

植物学报 ›› 2024, Vol. 59 ›› Issue (4): 558-573.DOI: 10.11983/CBB23129 cstr: 32102.14.CBB23129
赵来鹏1,2, 王柏柯2, 杨涛2, 李宁2, 杨海涛2, 王娟2,*( ), 闫会转1,*(
), 闫会转1,*( )
)
收稿日期:2023-09-15
接受日期:2023-12-19
出版日期:2024-07-10
发布日期:2024-07-10
通讯作者:
*王娟, 新疆农业科学院园艺作物研究所副研究员, 硕士生导师。长期从事番茄品质改良及抗逆性分子育种研究。以通讯作者和第一作者身份在Plant Physiology and Biochemistry和Environmental and Experimental Botany等国际期刊上发表研究论文20余篇。目前其研究团队以番茄为模式植物, 利用遗传学、基因组学及翻译组学等手段在方法学上不断创新, 解析番茄响应非生物胁迫及品质改良的分子机制, 利用基因组编辑技术开发番茄育种体系。E-mail: drjuanwang@126.com; hzhyan1118@163.com
基金资助:
Laipeng Zhao1,2, Baike Wang2, Tao Yang2, Ning Li2, Haitao Yang2, Juan Wang2,*( ), Huizhuan Yan1,*(
), Huizhuan Yan1,*( )
)
Received:2023-09-15
Accepted:2023-12-19
Online:2024-07-10
Published:2024-07-10
Contact:
*E-mail: drjuanwang@126.com; hzhyan1118@163.com
摘要: 植物在生长发育过程中面临各种非生物胁迫。其中干旱胁迫严重影响作物生长, 降低其产量。植物中以TB2/DP1结构域为特征的HVA22蛋白参与调控生长发育和非生物胁迫响应。然而, HVA22在番茄(Solanum lycopersicum)干旱胁迫响应中的功能尚不清楚。该研究探索了番茄SlHVA22l基因的功能。结果表明, 番茄SlHVA22l与其它双子叶植物中的HVA22l同源蛋白具有较高的序列相似性。表达模式分析显示, SlHVA22l基因表达受干旱胁迫和植物激素(ABA和MeJA)诱导。此外, 通过酵母(Saccharomyces cerevisiae)异源表达和病毒诱导基因沉默技术沉默番茄SlHVA22l基因, 验证了SlHVA22l基因的抗旱功能。干旱处理后沉默植株表现出较高的过氧化氢(H2O2)和丙二醛(MDA)含量, 以及较低的O2-.清除率, 且其超氧化物歧化酶(SOD)、过氧化物酶(POD)、过氧化氢酶(CAT)和抗坏血酸过氧化物酶(APX)的活性较对照显著降低。综上表明, SlHVA22l基因在番茄抵御干旱胁迫中发挥重要作用。
赵来鹏, 王柏柯, 杨涛, 李宁, 杨海涛, 王娟, 闫会转. SlHVA22l基因调节番茄耐旱性. 植物学报, 2024, 59(4): 558-573.
Laipeng Zhao, Baike Wang, Tao Yang, Ning Li, Haitao Yang, Juan Wang, Huizhuan Yan. Investigation of the Regulation of Drought Tolerance by the SlHVA22l Gene in Tomato. Chinese Bulletin of Botany, 2024, 59(4): 558-573.
| Primer name | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| SlHVA22l | GGCATGGCTAGTTTTTCCACATT | TGGGAGTGATGAAGTCCACAAA |
| SlPDS | GTGAACCCTGTCGGCCTTTA | AACTACGCTTGCTTCCGACA |
| Slactin | AGCAGGAACTTGAAACCGCT | CTCATGGATACCCGCAGCTT |
| SlDREB1A | GGTTTGTGTGCCGTTGGATT | AGGAACTTTACGCACTGGCT |
| SlHK1 | GCTCTGAGTGGGTTTGCTCT | GGCAAAGACTCTCTGGCCTT |
| SlAREB1 | AATGAGGGCAGGGATGGTTG | TCCATTGCTCTTCCCAAGTCC |
| SlPYL9 | CGGTTCATCCCGAGGTCATT | GTACCGCCAAACGCTCTGAT |
| SlAVP1 | CAACACCGCAAATATCGCCC | CGTCCATACCAGCCATCTCC |
| SlABI1 | AGTCAGCCGCAGTGTTTTTG | AGACAGTCCATCAAGCACGC |
| SlAAPK | TCTGGAGGGGAGCTGTTTGA | GTGCAGGACTTCCATCCAGT |
| SlMPK8 | CCAAGGTACCCAAGGCAACA | GCCTCGGACAAACAGGTTCT |
表1 qRT-PCR引物
Table 1 Primers for qRT-PCR
| Primer name | Forward primer (5'-3') | Reverse primer (5'-3') |
|---|---|---|
| SlHVA22l | GGCATGGCTAGTTTTTCCACATT | TGGGAGTGATGAAGTCCACAAA |
| SlPDS | GTGAACCCTGTCGGCCTTTA | AACTACGCTTGCTTCCGACA |
