

植物学报 ›› 2020, Vol. 55 ›› Issue (2): 137-146.DOI: 10.11983/CBB19109 cstr: 32102.14.CBB19109
收稿日期:2019-06-14
接受日期:2019-11-05
出版日期:2020-03-01
发布日期:2020-02-12
通讯作者:
李华
基金资助:
Yang Zhang,Huajie Liu,Ruili Xue,Haixia Li,Hua Li( )
)
Received:2019-06-14
Accepted:2019-11-05
Online:2020-03-01
Published:2020-02-12
Contact:
Hua Li
摘要: 半胱氨酸脱巯基酶(CDes)可催化降解半胱氨酸(Cys)生成硫化氢(H2S)。通过克隆小麦(Triticum aestivum)中的L-半胱氨酸脱巯基酶基因TaLCD, 并将其在拟南芥(Arabidopsis thaliana)中过表达, 探讨TaLCD对渗透胁迫条件下种子萌发和根系生长的影响, 并分析其对干旱胁迫的调节作用。结果显示, 盐胁迫条件下, TaLCD过表达植株种子萌发率显著高于野生型; 甘露醇处理条件下, TaLCD过表达植株的根长也显著高于野生型, 且TaLCD过表达显著提高植株抗旱性。此外, TaLCD过表达植株对ABA更加敏感, ABA处理下TaLCD过表达植株的种子萌发率及根长均显著低于野生型。干旱胁迫下, TaLCD过表达植株胁迫响应基因(COR47、RD29A、RAB18和RD22)及ABA信号途径相关基因(NCED3、HAB1、HAB2、ABI1、ABI2和ABF2)的表达水平均显著高于野生型。因此推测, TaLCD增强植株抗旱和抗盐能力可能依赖于ABA信号途径。
张扬,刘华杰,薛瑞丽,李海霞,李华. 小麦TaLCD基因的克隆及其对渗透胁迫的调节作用. 植物学报, 2020, 55(2): 137-146.
Yang Zhang,Huajie Liu,Ruili Xue,Haixia Li,Hua Li. Cloning of Wheat TaLCD Gene and Its Regulation on Osmotic Stress. Chinese Bulletin of Botany, 2020, 55(2): 137-146.
| Gene name | Primer sequences | |
|---|---|---|
| Forward (5'-3') | Reverse (5'-3') | |
| TaDCD | GAGGAAGGACGGAAGCCATAT | TCAGGGTCATCGCAAACAGAG |
| TaLCD | TCCATTACGCCTACGGAGCAG | CAAGCCGGACCTTACGACCA |
| ABF2 | ATCAGAAGGGATAGGGAAGAGTAAT | TTGGTCTGCCGTGAATATCTGT |
| HAB1 | GTGTTCTCGCCATGTCTAGGTC | CTATTTCGCAGACTTCTTGGTTG |
| HAB2 | GCAGAAGTCCTTATTGCGAGTC | CTCAGAAGTTGCCACCTCCATA |
| ABI1 | TGACGGCTGTGAAGAGAGTA | CCATCTCACACGCTTCTTCA |
| ABI2 | ATTCAGACCATTCACTGACCCTC | GCTCCGTCGCCAGAACAAG |
| NCED3 | TGGCTTCTTTCACGGCAAC | ACAACAATGGCGGGAGAGTTT |
| COR47 | GAGGTTACGGATCGTGGAT | GAGCTGTTGGATCGGTGA |
