

植物学报 ›› 2020, Vol. 55 ›› Issue (2): 126-136.DOI: 10.11983/CBB19242 cstr: 32102.14.CBB19242
收稿日期:2019-12-16
接受日期:2020-02-17
出版日期:2020-03-01
发布日期:2020-02-17
通讯作者:
王超
基金资助:
Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang( )
)
Received:2019-12-16
Accepted:2020-02-17
Online:2020-03-01
Published:2020-02-17
Contact:
Chao Wang
摘要: FAB1/PIKfyve是介导PI(3,5)P2 (磷脂酰肌醇3,5-二磷酸)生物合成的磷酸肌醇激酶。在动物和酵母(Saccharomyces cerevisiae)中, PI(3,5)P2参与调控胞内膜运输, 但在植物中的研究较少。该文通过分析拟南芥(Arabidopsis thaliana) FAB1的T-DNA插入突变体的表型解析PI(3,5)P2的生物学功能。拟南芥FAB1基因家族包含FAB1A、FAB1B、FAB1C和FAB1D四个基因。研究发现, fab1a/b呈现雄配子体致死的表型。利用遗传杂交获得fab1b/c/d三突变体, 发现FAB1B、FAB1C和FAB1D功能缺失导致根毛相比野生型变短, 经FAB1特异性抑制剂YM201636处理后的野生型中也观察到相似的短根毛表型。此外, fab1b/c/d三突变体中DR5转录水平降低。同时, 外源施加生长素类似物2,4-D和NAA能部分恢复fab1b/c/d植株短根毛的表型, 但fab1b/c/d突变体对生长素转运抑制剂(1-NOA和TIBA)的敏感性与野生型相似。此外, FAB1B/C/D功能缺失使根毛中ROS的含量减少且影响肌动蛋白的表达。上述结果表明, FAB1B/C/D通过调控生长素分布、ROS含量和肌动蛋白的表达影响拟南芥根毛伸长。
姚玉婷,马家琦,冯晓莉,潘建伟,王超. 磷酸肌醇激酶FAB1调控拟南芥根毛伸长. 植物学报, 2020, 55(2): 126-136.
Yuting Yao,Jiaqi Ma,Xiaoli Feng,Jianwei Pan,Chao Wang. A Role of Arabidopsis Phosphoinositide Kinase, FAB1, in Root Hair Growth. Chinese Bulletin of Botany, 2020, 55(2): 126-136.
| Primer name | Primer sequence (5'-3') | Purpose |
|---|---|---|
| F1 | GGCGAGGGATATTGA GTTCAG | Genotyping of fab1b-2 and RT-PCR |
| R1 | GTCATACATGTGGGA TCACCG | Genotyping of fab1b-2 and RT-PCR |
| F2 | TGGGAGAAAACAGCAA TGAAC | Genotyping of fab1c-2 and RT-PCR |
| R2 | CACGACAACTTCCCCG AAGCACAA | Genotyping of fab1c-2 and RT-PCR |