| Slactin | AGCAGGAACTTGAAACCGCT | CTCATGGATACCCGCAGCTT |
| SlDREB1A | GGTTTGTGTGCCGTTGGATT | AGGAACTTTACGCACTGGCT |
| SlHK1 | GCTCTGAGTGGGTTTGCTCT | GGCAAAGACTCTCTGGCCTT |
| SlAREB1 | AATGAGGGCAGGGATGGTTG | TCCATTGCTCTTCCCAAGTCC |
| SlPYL9 | CGGTTCATCCCGAGGTCATT | GTACCGCCAAACGCTCTGAT |
| SlAVP1 | CAACACCGCAAATATCGCCC | CGTCCATACCAGCCATCTCC |
| SlABI1 | AGTCAGCCGCAGTGTTTTTG | AGACAGTCCATCAAGCACGC |
| SlAAPK | TCTGGAGGGGAGCTGTTTGA | GTGCAGGACTTCCATCCAGT |
| SlMPK8 | CCAAGGTACCCAAGGCAACA | GCCTCGGACAAACAGGTTCT |

图1 SlHVA22l氨基酸序列分析 (A) 真核生物中HVA22l蛋白系统发育树; (B) 双子叶与单子叶植物中HVA22l蛋白系统发育树; (C) SlHVA22l与其它植物物种同源蛋白多序列比对; (D) TB2/DP1结构域。Cs: 甜橙; Gh: 陆地棉; Tc: 可可; Vv: 葡萄; Cas: 茶; Ah: 落花生; Coa: 小粒咖啡; Nt: 绒毛状烟草; Ca: 辣椒; Sl: 番茄; St: 马铃薯; Mb: 野蕉; Zo: 姜; Os: 水稻; Bd: 二穗短柄草; Ta: 小麦; Hv: 大麦; Sb: 高粱; Zm: 玉米; Si: 谷子; Sv: 狗尾草; Pv: 柳枝稷; Pm: 黍; Ph: 哈利谷草
Figure 1 Amino acid sequence analysis of SlHVA22l (A) Phylogenetic tree of the HVA22l protein in eukaryotic organisms; (B) Phylogenetic tree of HVA22l proteins in dicotyledonous and monocotyledonous plants; (C) Comparison of homologous proteins between SlHVA22l and other plant species through multiple sequence alignment; (D) The TB2/DP1 domain. Cs: Citrus sinensis; Gh: Gossypium hirsutum; Tc: Theobroma cacao; Vv: Vitis vinifera; Cas: Camellia sinensis; Ah: Arachis hypogaea; Coa: Coffea arabica; Nt: Nicotiana tomentosiformis; Ca: Capsicum annuum; Sl: Solanum lycopersicum; St: So. tuberosum; Mb: Musa balbisiana; Zo: Zingiber officinale; Os: Oryza sativa; Bd: Brachypodium distachyon; Ta: Triticum aestivum; Hv: Hordeum vulgare; Sb: Sorghum bicolor; Zm: Zea mays; Si: Setaria italica; Sv: Se. viridis; Pv: Panicum virgatum; Pm: P. miliaceum; Ph: P. hallii
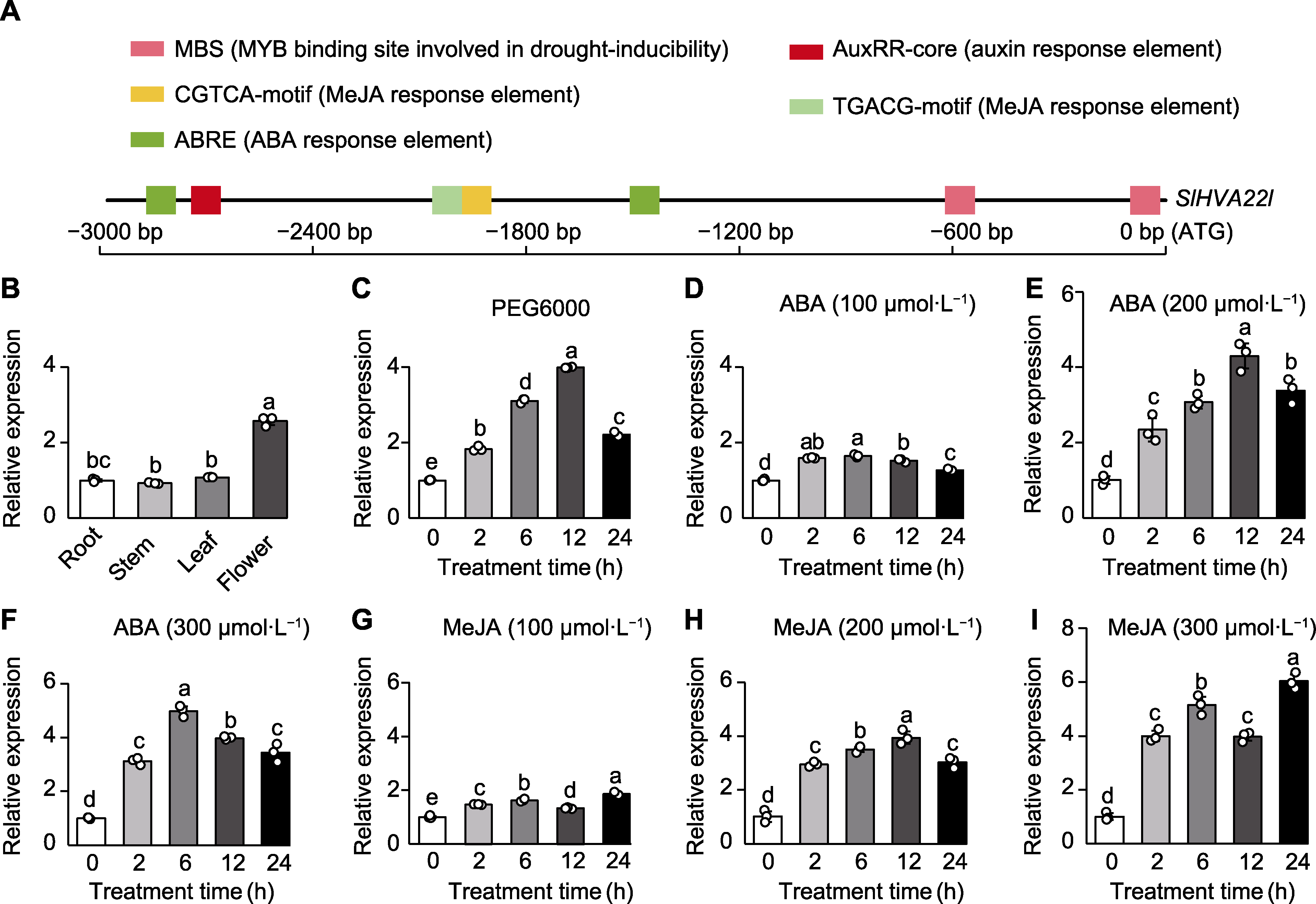
图2 SlHVA22l基因的顺式作用元件和表达模式分析 (A) SlHVA22l基因上游3 000 bp启动子区顺式作用元件分析; (B) SlHVA22l基因组织特异性表达; (C)-(I) PEG6000、ABA (100、200和300 µmol∙L-1)和MeJA (100、200和300 µmol∙L-1)处理0-24小时番茄SlHVA22l基因的表达变化。以Slactin为内参基因。不同小写字母表示各处理间差异显著(P<0.05)。
Figure 2 Cis-acting elements and expression pattern analysis of the SlHVA22l gene (A) Cis-acting elements within the 3 000 bp upstream promoter region of the SlHVA22l gene; (B) Tissue-specific expression of the SlHVA22l gene; (C)-(I) SlHVA22l expression pattern under PEG6000, ABA (100, 200, and 300 µmol∙L-1), and MeJA (100, 200, and 300 µmol∙L-1) treatments at 0-24 h. Slactin was used as a reference gene. Different lowercase letters indicate significant differences among different treatments (P<0.05).