| RAB18 | ATGGCGTCTTACCAGAACCGTCCA | TACCCTTGGCCACCTGATCC |
| RD29A | GTTACTGATCCCACCAAAGAAGA | GGAGACTCATCAGTCACTTCCA |
| RD22 | AGGGCTGTTTCCACTGAGG | CACCACAGATTTATCGTCAGACA |
| Actin2 | TACCCGATGGGCAAGTCA | TGCTCATACGGTCAGCGATA |
表1 qRT-PCR分析所用引物
Table 1 Primers used for qRT-PCR analysis
| Gene name | Primer sequences | |
|---|---|---|
| Forward (5'-3') | Reverse (5'-3') | |
| TaDCD | GAGGAAGGACGGAAGCCATAT | TCAGGGTCATCGCAAACAGAG |
| TaLCD | TCCATTACGCCTACGGAGCAG | CAAGCCGGACCTTACGACCA |
| ABF2 | ATCAGAAGGGATAGGGAAGAGTAAT | TTGGTCTGCCGTGAATATCTGT |
| HAB1 | GTGTTCTCGCCATGTCTAGGTC | CTATTTCGCAGACTTCTTGGTTG |
| HAB2 | GCAGAAGTCCTTATTGCGAGTC | CTCAGAAGTTGCCACCTCCATA |
| ABI1 | TGACGGCTGTGAAGAGAGTA | CCATCTCACACGCTTCTTCA |
| ABI2 | ATTCAGACCATTCACTGACCCTC | GCTCCGTCGCCAGAACAAG |
| NCED3 | TGGCTTCTTTCACGGCAAC | ACAACAATGGCGGGAGAGTTT |
| COR47 | GAGGTTACGGATCGTGGAT | GAGCTGTTGGATCGGTGA |
| RAB18 | ATGGCGTCTTACCAGAACCGTCCA | TACCCTTGGCCACCTGATCC |
| RD29A | GTTACTGATCCCACCAAAGAAGA | GGAGACTCATCAGTCACTTCCA |
| RD22 | AGGGCTGTTTCCACTGAGG | CACCACAGATTTATCGTCAGACA |
| Actin2 | TACCCGATGGGCAAGTCA | TGCTCATACGGTCAGCGATA |
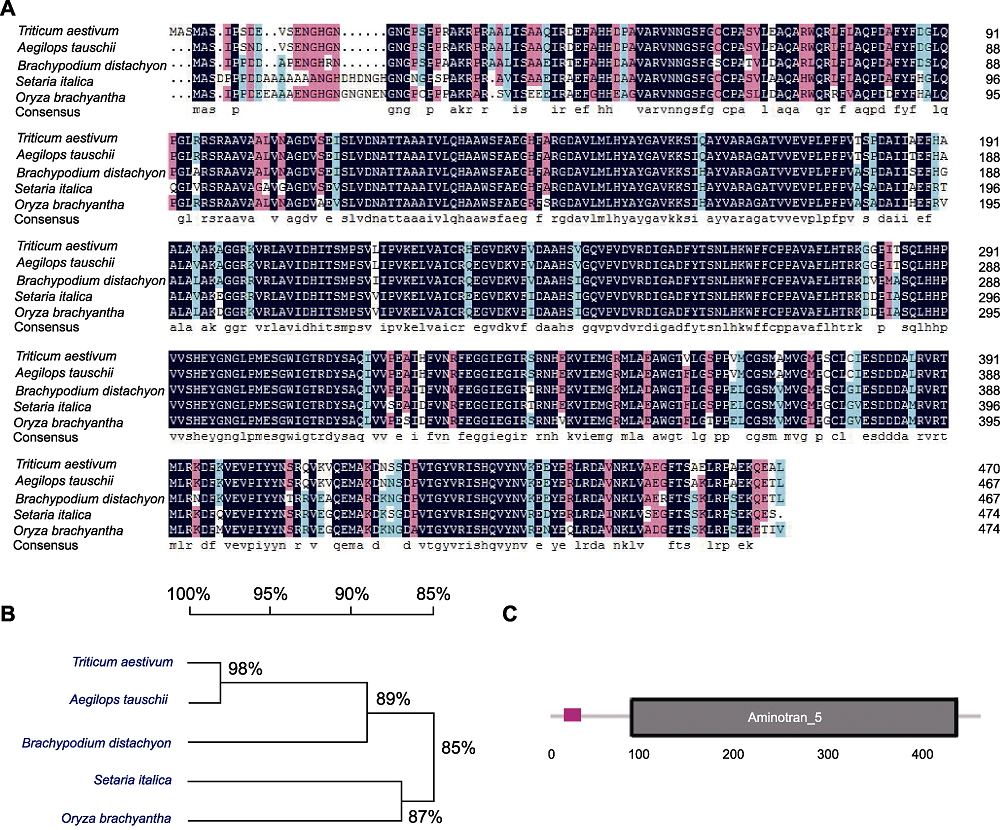
图1 TaLCD序列分析 (A) 小麦中LCD氨基酸序列与其它4个物种(粗山羊草、二穗短柄草、谷子和短花药野生稻)中LCD序列同源比对; (B) 5个物种间LCD进化树分析; (C) TaLCD的结构示意图, 黑框表示Aminotran_5结构域。
Figure 1 Sequence analysis of TaLCD (A) Comparison of the derived amino acid sequences of TaLCD in Triticum aestivum with other 4 species (Aegilops tauschii, Brachypodium distachyon, Setaria italica, and Oryza brachyantha); (B) Phylogenetic analysis of LCD in five species; (C) Schematic structures of TaLCD, black box indicates Aminotran_5 domain.
