

植物学报 ›› 2021, Vol. 56 ›› Issue (2): 142-146.DOI: 10.11983/CBB21042 cstr: 32102.14.CBB21042
收稿日期:2021-03-02
接受日期:2021-03-05
出版日期:2021-03-01
发布日期:2021-03-17
通讯作者:
唐定中
作者简介:*E-mail: dztang@genetics.ac.cn基金资助:Received:2021-03-02
Accepted:2021-03-05
Online:2021-03-01
Published:2021-03-17
Contact:
Dingzhong Tang
摘要: 植物先天免疫系统在抵御病原菌入侵过程中发挥至关重要的作用, 主要包括两个层次, 即病原菌相关分子模式和效应因子分别触发的PTI和ETI免疫反应。PTI和ETI分别由植物细胞膜表面模式识别受体(PRRs)和胞内免疫受体(NLRs)激活, 具有特异的激活机制, 但是两者激活的下游免疫事件相互重叠。PTI和ETI是否为泾渭分明的两道防线, 以及ETI与PTI下游事件为何如此相似, 一直是植物免疫领域最受关注的问题之一。最近, 中国科学院分子植物科学卓越创新中心辛秀芳团队与合作者利用拟南芥(Arabidopsis thaliana)与丁香假单胞杆菌(Pseudomonas syringae)互作系统对PTI和ETI在机制上的联系进行了研究。他们发现PRRs和共受体参与ETI, 而活性氧的产生是联系PRRs和NLRs所介导的免疫早期信号事件。他们还发现NLRs信号能够迅速增强PTI关键因子的转录和蛋白水平, PTI的增强在ETI免疫反应中不可或缺。该研究从机制上解析了植物免疫领域中长期悬而未决的PTI与ETI相似性之谜, 是该领域的一项突破性进展, 为未来作物分子设计育种提供了新的启示。
王伟, 唐定中. 两类免疫受体强强联手筑牢植物免疫防线. 植物学报, 2021, 56(2): 142-146.
Wei Wang, Dingzhong Tang. Synergistic Cooperation Between Cell Surface and Intracellular Immune Receptors Potentiates to Activate Robust Plant Defense. Chinese Bulletin of Botany, 2021, 56(2): 142-146.
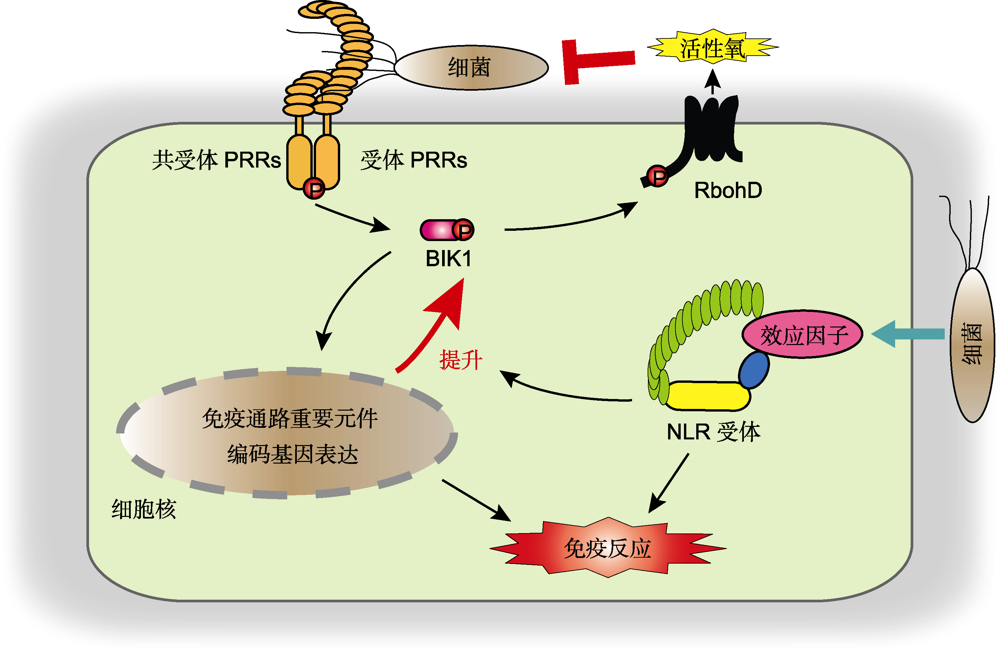
图1 植物先天免疫系统示意图 植物通过细胞膜表面PRRs受体和共受体识别病原菌PAMPs, 并磷酸化胞质型类受体激酶BIK1。激活的BIK1通过磷酸化RbohD正调控活性氧(ROS)爆发, 抑制病原菌生长。成功入侵的病原菌通过向植物细胞内分泌效应因子抑制免疫反应。宿主细胞内的NLRs受体识别特定的效应因子后, 不仅能够激活下游免疫反应, 而且能提升BIK1和RbohD等免疫关键蛋白的丰度, 增强活性氧爆发和免疫通路重要基因的表达, 导致更加强烈的免疫反应。
Figure 1 Schematic representation of the plant immune system Plants recognize the PAMPs of pathogens via the cell surface-localized PRRs, leads to phosphorylation of the receptor-like cytoplasmic kinase BIK1. The activated BIK1 positively regulates reactive oxygen species (ROS) burst by phosphorylation of RbohD, resulting in the inhibition of pathogen growth. Successful invaded pathogens deliver effectors into plant cell to inhibit immune responses. Upon perception of specific effectors, the host intracellular NLRs activate downstream immune responses, and boost the abundance of key immune-related proteins such as BIK1 and RbohD to enhance ROS burst and the transcripts of important genes in immune signaling, leading to robust immune responses.