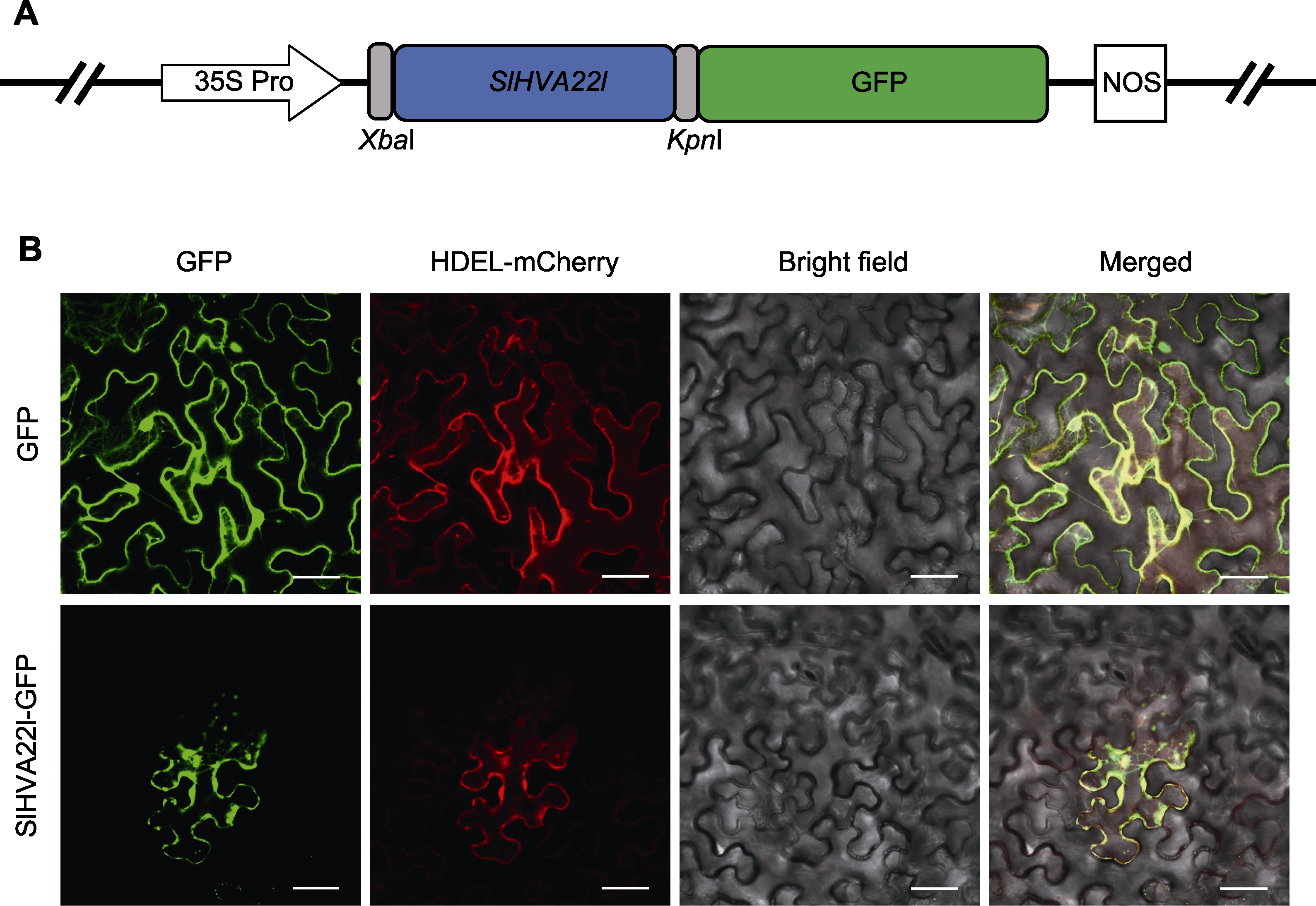
图3 SlHVA22l的亚细胞定位 (A) SlHVA22l在pCAMBIA1300-GFP载体中的示意图; (B) SlHVA22l蛋白在本氏烟草细胞中的定位。HDEL为内质网定位标记。Bars=50 μm
Figure 3 Subcellular localization of SlHVA22l (A) Schematic representation of SlHVA22l in the pCAMBIA1300-GFP vector; (B) Localization of the SlHVA22l protein in Ben’s tobacco cells. HDEL is an endoplasmic reticulum localization marker. Bars=50 μm
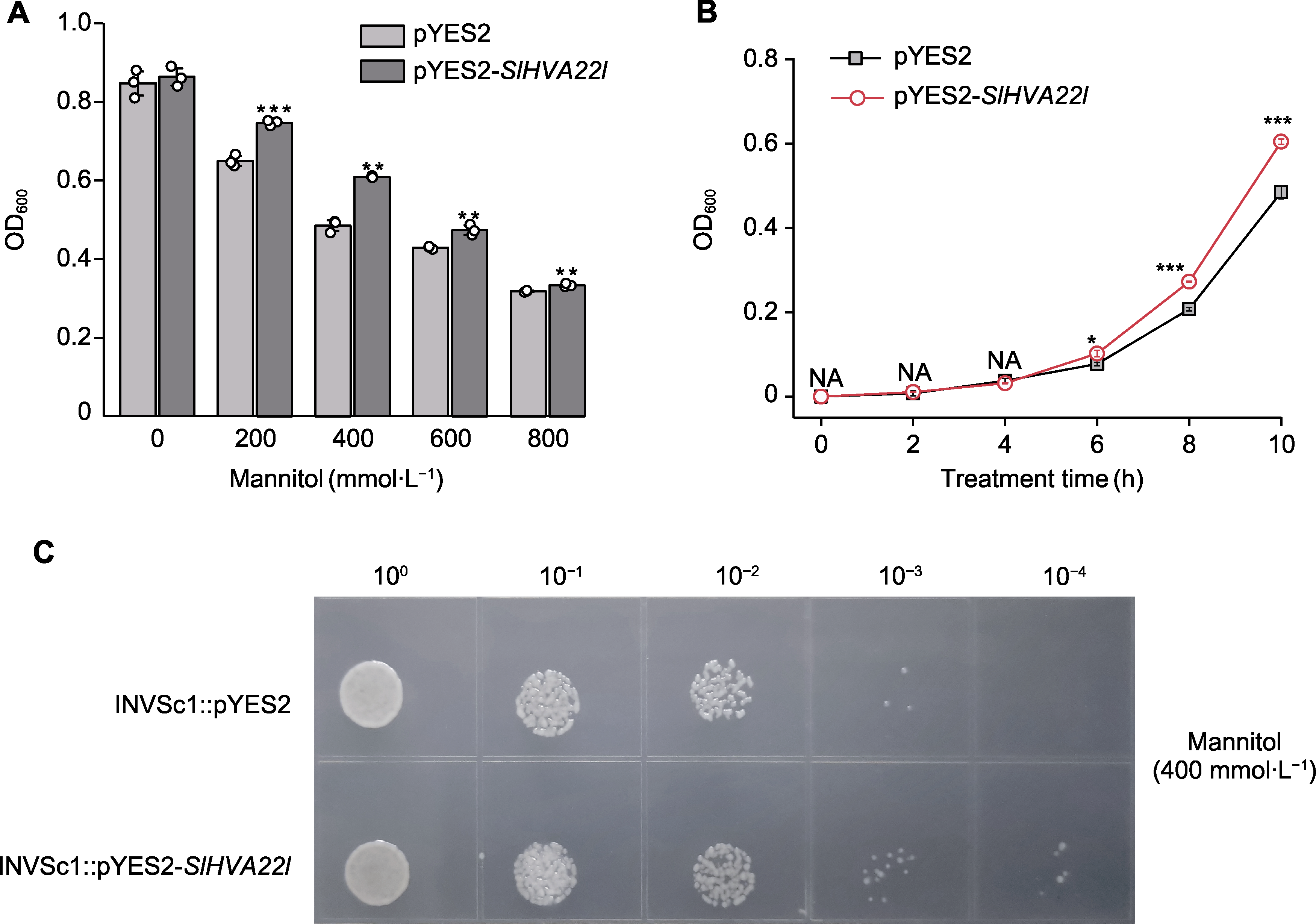
图4 SlHVA22l在酵母中的表达分析 (A) 重组酵母pYES2-SlHVA22l和对照菌pYES2对模拟干旱胁迫的响应; (B) 重组酵母pYES2-SlHVA22l和对照菌pYES2在干旱胁迫下的生长曲线; (C) 干旱胁迫下重组酵母pYES2-SlHVA22l和对照菌pYES2的滴板实验。* P<0.05; ** P<0.01; *** P<0.001
Figure 4 Expression analysis of SlHVA22l in Saccharomyces cerevisiae (A) Responses of recombinant pYES2-SlHVA22l and control pYES2 under simulated drought conditions; (B) Growth kinetics of recombinant pYES2-SlHVA22l and control pYES2 under drought stress; (C) Drip plate experiment of recombinant pYES2- SlHVA22l and control pYES2 under drought stress. * P<0.05; ** P<0.01; *** P<0.001
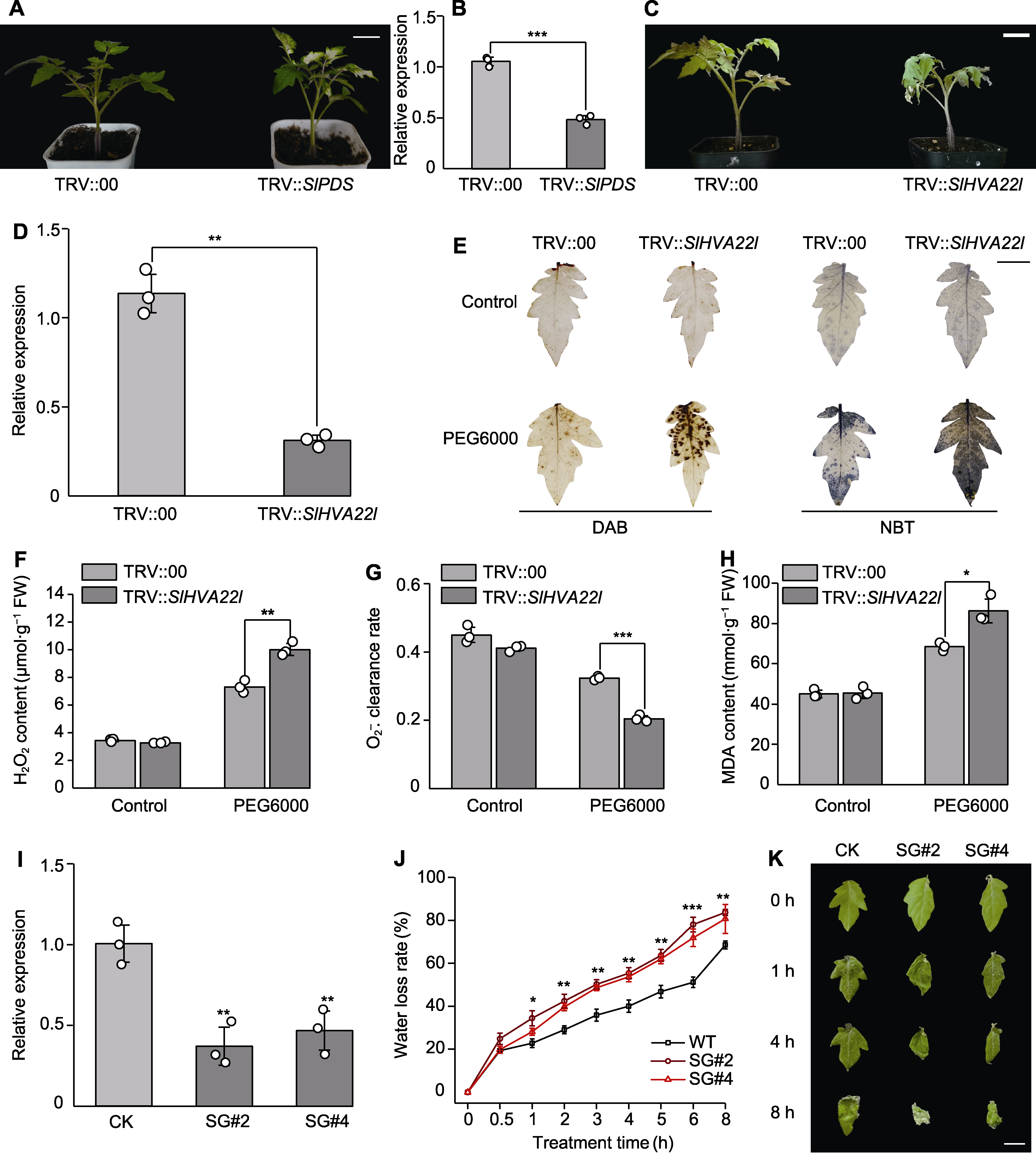
图5 沉默SlHVA22l基因对番茄耐旱性的影响 (A), (B) 将携带TRV::SlPDS的农杆菌注射2周龄番茄植株, 2周后的表型(A)及qRT-PCR检测SlPDS的沉默效率(B); (C) 对照植株和沉默植株在20% PEG6000处理48小时后的表型; (D) qRT-PCR检测SlHVA22l的沉默效率; (E) 干旱胁迫前后对照植株和沉默植株叶片DAB和NBT染色; (F) H2O2含量; (G) O2-. 清除率; (H) 丙二醛(MDA)含量; (I) 沉默株系鉴定; (J) 失水率; (K) 离体叶片失水表型。CK: TRV::00株系; SG: TRV::SlHVA22l株系。* P<0.05; ** P<0.01; *** P<0.001。(A), (C) Bars=3 cm; (E), (K) Bars=1 cm