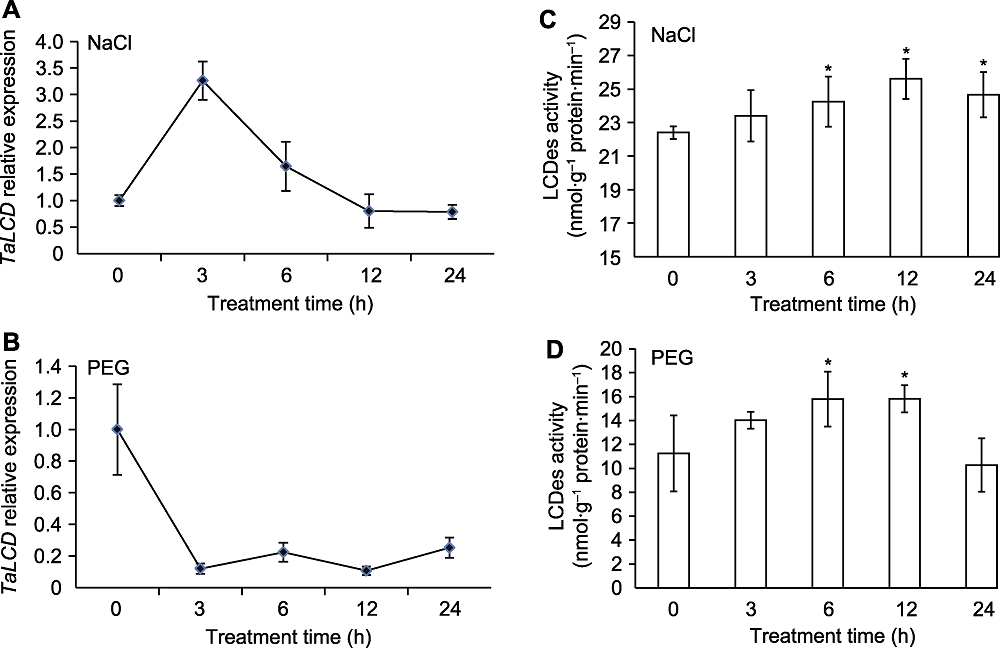
图2 NaCl和PEG处理下小麦TaLCD的表达及酶活变化 (A) NaCl处理下TaLCD的表达; (B) PEG处理下TaLCD的表达; (C) NaCl处理下TaLCD酶活变化; (D) PEG处理下TaLCD酶活变化。* 表示差异显著(P<0.05)。
Figure 2 The expression of TaLCD and enzyme activity of TaLCD in wheat under NaCl and PEG treatments (A) The expression of TaLCD under NaCl treatment; (B) The expression of TaLCD under PEG treatment; (C) The enzyme activity of TaLCD under NaCl treatment; (D) The enzyme activity of TaLCD under PEG treatment. * indicate significant differences (P<0.05).
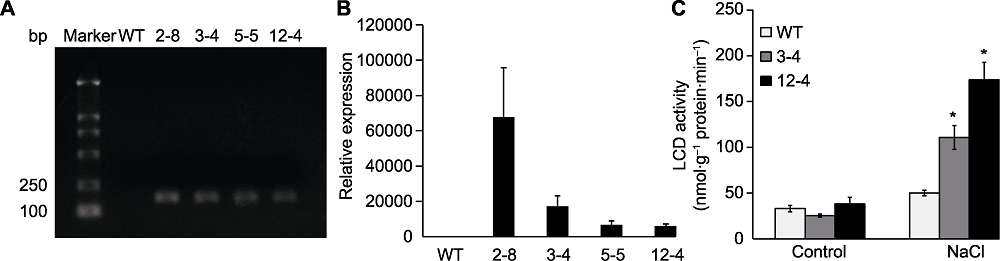
图3 TaLCD转基因拟南芥植株中TaLCD的表达及酶活 (A) RT-PCR; (B) qRT-PCR; (C) NaCl处理下TaLCD的酶活变化。Marker: DNA分子量标准; WT: 野生型; 2-8、3-4、5-5和12-4: TaLCD转基因株系; * 表示差异显著(P<0.05)。
Figure 3 Expression of TaLCD and enzyme activity of TaLCD in transgenic Arabidopsis (A) RT-PCR; (B) qRT-PCR; (C) Enzyme activity of TaLCD under NaCl treatment. Marker: DNA marker; WT: Wild type; 2-8, 3-4, 5-5, and 12-4: TaLCD transgenic lines; * indicate significant differences (P<0.05).