| F3 | AGGTTGGGATGAATGG TTTTG | Genotyping of fab1d-2 and RT-PCR |
| R3 | AGGTCGTGCCGTATC TCTTTC | Genotyping of fab1d-2 and RT-PCR |
| sgtDs3'-1 | GGTTCCCGTCCGATT TCGACT | Genotyping of fab1c-2 |
| LBb1.3 | ATTTTGCCGATTTCG GAAC | Genotyping of fab1b-2 and fab1d-2 |
| AtACTIN-F | GTCGTACAACCGGTA TTGTG | Internal control for RT-PCR |
| AtACTIN-R | GAGCTGGTCTTTGAG GTTTC | Internal control for RT-PCR |
表1 引物序列
Table 1 Primers used in this study
| Primer name | Primer sequence (5'-3') | Purpose |
|---|---|---|
| F1 | GGCGAGGGATATTGA GTTCAG | Genotyping of fab1b-2 and RT-PCR |
| R1 | GTCATACATGTGGGA TCACCG | Genotyping of fab1b-2 and RT-PCR |
| F2 | TGGGAGAAAACAGCAA TGAAC | Genotyping of fab1c-2 and RT-PCR |
| R2 | CACGACAACTTCCCCG AAGCACAA | Genotyping of fab1c-2 and RT-PCR |
| F3 | AGGTTGGGATGAATGG TTTTG | Genotyping of fab1d-2 and RT-PCR |
| R3 | AGGTCGTGCCGTATC TCTTTC | Genotyping of fab1d-2 and RT-PCR |
| sgtDs3'-1 | GGTTCCCGTCCGATT TCGACT | Genotyping of fab1c-2 |
| LBb1.3 | ATTTTGCCGATTTCG GAAC | Genotyping of fab1b-2 and fab1d-2 |
| AtACTIN-F | GTCGTACAACCGGTA TTGTG | Internal control for RT-PCR |
| AtACTIN-R | GAGCTGGTCTTTGAG GTTTC | Internal control for RT-PCR |
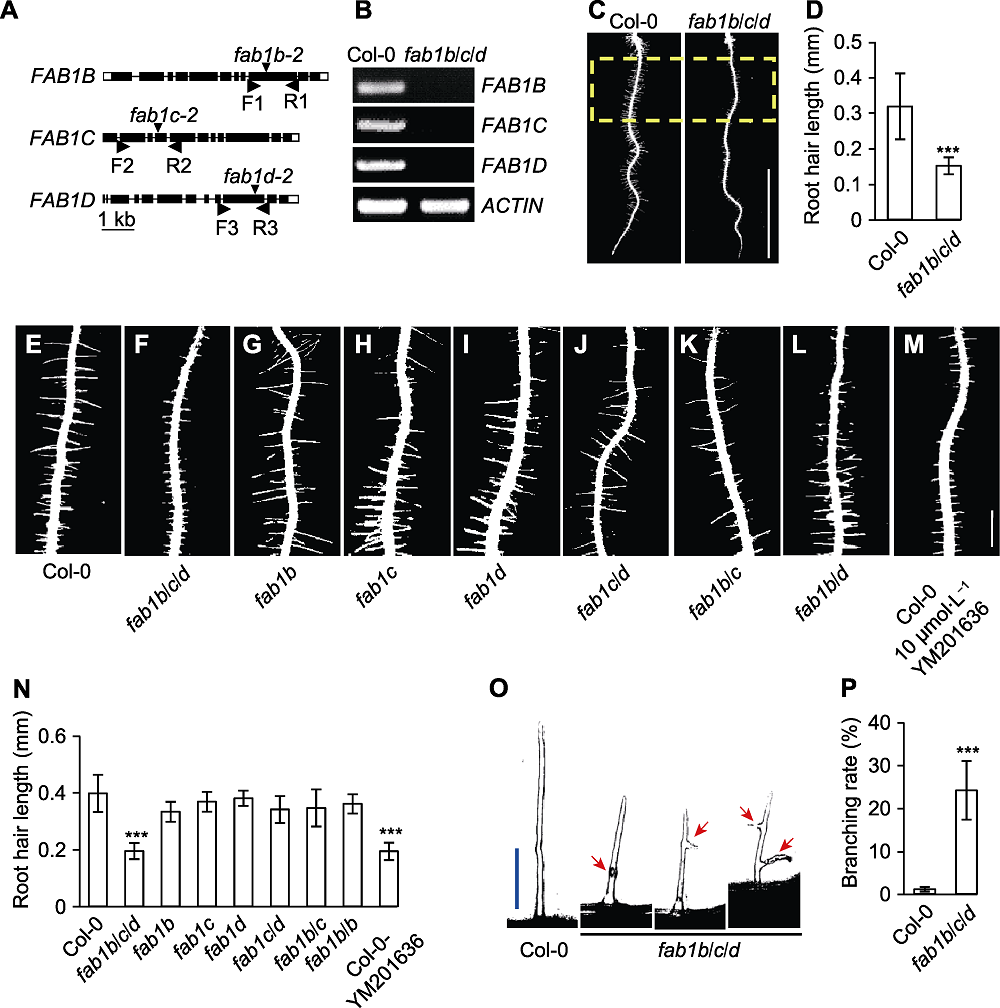
图1 FAB1B、FAB1C和FAB1D调控拟南芥根毛伸长 (A) FAB1基因结构和T-DNA插入位点; (B) RT-PCR检测FAB1B、FAB1C和FAB1D的表达; (C) 5天龄幼苗根毛长度(黄色虚线框代表定量区域) (Bar=5 mm); (D) 定量根毛长度; (E)-(M) FAB1单突变体、双突变体及YM201636 (FAB1特异性抑制剂)处理的野生型幼苗根毛表型(Bar=0.5 mm); (N) 定量(E)-(M)突变体根毛长度; (O) 根毛形态分析, 红色箭头表示根毛上的凸起和分叉(Bar=75 µm); (P) 定量分叉根毛占总根毛数量的百分比。*** P<0.001 (Student’s t-test)