Figure 5 The effect of SlHVA22l gene silencing on drought tolerance in tomato (A), (B) TRV::SlPDS-carrying Agrobacterium was introduced into 2-week-old tomato plants, after two weeks, phenotypic changes (A) and the efficiency of SlPDS silencing was quantified using qRT-PCR (B); (C) The phenotypes of the control and silenced plants were visually documented and recorded 48 hours after treatment with a 20% PEG6000 solution; (D) The silencing efficiency of SlHVA22l was assessed via qRT-PCR analysis; (E) DAB and NBT staining of leaves from drought-stressed control and silenced plants, respectively, were performed before and after the stress treatment; (F) H2O2 contents; (G) O2-. clearance rate; (H) Malondialdehyde (MDA) contents; (I) Identification of silenced plants; (J) Water loss rate; (K) Dehydration phenotype of detached leaves. CK: TRV::00; SG: TRV::SlHVA22l. * P<0.05; ** P<0.01; *** P<0.001. (A), (C) Bars=3 cm; (E), (K) Bars=1 cm
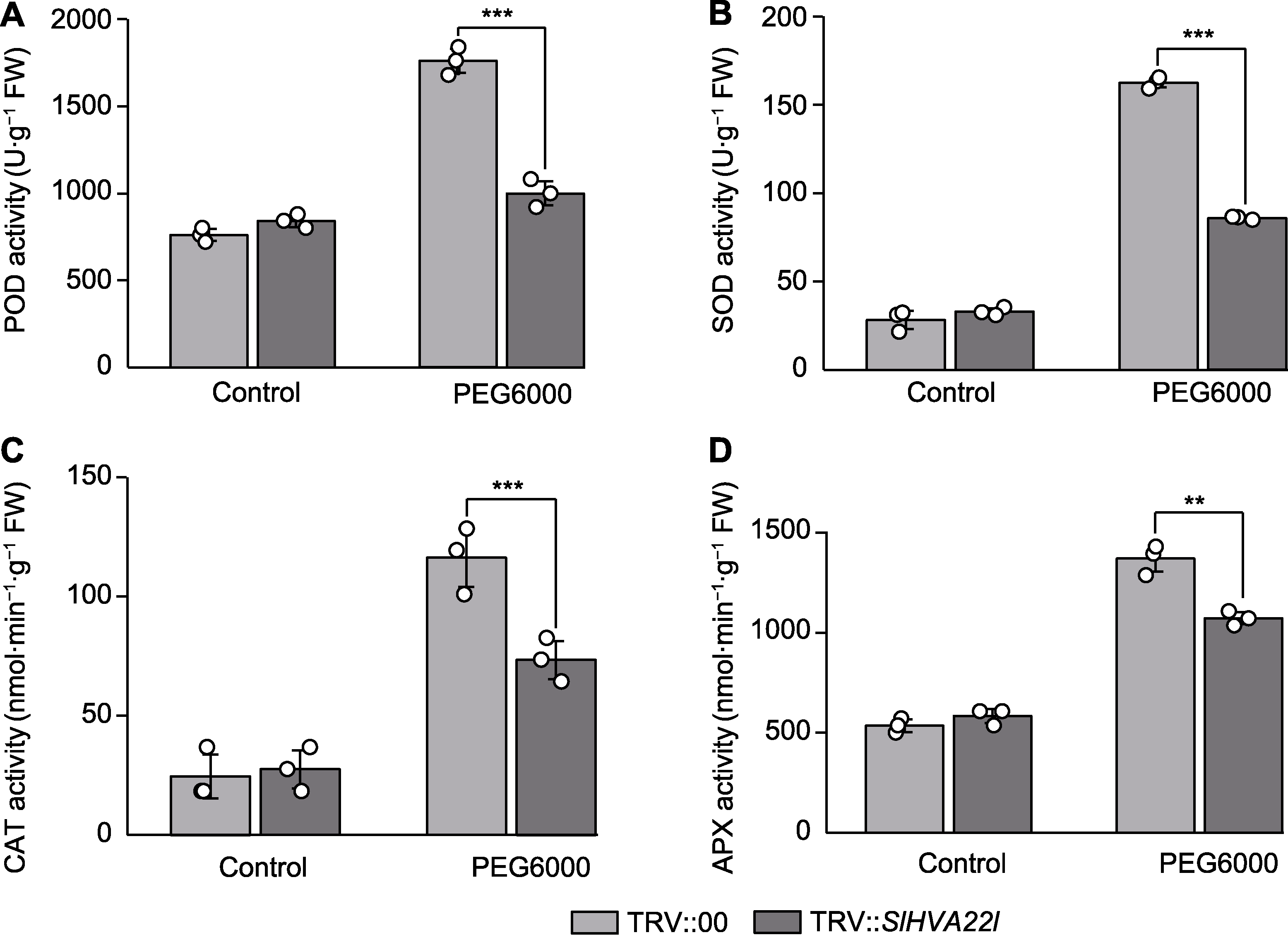
图6 干旱胁迫下SlHVA22l沉默番茄叶片的抗氧化酶活性 (A) 过氧化物酶(POD)活性; (B) 超氧化物歧化酶(SOD)活性; (C) 过氧化氢酶(CAT)活性; (D) 抗坏血酸过氧化物酶(APX)活性。** P<0.01; *** P<0.001
Figure 6 The antioxidant enzyme activities in SlHVA22l silenced tomato leaf under drought stress (A) Peroxidase (POD) activity; (B) Superoxide dismutase (SOD) activity; (C) Catalase (CAT) activity; (D) Ascorbate peroxidase (APX) activity. ** P<0.01; *** P<0.001
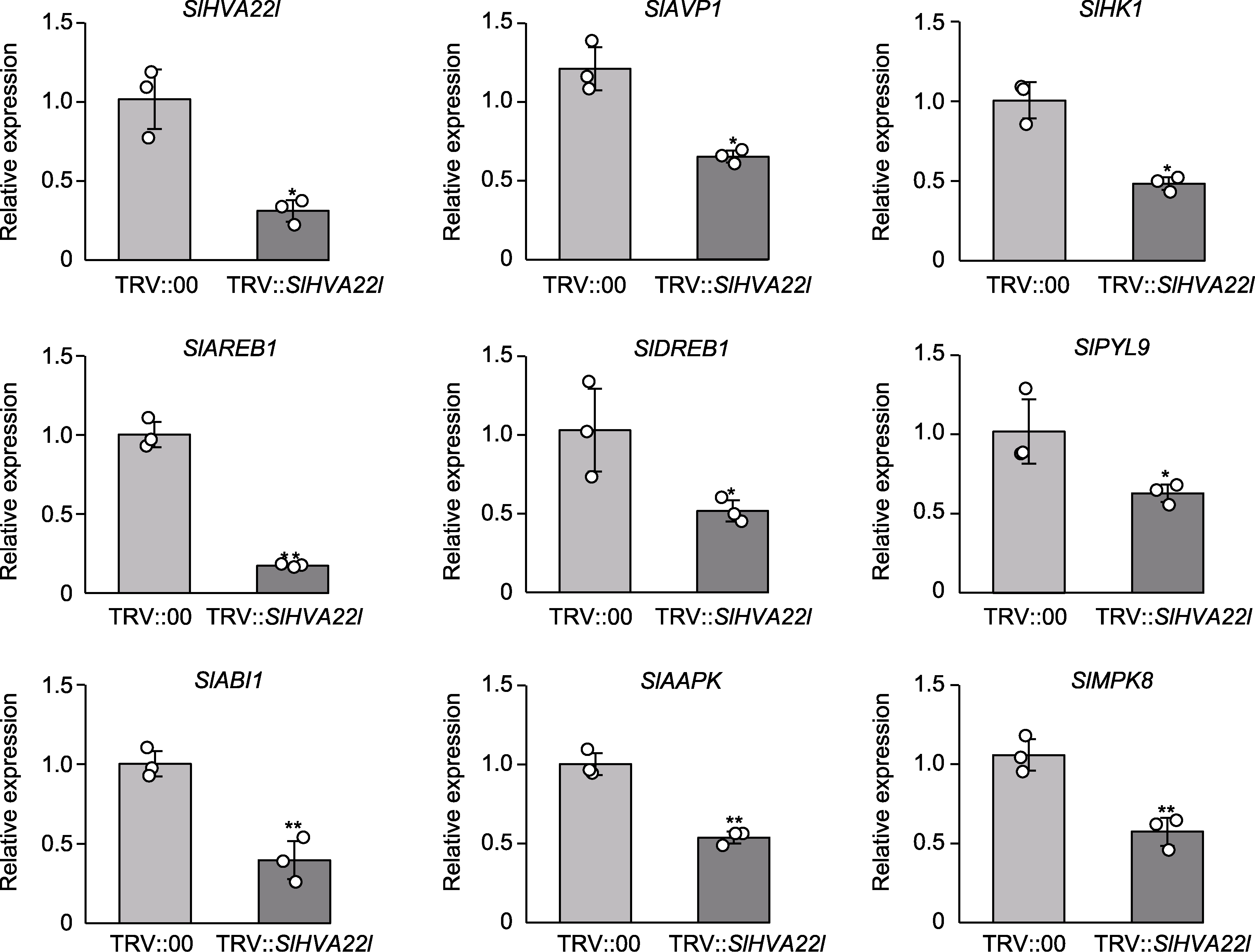
图7 SlHVA22l沉默番茄植株中干旱胁迫相关基因的表达水平 * P<0.05; ** P<0.01