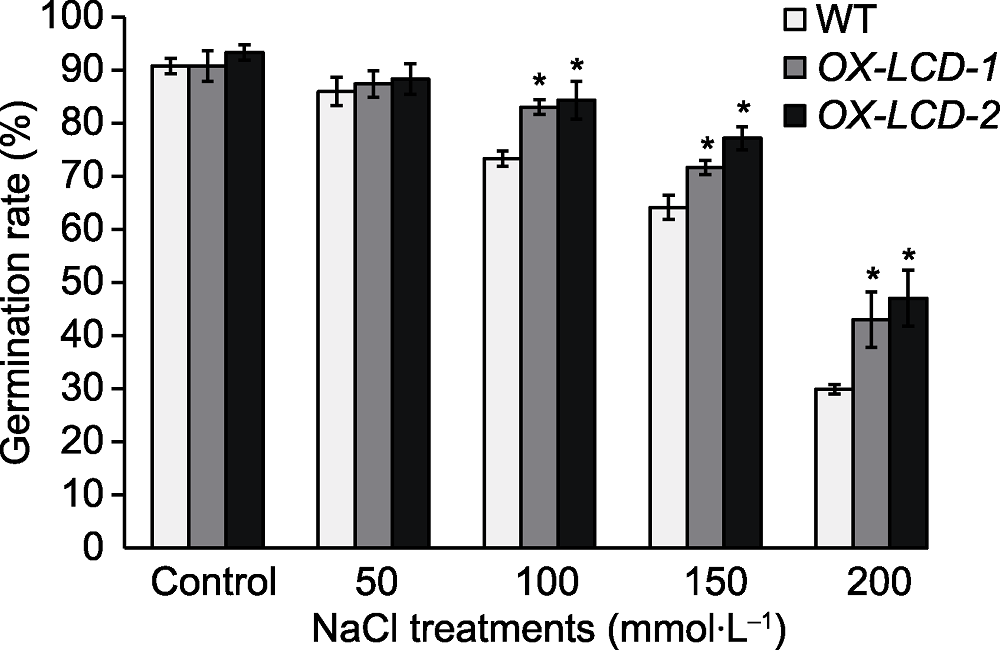
图4 NaCl处理对TaLCD转基因拟南芥种子萌发率的影响 WT: 野生型; OX-LCD-1和OX-LCD-2: 转基因株系; * 表示差异显著(P<0.05)。
Figure 4 Seed germination rate of Arabidopsis lines expressing TaLCD under different concentrations of NaCl treatment WT: Wild type; OX-LCD-1 and OX-LCD-2: Transgenic lines; * indicate significant differences (P<0.05).