Figure 1 FAB1B, FAB1C and FAB1D regulate root hair growth in Arabidopsis (A) FAB1 gene structure and T-DNA insertion sites; (B) Analysis of gene expression of FAB1B, FAB1C and FAB1D by RT-PCR; (C) Assay of root hair length in 5-day-old seedlings (the yellow dotted box represents quantitative area) (Bar=5 mm); (D) Quantification of root hair length; (E)-(M) The root hair phenotype of the FAB1 single mutants, double mutants and YM201636 (FAB1-specific inhibitor) treatment of Col-0 seedling (Bar=0.5 mm); (N) Quantification of (E)-(M) mutant root hair length; (O) Images of the typical root hair morphologies, the root hair morphologies were categorized as swollen or branched (red arrow indicates) (Bar=75 μm); (P) Quantification of frequency of branched root hairs (%). *** P<0.001 (Student’s t-test)
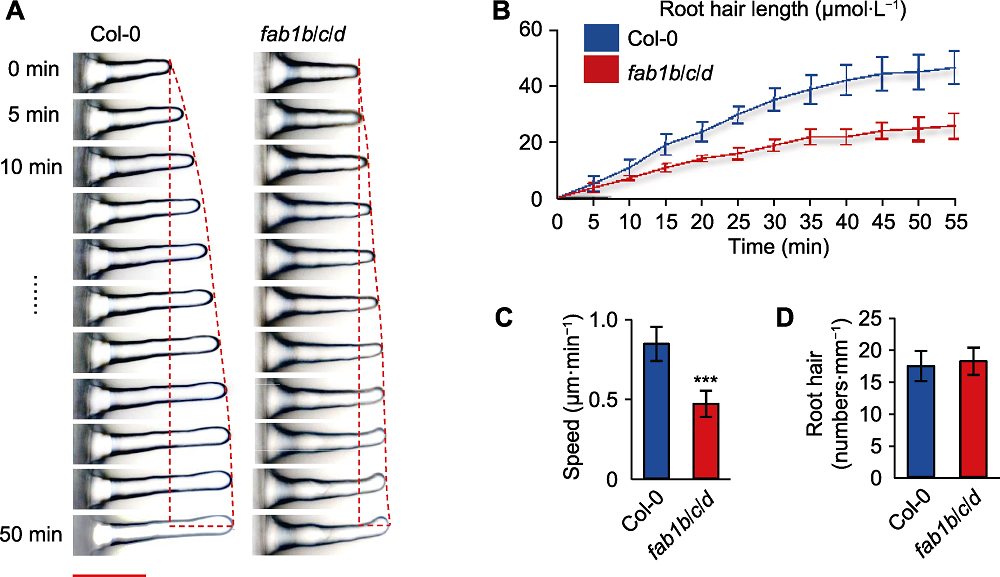
图2 拟南芥fab1b/c/d突变体根毛生长速率减慢 (A) 根毛伸长动力学分析(使用荧光显微镜连续50分钟记录野生型Col-0和fab1b/c/d突变体的动态生长趋势, 并每隔5分钟拍照1次) (Bar=50 µm); (B) 单位时间根毛长度; (C) 根毛生长速率(*** P<0.001, Student’s t-test); (D) 单位长度内根毛数量。
Figure 2 Slow growth of Arabidopsis fab1b/c/d mutant root hairs (A) Growth dynamics of individual Col-0 and fab1b/c/d root hairs (fluorescence microscopy was used to assess root hair elongation, showing consecutive frames of growing root hairs for a period of 50 minutes, pictures were taken every 5 minutes) (Bar=50 µm); (B) Root hair length per unit time; (C) Root hairs growth speed (*** P<0.001, Student’s t-test); (D) Average root hairs number.