Figure 7 Expression levels of drought stress-related genes in SlHVA22l silenced tomato plants * P<0.05; ** P<0.01
| [1] |
Argasinska J, Rana AA, Gilchrist MJ, Lachani K, Young A, Smith JC (2009). Loss of REEP4 causes paralysis of the Xenopus embryo. Int J Dev Biol 53, 37-43.
DOI PMID |
| [2] |
Casaretto JA, Ho THD (2005). Transcriptional regulation by abscisic acid in barley (Hordeum vulgare L.) seeds involves autoregulation of the transcription factor HvABI5. Plant Mol Biol 57, 21-34.
DOI PMID |
| [3] | Chaudhary J, Khatri P, Singla P, Kumawat S, Kumari A, Vikram A, Jindal SK, Kardile H, Kumar R, Sonah H (2019). Advances in omics approaches for abiotic stress tolerance in tomato. Biology 8, 90. |
| [4] | Chen CN, Chu CC, Zentella R, Pan SM, Ho THD (2002). AtHVA22 gene family in Arabidopsis: phylogenetic relationship, ABA and stress regulation, and tissue-specific expression. Plant Mol Biol 49, 631-642. |
| [5] | Choudhury FK, Rivero RM, Blumwald E, Mittler R (2017). Reactive oxygen species, abiotic stress and stress combination. Plant J 90, 856-867. |
| [6] | Danquah A, de Zélicourt A, Boudsocq M, Neubauer J, Frei dit Frey N, Leonhardt N, Pateyron S, Gwinner F, Tamby JP, Ortiz-Masia D, Marcote MJ, Hirt H, Colcombet J (2015). Identification and characterization of an ABA-activated MAP kinase cascade in Arabidopsis thaliana. Plant J 82, 232-244. |
| [7] | Eggert E, Obata T, Gerstenberger A, Gier K, Brandt T, Fernie AR, Schulze W, Kühn C (2016). A sucrose transporter-interacting protein disulphide isomerase affects redox homeostasis and links sucrose partitioning with abiotic stress tolerance. Plant Cell Environ 39, 1366- 1380. |