| [1] |
Cesari S (2018). Multiple strategies for pathogen perception by plant immune receptors. New Phytol 219,17-24.
DOI URL |
| [2] |
Couto D, Zipfel C (2016). Regulation of pattern recognition receptor signaling in plants. Nat Rev Immunol 16,537- 552.
DOI URL |
| [3] |
Cui HT, Tsuda K, Parker JE (2015). Effector-triggered immunity: from pathogen perception to robust defense. Annu Rev Plant Biol 66,487-511.
DOI URL |
| [4] |
Daudi A, Cheng ZY, O'Brien JA, Mammarella N, Khan S, Ausubel FM, Bolwell GP (2012). The apoplastic oxidative burst peroxidase in Arabidopsis is a major component of pattern-triggered immunity. Plant Cell 24,275-287.
DOI URL |
| [5] |
Day B, Dahlbeck D, Huang J, Chisholm ST, Li DH, Staskawicz BJ (2005). Molecular basis for the RIN4 negative regulation of RPS2 disease resistance. Plant Cell 17,1292-1305.
DOI URL |
| [6] |
Jones JDG, Dangl JL (2006). The plant immune system. Nature 444,323-329.
DOI URL |
| [7] |
Kadota Y, Liebrand TWH, Goto Y, Sklenar J, Derbyshire P, Menke FLH, Torres MA, Molina A, Zipfel C, Coaker G, Shirasu K (2019). Quantitative phosphoproteomic analysis reveals common regulatory mechanisms bet- ween effector- and PAMP-triggered immunity in plants. New Phytol 221,2160-2175.
DOI URL |
| [8] |
Kim HS, Desveaux D, Singer AU, Patel P, Sondek J, Dangl JL (2005). The Pseudomonas syringae effector AvrRpt2 cleaves its C-terminally acylated target, RIN4, from Arabidopsis membranes to block RPM1 activation. Proc Natl Acad Sci USA 102,6496-6501.
DOI URL |
| [9] |
Lu DP, Wu SJ, Gao XQ, Zhang YL, Shan LB, He P (2010). A receptor-like cytoplasmic kinase, BIK1, associates with a flagellin receptor complex to initiate plant innate immunity. Proc Natl Acad Sci USA 107,496-501.
DOI URL |
| [10] |
Monteiro F, Nishimura MT (2018). Structural, functional, and genomic diversity of plant NLR proteins: an evolved resource for rational engineering of plant immunity. Annu Rev Phytopathol 56,243-267.
DOI URL |
| [11] | Ngou BPM, Ahn HK, Ding PT, Jones JDG (2021). Mutual potentiation of plant immunity by cell-surface and intracellular receptors. Nature doi: 10.1038/s41586-021-03315-7. |
| [12] |
Qi JS, Wang JL, Gong ZZ, Zhou JM (2017). Apoplastic ROS signaling in plant immunity. Curr Opin Plant Biol 38,92-100.
DOI URL |
| [13] |
Qi YP, Tsuda K, Glazebrook J, Katagiri F (2011). Physical association of pattern-triggered immunity (PTI) and effec-tor-triggered immunity (ETI) immune receptors in Arabidopsis. Mol Plant Pathol 12, 702-708.
DOI URL |
| [14] |
Shi H, Shen QJ, Qi YP, Yan HJ, Nie HZ, Chen YF, Zhao T, Katagiri F, Tang DZ (2013). BR-SIGNALING KINASE 1 physically associates with FLAGELLIN SENSING 2 and regulates plant innate immunity in Arabidopsis. Plant Cell 25, 1143-1157.