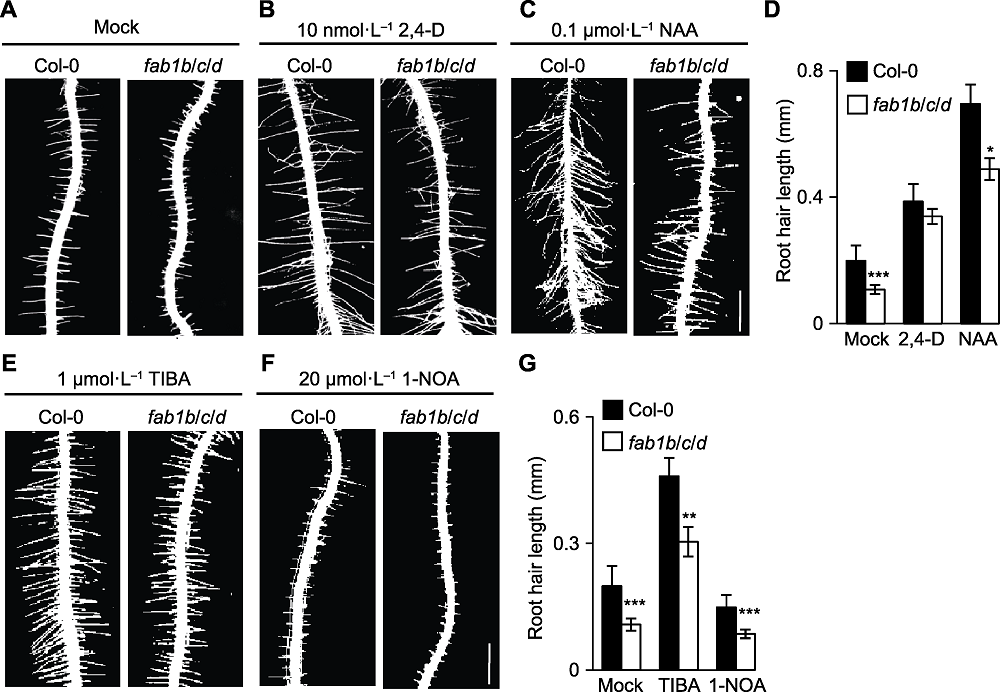
图3 外源生长素能部分恢复拟南芥fab1b/c/d突变体的短根毛表型 (A)-(C) 外源添加DMSO (Mock)、10 nmol·L-1 2,4-D或0.1 μmol·L-1 NAA可部分恢复fab1b/c/d突变体根毛表型(Bar=0.5 mm); (D) 定量分析根毛长度; (E), (F) 生长素运输抑制剂TIBA (生长素输出抑制剂)和1-NOA (生长素输入抑制剂)处理后根毛表型(Bar=0.5 mm); (G) 定量分析根毛长度。* P<0.05; ** P<0.01; *** P<0.001 (Student’s t-test)
Figure 3 Exogenous auxin application partially rescued the root hair defect of Arabidopsis fab1b/c/d mutants (A)-(C) Root hairs of fab1b/c/d treated with DMSO (Mock), 10 nmol·L-1 2,4-D and 0.1 μmol·L-1 NAA, respectively, the phenotype of root hairs was rescued partially (Bar=0.5 mm); (D) Quantification of root hair length; (E), (F) Col-0 and fab1b/c/d seedlings were transferred to plates containing TIBA (auxin efflux inhibitors) and 1-NOA (auxin influx inhibitor) (Bar=0.5 mm); (G) Quantitative analysis of root hair length. * P<0.05; ** P<0.01; *** P<0.001 (Student’s t-test)
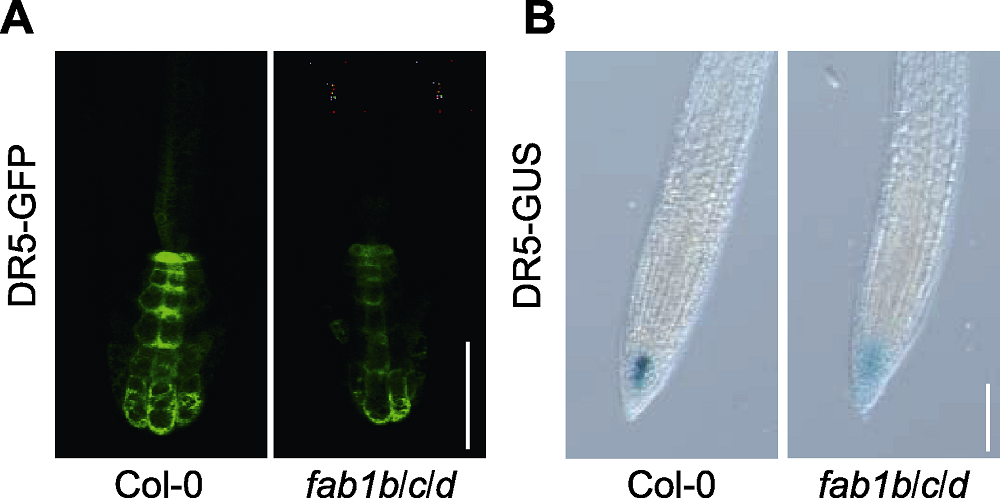
图4 FAB1影响拟南芥生长素的分布 (A) 利用DR5-GFP分析Col-0和fab1b/c/d根部的生长素分布变化(Bar=75 µm); (B) DR5-GUS在Col-0和fab1b/c/d中的表达情况(Bar=100 µm)
Figure 4 FAB1 affects auxin distribution in Arabidopsis (A) The expression analysis of DR5-GFP for auxin distribution in the root (Bar=75 µm); (B) DR5-GUS expression in Col-0 and fab1b/c/d (Bar=100 µm)
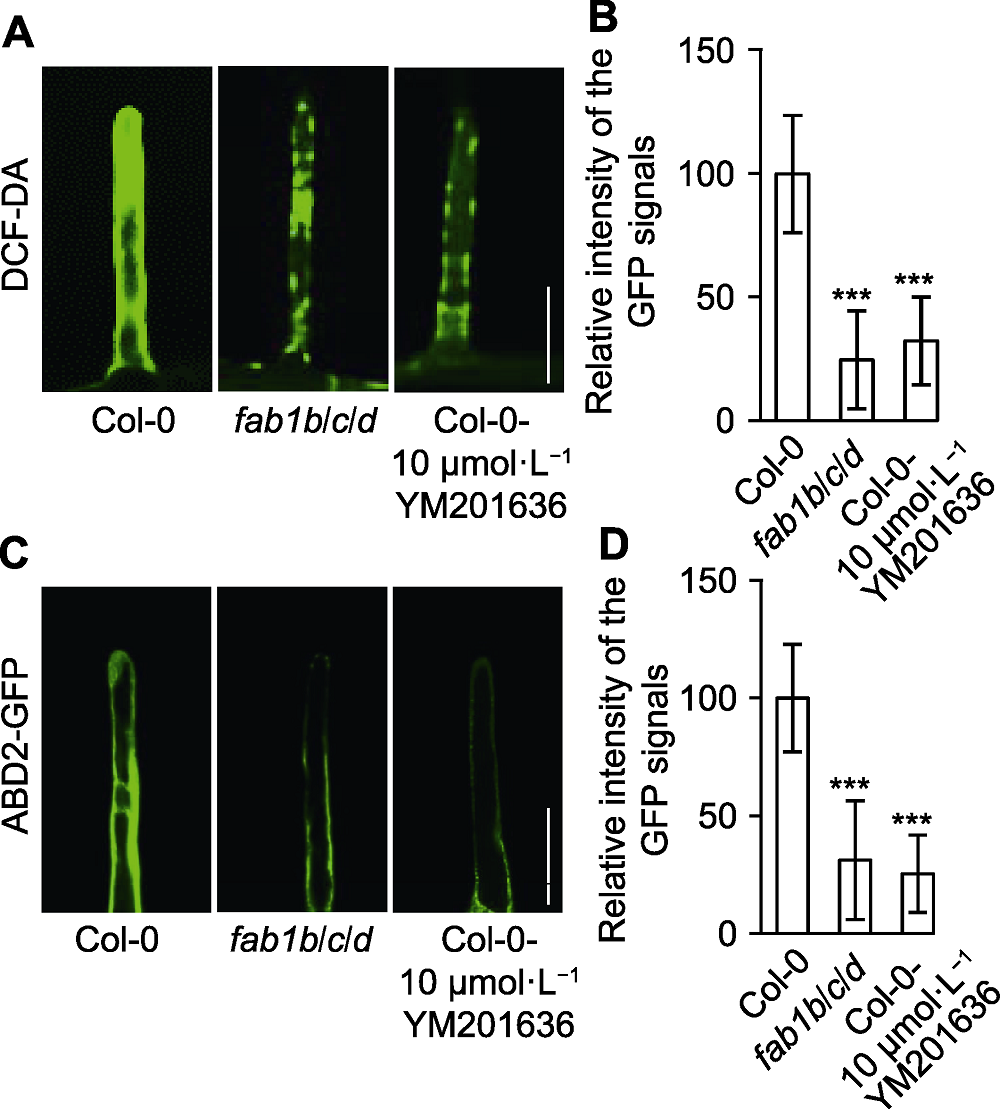
图5 拟南芥fab1b/c/d突变体根毛活性氧(ROS)含量及肌动蛋白稳定性改变 (A) 用DCF-DA (ROS染液)检测Col-0、fab1b/c/d和Col-0- YM201636根毛中的ROS含量(Bar=25 µm); (B) 定量分析DCF-DA荧光强度; (C) Col-0、fab1b/c/d和Col-0-YM201636的根毛肌动蛋白细胞骨架标记ABD2的分布(Bar=25 µm); (D) 定量分析ABD2-GFP荧光强度。*** P<0.001 (Student’s t-test)
Figure 5 Reactive oxygen species (ROS) intensity and actin stability were altered in root hairs of Arabidopsis fab1b/c/d seedlings (A) Total ROS generated by oxidation of DCF-DA in wild type, fab1b/c/d and Col-0-YM201636 root (Bar=25 µm); (B) Relative intensity of the GFP signals; (C) Distribution of actin cytoskeleton marker ABD2 in root hair of Col-0, fab1b/c/d and Col-0-YM201636 treatment (Bar=25 µm); (D) Average relative intensity of the ABD2-GFP signals. *** P<0.001 (Student’s t-test)
| [1] | 李林, 谭康, 唐秀光, 晁晓婷, 汶晨曦, 白壮东, 丰华玲, 刘文哲, 苏慧 ( 2016). 拟南芥根毛衰老死亡过程的PCD检测. 植物学报 51, 194-201. |
| [2] | 王立德, 廖红, 王秀荣, 严小龙 ( 2004). 植物根毛的发生、发育及养分吸收. 植物学通报 21, 649-659. |
| [3] | Bak G, Lee EJ, Lee Y, Kato M, Segami S, Sze H, Maeshima M, Hwang JU, Lee Y ( 2013). Rapid structural changes and acidification of guard cell vacuoles during stomatal closure require phosphatidylinositol 3,5-bisphosphate. Plant Cell 25, 2202-2216. |