| [8] | Fukuda T, Saigusa T, Furukawa K, Inoue K, Yamashita SI, Kanki T (2023). Hva22, a REEP family protein in fission yeast, promotes reticulophagy in collaboration with a receptor protein. Autophagy 19, 2657-2667. |
| [9] | Gomes Ferreira MD, Araújo Castro J, Santana Silva RJ, Micheli F (2019). HVA22 from citrus: a small gene family whose some members are involved in plant response to abiotic stress. Plant Physiol Biochem 142, 395-404. |
| [10] | Grzesiak MT, Hordyńska N, Maksymowicz A, Grzesiak S, Szechyńska-Hebda M (2019). Variation among spring wheat (Triticum aestivum L.) genotypes in response to the drought stress. II-root system structure. Plants 8, 584. |
| [11] | Guo WJ, Ho THD (2008). An abscisic acid-induced protein, HVA22, inhibits gibberellin-mediated programmed cell death in cereal aleurone cells. Plant Physiol 147, 1710- 1722. |
| [12] |
Hata T, Nan HT, Koh K, Ishiura H, Tsuji S, Takiyama Y (2022). A clinical and genetic study of SPG31 in Japan. J Hum Genet 67, 421-425.
DOI PMID |
| [13] |
Iovieno P, Punzo P, Guida G, Mistretta C, Van Oosten MJ, Nurcato R, Bostan H, Colantuono C, Costa A, Bagnaresi P, Chiusano ML, Albrizio R, Giorio P, Batelli G, Grillo S (2016). Transcriptomic changes drive physiological responses to progressive drought stress and rehydration in tomato. Front Plant Sci 7, 371.