DOI URL |
| [15] |
Tang DZ, Wang GX, Zhou JM (2017). Receptor kinases in plant-pathogen interactions: more than pattern recogni- tion. Plant Cell 29,618-637.
DOI URL |
| [16] |
Torres MA, Dangl JL, Jones JDG (2002). Arabidopsis gp91 phox homologues AtrbohD and AtrbohF are required for accumulation of reactive oxygen intermediates in the plant defense response. Proc Natl Acad Sci USA 99,517-522.
DOI URL |
| [17] |
Wang W, Feng BM, Zhou JM, Tang DZ (2020). Plant immune signaling: advancing on two frontiers. J Integr Plant Biol 62,2-24.
DOI URL |
| [18] | Wei HL, Chakravarthy S, Mathieu J, Helmann TC, Stodghill P, Swingle B, Martin GB, Collmer A (2015). Pseudomonas syringae pv. tomato DC3000 Type III secretion effector polymutants reveal an interplay between HopAD1 and AvrPtoB. Cell Host Microbe 17, 752-762. |
| [19] | Yuan M, Jiang Z, Bi G, Nomura K, Liu M, Wang Y, Cai B, Zhou JM, He SY, Xin XF (2021). Pattern-recognition receptors are required for NLR-mediated plant immunity. Nature doi: 10.1038/s41586-021-03316-6. |
| [20] |
Zhang J, Li W, Xiang TT, Liu ZX, Laluk K, Ding XJ, Zou Y, Gao MH, Zhang XJ, Chen S, Mengiste T, Zhang YL, Zhou JM (2010). Receptor-like cytoplasmic kinases integrate signaling from multiple plant immune receptors and are targeted by a Pseudomonas syringae effector. Cell Host Microbe 7,290-301.
DOI URL |
| [21] |
Zhou JM, Zhang YL (2020). Plant immunity: danger percep- tion and signaling. Cell 181,978-989.
DOI URL |
| [1] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [2] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| [3] | 周玉萍, 颜嘉豪, 田长恩. 保卫细胞中ABA信号调控机制研究进展[J]. 植物学报, 2022, 57(5): 684-696. |
| [4] | 胡海涛, 钱婷婷, 杨玲. 基于H2DCFDA荧光探针的植物活性氧检测方法[J]. 植物学报, 2022, 57(3): 320-326. |
| [5] | 覃磊, 彭志红, 夏石头. 植物NLR免疫受体的识别、免疫激活与信号调控[J]. 植物学报, 2022, 57(1): 12-23. |
| [6] | 周俭民. 免疫信号轴揭示水稻与病原菌斗争的秘密[J]. 植物学报, 2021, 56(5): 513-515. |
| [7] | 王伟, 孙蒙祥. 花粉外被蛋白B类小肽, 花粉打开柱头大门的一把钥匙[J]. 植物学报, 2021, 56(2): 147-150. |
| [8] | 杨程惠子,唐先宇,李威,夏石头. NLR及其在植物抗病中的调控作用[J]. 植物学报, 2020, 55(4): 497-504. |
| [9] | 崔亚宁, 钱虹萍, 赵艳霞, 李晓娟. 模式识别受体的胞内转运及其在植物免疫中的作用[J]. 植物学报, 2020, 55(3): 329-339. |
| [10] | 姚玉婷,马家琦,冯晓莉,潘建伟,王超. 磷酸肌醇激酶FAB1调控拟南芥根毛伸长[J]. 植物学报, 2020, 55(2): 126-136. |
| [11] | 李伟滔, 贺闽, 陈学伟. ZmFBL41 Chang7-2: 玉米抗纹枯病的关键利器[J]. 植物学报, 2019, 54(5): 547-549. |
| [12] | 夏石头, 李昕. 开启防御之门: 植物抗病小体[J]. 植物学报, 2019, 54(3): 288-292. |
| [13] | 代宇佳,罗晓峰,周文冠,陈锋,帅海威,杨文钰,舒凯. 生物和非生物逆境胁迫下的植物系统信号[J]. 植物学报, 2019, 54(2): 255-264. |
| [14] | 马丹颖,季东超,徐勇,陈彤,田世平. 活性氧调控植物细胞自噬的研究进展[J]. 植物学报, 2019, 54(1): 81-92. |
| [15] | 何光明, 邓兴旺. 死亡信号传递: 叶绿体与线粒体间信号交流调控植物程序性细胞死亡[J]. 植物学报, 2018, 53(4): 441-444. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