| [4] | Balla T ( 2013). Phosphoinositides: tiny lipids with giant impact on cell regulation. Physiol Rev 93, 1019-1173. |
| [5] | Carol RJ, Dolan L ( 2006). The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot 57, 1829-1834. |
| [6] | Casimiro I, Beeckman T, Graham N, Bhalerao R, Zhang HM, Casero P, Sandberg G, Bennett MJ ( 2003). Dissecting Arabidopsis lateral root development. Trends Plant Sci 8, 165-171. |
| [7] | Cui SK, Suzaki T, Tominaga-Wada R, Yoshida S ( 2018). Regulation and functional diversification of root hairs. Semin Cell Dev Biol 83, 115-122. |
| [8] | De Craene JO, Bertazzi D, Bär S, Friant S ( 2017). Phosphoinositides, major actors in membrane trafficking and lipid signaling pathways. Int J Mol Sci 18, 634. |
| [9] | Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M ( 2005). Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9, 109-119. |
| [10] | Dove SK, Cooke FT, Douglas MR, Sayers LG, Parker PJ, Michell RH ( 1997). Osmotic stress activates phosphatidylinositol-3,5-bisphosphate synthesis. Nature 390, 187-192. |
| [11] | Dove SK, Dong KZ, Kobayashi T, Williams FK, Michell RH ( 2009). Phosphatidylinositol 3,5-bisphosphate and Fab1p/PIKfyve under PPIn endo-lysosome function. Biochem J 419, 1-13. |
| [12] | Ganguly A, Lee SH, Cho M, Lee OR, Yoo H, Cho HT ( 2010). Differential auxin-transporting activities of PIN- FORMED proteins in Arabidopsis root hair cells. Plant Physiol 153, 1046-1061. |
| [13] | Gary JD, Wurmser AE, Bonangelino CJ, Weisman LS, Emr SD ( 1998). Fab1p is essential for PtdIns(3)P5-kinase activity and the maintenance of vacuolar size and membrane homeostasis. J Cell Biol 143, 65-79. |
| [14] | Griersona C, Nielsen E, Ketelaarc T, Schiefelbein J ( 2014). Root hairs. Arabidopsis Book 12, e0172. |
| [15] | Hasegawa J, Strunk BS, Weisman LS ( 2017). PI5P and PI(3,5)P2: minor, but essential phosphoinositides. Cell Struct Funct 42, 49-60. |
| [16] | Hirano T, Konno H, Takeda S, Dolan L, Kato M, Aoyama T, Higaki T, Takigawa-Imamura H, Sato MH ( 2018). PtdIns(3,5)P2 mediates root hair shank hardening in Arabidopsis. Nat Plants 4, 888-897. |
| [17] | Hirano T, Matsuzawa T, Takegawa K, Sato MH ( 2011). Loss-of-function and gain-of-function mutations in FAB1A/B impair endomembrane homeostasis, conferring pleiotropic developmental abnormalities in Arabidopsis. Plant Physiol 155, 797-807. |
| [18] | Hirano T, Munnik T, Sato MH ( 2015). Phosphatidylinositol 3-phosphate 5-kinase, FAB1/PIKfyve kinase mediates endosome maturation to establish endosome-cortical microtubule interaction in Arabidopsis. Plant Physiol 169, 1961-1974. |