DOI PMID |
| [14] | Jiang LL, Wang YB, Zhang SH, He R, Li W, Han J, Cheng XG (2017). Tomato SlDREB1 gene conferred the transcriptional activation of drought-induced gene and an enhanced tolerance of the transgenic Arabidopsis to drought stress. Plant Growth Regul 81, 131-145. |
| [15] | Jiao P, Liu TY, Zhao CL, Fei JB, Guan SY, Ma YY (2023). ZmTCP14, a TCP transcription factor, modulates drought stress response in Zea mays L. Environ Exp Bot 208, 105232. |
| [16] | Kumar MN, Jane WN, Verslues PE (2013). Role of the putative osmosensor Arabidopsis Histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol 161, 942-953. |
| [17] | Letunic I, Bork P (2021). Interactive tree of life (iTOL) v5: an online tool for phylogenetic tree display and annotation. Nucleic Acids Res 49, W293-W296. |
| [18] | Li Q, Shen H, Yuan SJ, Dai XG, Yang CX (2023). miRNAs and lncRNAs in tomato: roles in biotic and abiotic stress responses. Front Plant Sci 13, 1094459. |
| [19] | Liang MW, Li HJ, Zhou F, Li HY, Liu J, Hao Y, Wang YD, Zhao HP, Han SC (2015). Subcellular distribution of NTL transcription factors in Arabidopsis thaliana. Traffic 16, 1062- 1074. |
| [20] |
Liu MM, Yu HY, Zhao GJ, Huang QF, Lu YE, Ouyang B (2017). Profiling of drought-responsive microRNA and mRNA in tomato using high-throughput sequencing. BMC Genomics 18, 481.
DOI PMID |
| [21] |
Livak KJ, Schmittgen TD (2001). Analysis of relative gene expression data using real-time quantitative PCR and the 2-∆∆CT method. Methods 25, 402-408.
DOI PMID |
| [22] | Lu P (2013). Physiological functional analysis of a stress- induced protein, HVA22, in Escherichia coli. Acce Inter J 1, 14-23. |
| [23] | Massimi M (2021). Tomato (Lycopersicon esculentum Mill.) anatomical, physiological, biochemical and production responses to drought stress-a mini-review essay. Int J Hortic Sci 27, 40-45. |
| [24] | Meng FW, Zhao QQ, Zhao X, Yang C, Liu R, Pang JH, Zhao WS, Wang Q, Liu MX, Zhang ZG, Kong ZS, Liu J (2022). A rice protein modulates endoplasmic reticulum homeostasis and coordinates with a transcription factor to initiate blast disease resistance. Cell Rep 39, 110941. |
| [25] | Nemeskéri E, Helyes L (2019). Physiological responses of selected vegetable crop species to water stress. Agronomy 9, 447. |
| [26] | Nemeskéri E, Neményi A, Bőcs A, Pék Z, Helyes L (2019). Physiological factors and their relationship with the productivity of processing tomato under different water supplies. Water 11, 586. |
| [27] |
Nicolas P, Shinozaki Y, Powell A, Philippe G, Snyder SI, Bao K, Zheng Y, Xu YM, Courtney L, Vrebalov J, Casteel CL, Mueller LA, Fei ZJ, Giovannoni JJ, Rose JKC, Catalá C (2022). Spatiotemporal dynamics of the tomato fruit transcriptome under prolonged water stress. Plant Physiol 190, 2557-2578.
DOI PMID |
| [28] | Orellana S, Yañez M, Espinoza A, Verdugo I, González E, Ruiz-Lara S, Casaretto JA (2010). The transcription factor SlAREB1 confers drought, salt stress tolerance and regulates biotic and abiotic stress-related genes in tomato. Plant Cell Environ 33, 2191-2208. |
| [29] |
Park S, Li JS, Pittman JK, Berkowitz GA, Yang HB, Undurraga S, Morris J, Hirschi KD, Gaxiola RA (2005). Up-regulation of a H+-pyrophosphatase (H+-PPase) as a strategy to engineer drought-resistant crop plants. Proc Natl Acad Sci USA 102, 18830-18835.
DOI PMID |
| [30] |
Reichardt S, Piepho HP, Stintzi A, Schaller A (2020). Peptide signaling for drought-induced tomato flower drop. Science 367, 1482-1485.
DOI PMID |
| [31] | Ripoll J, Urban L, Brunel B, Bertin N (2016). Water deficit effects on tomato quality depend on fruit developmental stage and genotype. J Plant Physiol 190, 26-35. |
| [32] | Rombauts S, Déhais P, Van Montagu M, Rouzé P (1999). PlantCARE, a plant cis-acting regulatory element database. Nucleic Acids Res 27, 295-296. |
| [33] | Sharon K, Suvarna S (2017). Cloning of HVA22 homolog from aloe vera and preliminary study of transgenic plant development. Int J Pure App Biosci 5, 1113-1121. |
| [34] | Shen QX, Chen CN, Brands A, Pan SM, Tuan-Hua DH (2001). The stress- and abscisic acid-induced barley gene HVA22: developmental regulation and homologues in diverse organisms. Plant Mol Biol 45, 327-340. |
| [35] |
Shen QX, Uknes S, Ho THD (1993). Hormone respon-se complex in a novel abscisic acid and cycloheximide-inducible barley gene. J Biol Chem 268, 23652-23660.
PMID |
| [36] | Sun HM, Li JT, Li X, Lv Q, Chen LP, Wang BX, Li LQ (2022a). RING E3 ubiquitin ligase TaSADR1 negatively regulates drought resistance in transgenic Arabidopsis. Plant Physiol Biochem 170, 255-265. |
| [37] | Sun ZH, Feng ZK, Ding YL, Qi YP, Jiang S, Li Z, Wang Y, Qi JS, Song CP, Yang SH, Gong ZZ (2022b). RAF22, ABI1 and OST1 form a dynamic interactive network that optimizes plant growth and responses to drought stress in Arabidopsis. Mol Plant 15, 1192-1210. |
| [38] |
Tamura K, Stecher G, Kumar S (2021). MEGA11: molecular evolutionary genetics analysis version 11. Mol Biol Evol 38, 3022-3027.
DOI PMID |
| [39] |
Wagih O (2017). ggseqlogo: a versatile R package for drawing sequence logos. Bioinformatics 33, 3645-3647.
DOI PMID |
| [40] | Wai AH, Waseem M, Cho LH, Kim ST, Lee DJ, Kim CK, Chung MY (2022). Comprehensive genome-wide analysis and expression pattern profiling of the SlHVA22 gene family unravels their likely involvement in the abiotic stress adaptation of tomato. Int J Mol Sci 23, 12222. |
| [41] | Wang L, Zheng B, Yuan Y, Xu QL, Chen P (2020a). Transcriptome profiling of Fagopyrum tataricum leaves in response to lead stress. BMC Plant Biol 20, 54. |
| [42] | Wang M, Yuan JR, Qin LM, Shi WM, Xia GM, Liu SW (2020b). TaCYP81D5, one member in a wheat cytochrome P450 gene cluster, confers salinity tolerance via reactive oxygen species scavenging. Plant Biotechnol J 18, 791-804. |
| [43] | Wang YJ, Zhang XY, Huang GR, Liu XY, Guo R, Gu FX, Zhong XL, Mei XR (2019). Characteristics of phosphatidic acid and the underlying mechanisms of ABA-induced stomatal movement in plants. Chin Bull Bot 54, 245-254. (in Chinese) |
|
王雅静, 张欣莹, 黄桂荣, 刘晓英, 郭瑞, 顾峰雪, 钟秀丽, 梅旭荣 (2019). 植物磷脂酸的特性及其在ABA诱导气孔运动中的作用. 植物学报 54, 245-254.