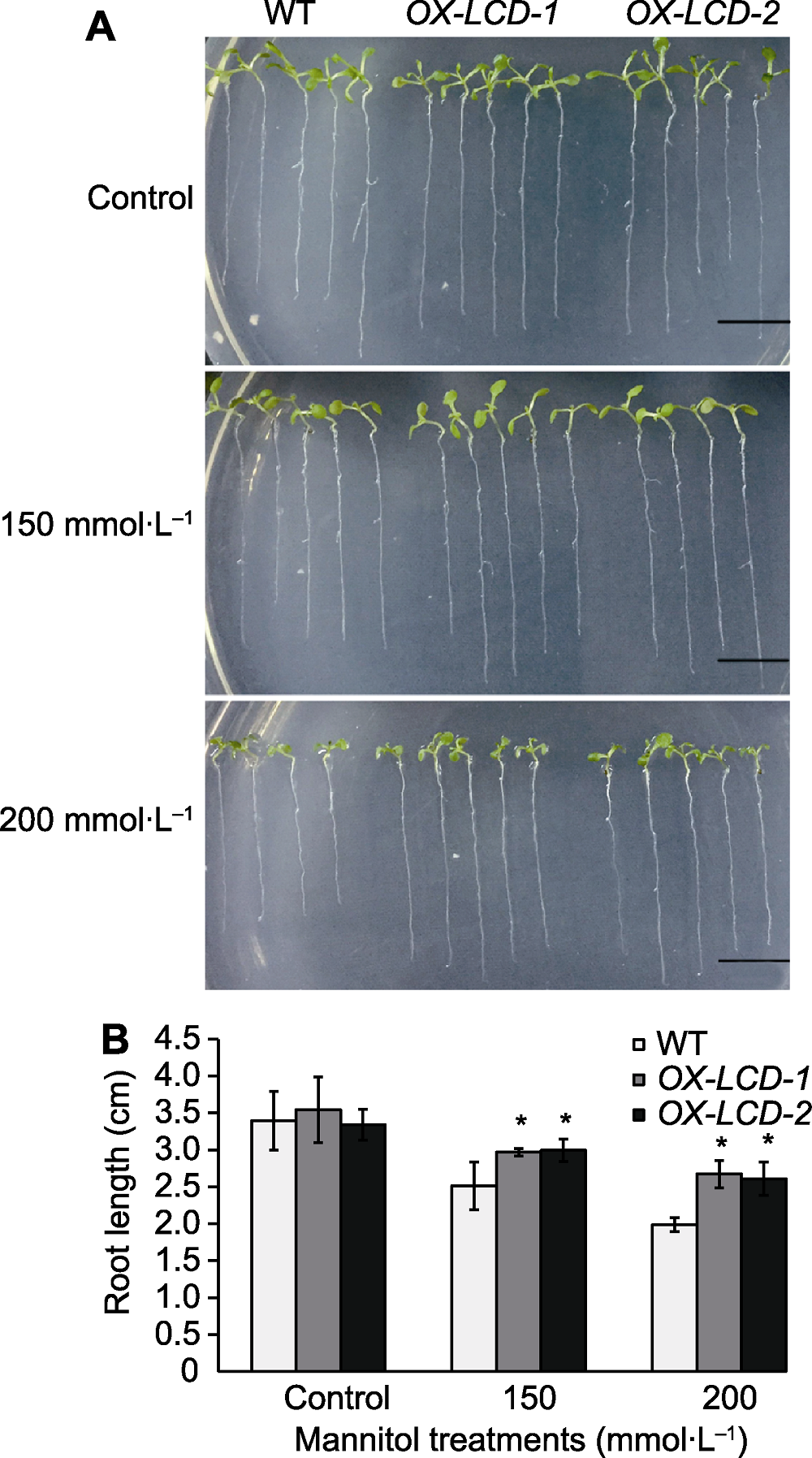
图5 不同浓度甘露醇处理下TaLCD转基因拟南芥的根长 (A) 表型变化(Bars=1 cm); (B) 根长变化数据统计。WT: 野生型; OX- LCD-1和OX-LCD-2: 转基因株系; * 表示差异显著(P<0.05)。
Figure 5 Root growth of Arabidopsis lines expressing TaLCD under different concentrations of mannitol treatment (A) Phenotype of root growth (Bars=1 cm); (B) Root elongation measurements. WT: Wild type; OX-LCD-1 and OX-LCD-2: Transgenic lines; * indicate significant differences (P<0.05).