| [19] | Hirano T, Stecker K, Munnik T, Xu HX, Sato MH ( 2017). Visualization of phosphatidylinositol 3,5-bisphosphate dynamics by a tandem ML1N-based fluorescent protein probe in Arabidopsis. Plant Cell Physiol 58, 1185-1195. |
| [20] | Ishida T, Kurata T, Okada K, Wada T ( 2008). A genetic regulatory network in the development of trichomes and root hairs. Annu Rev Plant Biol 59, 365-386. |
| [21] | Jin N, Lang MJ, Weisman LS ( 2016). Phosphatidylinositol 3,5-bisphosphate: regulation of cellular events in space and time. Biochem Soc Trans 44, 177-184. |
| [22] | Jones AR, Kramer EM, Knox K, Swarup R, Bennett MJ, Lazarus CM, Leyser HMO, Grierson CS ( 2009). Auxin transport through non-hair cells sustains root-hair development. Nat Cell Biol 11, 78-84. |
| [23] | Jones MA, Raymond MJ, Yang ZB, Smirnoff N ( 2007). NADPH oxidase-dependent reactive oxygen species formation required for root hair growth depends on ROP GTPase. J Exp Bot 58, 1261-1270. |
| [24] | Ketelaar T, de Ruijter NCA, Emons AMC ( 2003). Unstable F-Actin specifies the area and microtubule direction of cell expansion in Arabidopsis root hairs. Plant Cell 15, 285-292. |
| [25] | Kirsch SA, Kugemann A, Carpaneto A, Böckmann RA, Dietrich P ( 2018). Phosphatidylinositol-3,5-bisphosphate lipid-binding-induced activation of the human two-pore channel 2. Cell Mol Life Sci 75, 3803-3815. |
| [26] | Kusano H, Testerink C, Vermeer JEM, Tsuge T, Shimada H, Oka A, Munnik T, Aoyama T ( 2008). The Arabidopsis phosphatidylinositol phosphate 5-Kinase PIP5K3 is a key regulator of root hair tip growth. Plant Cell 20, 367-380. |
| [27] | Lee RDW, Cho HT ( 2013). Auxin, the organizer of the hormonal/environmental signals for root hair growth. Front Plant Sci 4, 448. |
| [28] | Lee SH, Cho HT ( 2006). PINOID positively regulates auxin efflux in Arabidopsis root hair cells and tobacco cells. Plant Cell 18, 1604-1616. |
| [29] | Lee Y, Bak G, Choi Y, Chuang WI, Cho HT, Lee Y ( 2008). Roles of phosphatidylinositol 3-kinase in root hair growth. Plant Physiol 147, 624-635. |
| [30] | Mangano S, Denita-Juarez SP, Choi HS, Marzol E, Hwang Y, Ranocha P, Velasquez SM, Borassi C, Barberini ML, Aptekmann AA, Muschietti JP, Nadra AD, Dunand C, Cho HT, Estevez JM ( 2017). Molecular link between auxin and ROS-mediated polar growth. Proc Natl Acad Sci USA 114, 5289-5294. |
| [31] | McCartney AJ, Zhang YL, Weisman LS ( 2014). Phosphatidylinositol 3,5-bisphosphate: low abundance, high significance. BioEssays 36, 52-64. |
