, Yujia Gu, Tianqi Lu, Wei Li, Shuai Yang, Chaoyu Zhu, Chengxiang Hu, Sanfeng Li, Yuexing Wang, Yuchun Rao. Mapping of QTL for Cell Wall Related Components in Rice Stem and Analysis of Candidate Genes [J]. Chinese Bulletin of Botany, 2023, 58(6): 882-892. |
| [13] | Tian Chuanyu, Fang Yanli, Shen Qing, Wang Hongjie, Chen Xifeng, Guo Wei, Zhao Kaijun, Wang Chunlian, Ji Zhiyuan. Genotypic Diversity and Pathogenisity of Xanthomonas oryzae pv. oryzae Isolated from Southern China in 2019-2021 [J]. Chinese Bulletin of Botany, 2023, 58(5): 743-749. |
| [14] | Dai Ruohui, Qian Xinyu, Sun Jinglei, Lu Tao, Jia Qiwei, Lu Tianqi, Lu Mei, Rao Yuchun. Research Progress on the Mechanisms of Leaf Color Regulation and Related Genes in Rice [J]. Chinese Bulletin of Botany, 2023, 58(5): 799-812. |
| [15] | Yuping Yan, Xiaoqi Yu, Deyong Ren, Qian Qian. Genetic Mechanisms and Breeding Utilization of Grain Number Per Panicle in Rice [J]. Chinese Bulletin of Botany, 2023, 58(3): 359-372. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||