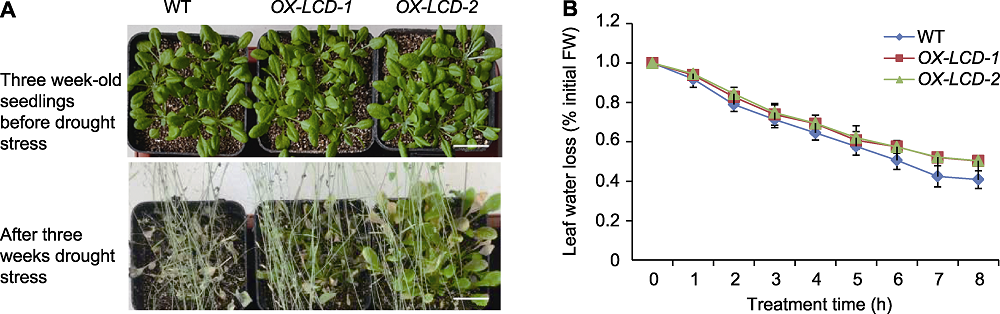
图6 TaLCD转基因拟南芥的抗旱性 (A) 生长3周的野生型(WT)、OX-LCD-1和OX-LCD-2植株在干旱处理后的表型(Bars=2 cm); (B) 叶片失水率
Figure 6 Drought resistance of Arabidopsis lines expressing TaLCD (A) Phenotype of three week-old wild type (WT), OX-LCD-1 and OX-LCD-2 plants after drought treatment (Bars=2 cm); (B) Leaf water loss
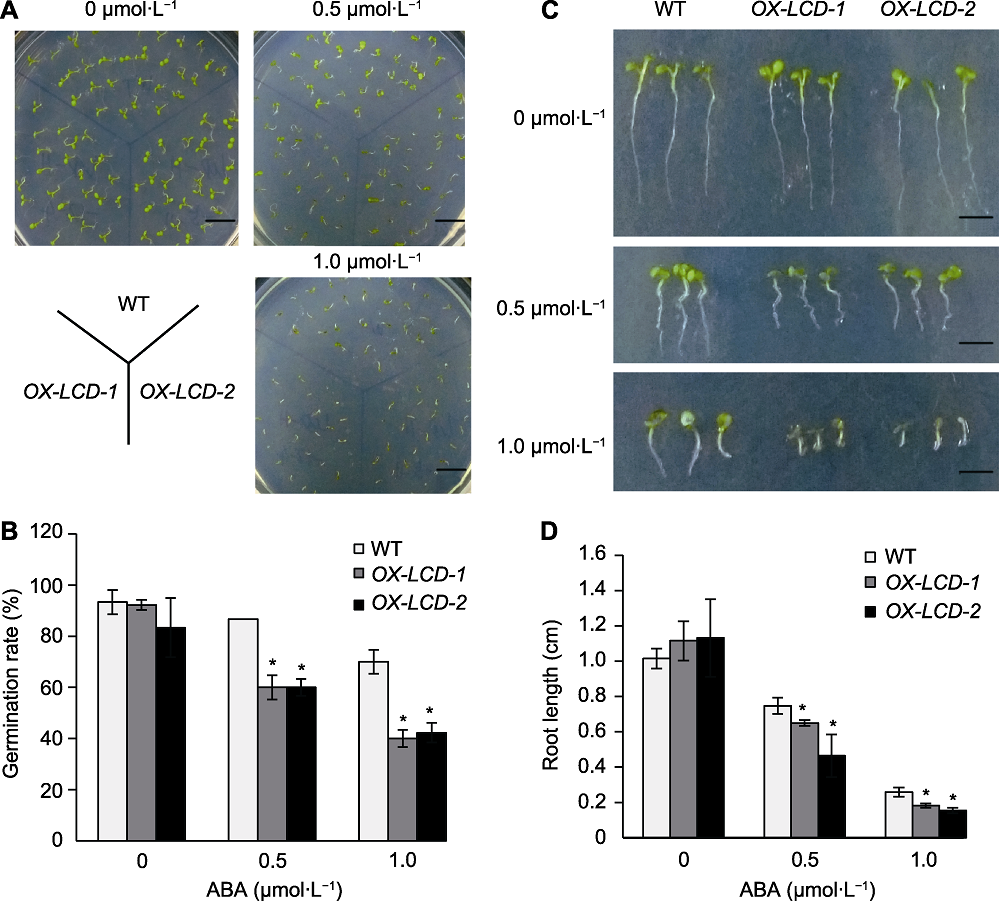
图7 不同浓度ABA处理下TaLCD转基因拟南芥的种子萌发率及生长状况 (A) 生长10天后的表型(Bars=1 cm); (B) 培养4天后的种子萌发率; (C) 生长10天后的根长表型(Bars=0.4 cm); (D) 生长10天后的根长。WT: 野生型; ABA: 脱落酸; * 表示差异显著(P<0.05)。
Figure 7 Seed germination and growth of Arabidopsis lines expressing TaLCD under different concentrations of ABA treatment (A) Phenotype on the 10th day (Bars=1 cm); (B) Seed germination rate after 4 d incubation; (C) Phenotype of root growth on the 10th day (Bars=0.4 cm); (D) Root length on the 10th day. WT: Wild type; ABA: Abscisic acid; * indicate significant differences (P<0.05).