| [32] | Mendrinna A, Persson S ( 2015). Root hair growth: it's a one way street. F1000Prime Rep 7, 23. |
| [33] | Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S ( 2007). Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci USA 104, 20996-21001. |
| [34] | Mueller-Roeber B, Pical C ( 2002). Inositol phospholipid metabolism in Arabidopsis. Characterized and putative isoforms of inositol phospholipid kinase and phosphoinositide-specific phospholipase C. Plant Physiol 130, 22-46. |
| [35] | Nakamura M, Claes AR, Grebe T, Hermkes R, Viotti C, Ikeda Y, Grebe M ( 2018). Auxin and ROP GTPase signaling of polar nuclear migration in root epidermal hair cells. Plant Physiol 176, 378-391. |
| [36] | Nishimura T, Hayashi K, Suzuki H, Gyohda A, Takaoka C, Sakaguchi Y, Matsumoto S, Kasahara H, Sakai T, Kato J, Kamiya Y, Koshiba T ( 2014). Yucasin is a potent inhibitor of YUCCA, a key enzyme in auxin biosynthesis. Plant J 77, 352-366. |
| [37] | Niu YF, Jin CW, Jin GL, Zhou QY, Lin XY, Tang CX, Zhang YS ( 2011). Auxin modulates the enhanced development of root hairs in Arabidopsis thaliana(L.) Heynh. under elevated CO2. Plant Cell Environ 34, 1304-1317. |
| [38] | Payrastre B, Missy K, Giuriato S, Bosin S, Plantavid M, Gratacap MP ( 2001). Phosphoinositides: key players in cell signaling, in time and space. Cell Signal 13, 377-387. |
| [39] | Pei WK, Du F, Zhang Y, He T, Ren HY ( 2012). Control of the actin cytoskeleton in root hair development. Plant Sci 187, 10-18. |
| [40] | Qin H, Huang RF ( 2018). Auxin controlled by ethylene steers root development. Int J Mol Sci 19, 3656. |
| [41] | Rahman A, Hosokawa S, Oono Y, Amakawa T, Goto N, Tsurumi S ( 2002). Auxin and ethylene response interactions during Arabidopsis root hair development dissected by auxin influx modulators. Plant Physiol 130, 1908-1917. |
| [42] | Schoenaers S, Balcerowicz D, Breen G, Hill K, Zdanio M, Mouille G, Holman TJ, Oh J, Wilson MH, Nikonorova N, Vu LD, De Smet I, Swarup R, De Vos WH, Pintelon I, Adriaensen D, Grierson C, Bennett MJ, Vissenberg K ( 2018). The auxin-regulated CrRLK1L kinase ERULUS controls cell wall composition during root hair tip growth. Curr Biol 28, 722-732. |
| [43] | Serrazina S, Dias FV, Malhó R ( 2014). Characterization of FAB1 phosphatidylinositol kinases in Arabidopsis pollen tube growth and fertilization. New Phytol 203, 784-793. |
| [44] | Shibata M, Sugimoto K ( 2019). A gene regulatory network for root hair development. J Plant Res 132, 301-309. |