DOI |
|
| [44] | Wang YQ, Cao XY, Zhang DK, Li YQ, Wang QQ, Ma F, Xu X, Zhan XQ, Hu TX (2023). SlGATA17, a tomato GATA protein, interacts with SlHY5 to modulate salinity tolerance and germination. Environ Exp Bot 206, 105191. |
| [45] | Wei XT, Fan XH, Zhang HL, Jiao P, Jiang ZZ, Lu X, Liu SY, Guan SY, Ma YY (2022). Overexpression of ZmSRG7 improves drought and salt tolerance in maize (Zea mays L.). Int J Mol Sci 23, 13349. |
| [46] | Xiong HY, Yu JP, Miao JL, Li JJ, Zhang HL, Wang X, Liu PL, Zhao Y, Jiang CH, Yin ZG, Li Y, Guo Y, Fu BY, Wang WS, Li ZK, Ali J, Li ZC (2018). Natural variation in OsLG3 increases drought tolerance in rice by inducing ROS scavenging. Plant Physiol 178, 451-467. |
| [47] |
Yamaguchi-Shinozaki K, Shinozaki K (2006). Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu Rev Plant Biol 57, 781-803.
PMID |
| [48] |
Yang YG, Lv WT, Li MJ, Wang B, Sun DM, Deng X (2013). Maize membrane-bound transcription factor Zmbzip17 is a key regulator in the cross-talk of ER quality control and ABA signaling. Plant Cell Physiol 54, 2020-2033.
DOI PMID |
| [49] | Zhang HJ, Yuan YC, Xing HX, Xin M, Saeed M, Wu Q, Wu J, Zhuang T, Zhang XP, Mao LL, Sun XZ, Song XL, Wang ZW (2023). Genome-wide identification and expression analysis of the HVA22 gene family in cotton and functional analysis of GhHVA22E1D in drought and salt tolerance. Front Plant Sci 14, 1139526. |
| [50] | Zhang YG, Zhang Y, Ayibaiheremu Mutailifu, Zhang DY (2021). Heterologous overexpression of desiccation-tole- rance moss ScABI3gene changes stomatal phenotype and improves drought resistance in transgenic Arabidopsis. Chin Bull Bot 56, 414-421. (in Chinese) |
| 张一弓, 张怡, 阿依白合热木·木台力甫, 张道远 (2021). 异源过表达齿肋赤藓ScABI3基因改变拟南芥气孔表型并提高抗旱性. 植物学报 56, 414-421. | |
| [51] | Zhao LP, Wang BK, Yang T, Yan HZ, Yu QH, Wang J (2023). Genome-wide identification and analysis of the evolution and expression pattern of the HVA22 gene family in three wild species of tomatoes. PeerJ 11, e14844. |
| [52] | Zhou P, An Y, Wang ZL, Du HM, Huang BR (2014). Characterization of gene expression associated with drought avoidance and tolerance traits in a perennial grass species. PLoS One 9, e103611. |
| [1] | 王子韵, 吕燕文, 肖钰, 吴超, 胡新生. 植物基因表达调控与进化机制研究进展[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 樊蓓, 任敏, 王延峰, 党峰峰, 陈国梁, 程国亭, 杨金雨, 孙会茹. 番茄SlWRKY45转录因子在响应低温和干旱胁迫中的功能(长英文摘要)[J]. 植物学报, 2025, 60(2): 186-203. |
| [3] | 龙吉兰, 蒋铮, 刘定琴, 缪宇轩, 周灵燕, 冯颖, 裴佳宁, 刘瑞强, 周旭辉, 伏玉玲. 干旱下植物根系分泌物及其介导的根际激发效应研究进展[J]. 植物生态学报, 2024, 48(7): 817-827. |
| [4] | 廖人玉, 王佳伟. 从损伤到重生——REF1小肽如何激发植物的内在再生潜能[J]. 植物学报, 2024, 59(3): 347-350. |
| [5] | 段政勇, 丁敏, 王宇卓, 丁艺冰, 陈凌, 王瑞云, 乔治军. 糜子SBP基因家族全基因组鉴定及表达分析[J]. 植物学报, 2024, 59(2): 231-244. |
| [6] | 蔡淑钰, 刘建新, 王国夫, 吴丽元, 宋江平. 褪黑素促进镉胁迫下番茄种子萌发的调控机理[J]. 植物学报, 2023, 58(5): 720-732. |
| [7] | 张盈川, 吴晓明玉, 陶保龙, 陈丽, 鲁海琴, 赵伦, 文静, 易斌, 涂金星, 傅廷栋, 沈金雄. Bna-miR43介导甘蓝型油菜响应干旱胁迫[J]. 植物学报, 2023, 58(5): 701-711. |
| [8] | 吴楠, 覃磊, 崔看, 李海鸥, 刘忠松, 夏石头. 甘蓝型油菜EXA1的克隆及其对植物抗病的调控作用[J]. 植物学报, 2023, 58(3): 385-393. |
| [9] | 陈图强, 徐贵青, 刘深思, 李彦. 干旱胁迫下梭梭水力性状调整与非结构性碳水化合物动态[J]. 植物生态学报, 2023, 47(10): 1407-1421. |
| [10] | 王菲菲, 周振祥, 洪益, 谷洋洋, 吕超, 郭宝健, 朱娟, 许如根. 大麦NF-YC基因鉴定及在盐胁迫下的表达分析[J]. 植物学报, 2023, 58(1): 140-149. |
| [11] | 周洁, 杨晓东, 王雅芸, 隆彦昕, 王妍, 李浡睿, 孙启兴, 孙楠. 梭梭和骆驼刺对干旱的适应策略差异[J]. 植物生态学报, 2022, 46(9): 1064-1076. |
| [12] | 郭书亚, 艾金祥, 陈虹宇, 邵烨瑶, 汪妍, 王倩, 叶怡彤, 张雅婷, 丁哲晓, 吴昊辰, 吴玉环, 张建新, 饶米德, 刘鹏. 基于主成分-聚类-逐步回归分析构建番茄苗期耐铝性综合评价体系[J]. 植物学报, 2022, 57(4): 479-489. |
| [13] | 王晓敏, 李洪磊, 王林, 周鹏泽, 白圣懿, 李国花, 郑福顺, 陶小荣, 程国新, 高艳明, 李建设. 银川番茄斑萎病毒的分子鉴定[J]. 植物学报, 2021, 56(6): 715-721. |
| [14] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [15] | 李佳馨, 李霞, 谢寅峰. 外源海藻糖增强高表达转玉米C4型PEPC水稻耐旱性的机制[J]. 植物学报, 2021, 56(3): 296-314. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||