| [45] | Stenzel I, Ischebeck T, König S, Holubowska A, Sporysz M, Hause B, Heilmann I ( 2008). The type B phosphatidylinositol-4-phosphate 5-kinase 3 is essential for root hair formation in Arabidopsis thaliana. Plant Cell 20, 124-141. |
| [46] | Takasuga S, Horie Y, Sasaki J, Sun-Wada GH, Kawamura N, Iizuka R, Mizuno K, Equchi S, Kofuji S, Kimura H, Yamazaki M, Horie C, Odanaga E, Sato Y, Chida S, Kontani K, Harada A, Katada T, Suzuki A, Wada Y, Ohnishi H, Sasaki T ( 2013). Critical roles of type III phosphatidylinositol phosphate kinase in murine embryonic visceral endoderm and adult intestine. Proc Natl Acad Sci USA 110, 1726-1731. |
| [47] | Velasquez SM, Barbez E, Kleine-Vehn J, Estevez JM ( 2016). Auxin and cellular elongation. Plant Physiol 170, 1206-1215. |
| [48] | Vijayakumar P, Datta S, Dolan L ( 2016). ROOT HAIR DEFECTIVE SIX-LIKE 4 (RSL4) promotes root hair elongation by transcriptionally regulating the expression of genes required for cell growth. New Physiol 212, 944-953. |
| [49] | Whitley P, Hinz S, Doughty J ( 2009). Arabidopsis FAB1/PIKfyve proteins are essential for development of viable pollen. Plant Physiol 151, 1812-1822. |
| [50] | Zhang YL, Li E, Feng QN, Zhao XY, Ge FR, Zhang Y, Li S ( 2015). Protein palmitoylation is critical for the polar growth of root hairs in Arabidopsis. BMC Plant Biol 15, 50. |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [3] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [4] | 周玉滢, 陈辉, 刘斯穆. 植物非典型Aux/IAA蛋白应答生长素研究进展[J]. 植物学报, 2024, 59(4): 651-658. |
| [5] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [6] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [7] | 张悦婧, 桑鹤天, 王涵琦, 石珍珍, 李丽, 王馨, 孙坤, 张继, 冯汉青. 植物对非生物胁迫系统性反应中信号传递的研究进展[J]. 植物学报, 2024, 59(1): 122-133. |
| [8] | 孔祥培, 张蒙悦, 丁兆军. 柳暗花明:胞外生长素信号感受的新突破[J]. 植物学报, 2023, 58(6): 861-865. |
| [9] | 园园, 恩和巴雅尔, 齐艳华. 植物GH3基因家族生物学功能研究进展[J]. 植物学报, 2023, 58(5): 770-782. |
| [10] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [11] | 周淑瑶, 李建明, 毛娟. AtGH3.17调控拟南芥生长素和油菜素甾醇的响应[J]. 植物学报, 2023, 58(3): 373-384. |
| [12] | 张慧, 曾文静, 龚新桃, 马泽清. 亚热带典型树种根毛特征及其与共生真菌的关系[J]. 植物生态学报, 2023, 47(1): 88-100. |
| [13] | 周玉萍, 颜嘉豪, 田长恩. 保卫细胞中ABA信号调控机制研究进展[J]. 植物学报, 2022, 57(5): 684-696. |
| [14] | 叶青, 闫晓燕, 陈慧泽, 冯金林, 韩榕. 氮掺杂石墨烯量子点对拟南芥主根生长方向的影响[J]. 植物学报, 2022, 57(5): 623-634. |
| [15] | 刘晓龙, 季平, 杨洪涛, 丁永电, 付佳玲, 梁江霞, 余聪聪. 脱落酸对水稻抽穗开花期高温胁迫的诱抗效应[J]. 植物学报, 2022, 57(5): 596-610. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||