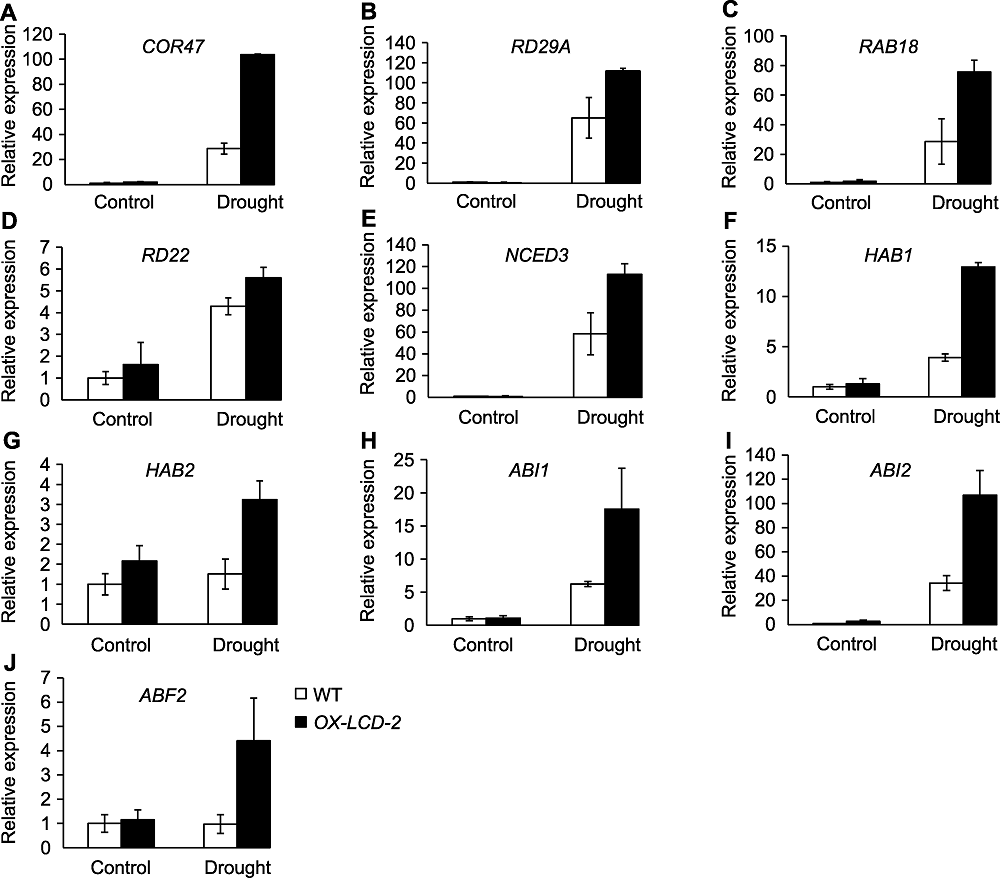
图8 干旱胁迫对TaLCD转基因拟南芥中胁迫响应基因及ABA信号途径相关基因表达的影响 (A)-(D) 胁迫响应基因; (E)-(J) ABA信号途径相关基因。WT: 野生型; OX-LCD-2: 转基因株系
Figure 8 The expression of stress response genes and ABA signaling related genes in Arabidopsis lines expressing TaLCD under drought stress (A)-(D) Stress response genes; (E)-(J) ABA signaling related genes. WT: Wild type; OX-LCD-2: Transgenic lines
| [1] | 侯智慧, 刘菁, 侯丽霞, 李希东, 刘新 ( 2011). H2S可能作为H2O2的下游信号介导茉莉酸诱导的蚕豆气孔关闭. 植物学报 46, 396-406. |
| [2] | 刘菁, 侯智慧, 赵方贵, 刘新 ( 2011). H2S介导ABA诱导蚕豆气孔运动的生理机制研究. 西北植物学报 31, 298-304. |
| [3] | 单长卷, 周岩 ( 2011). 外源硫化氢对水分胁迫下玉米种子萌发和生长的影响. 广东农业科学 38(20), 28-30. |
| [4] | Chen J, Wang WH, Wu FH, You CY, Liu TW, Dong XJ, He JX, Zheng HL ( 2013). Hydrogen sulfide alleviates aluminum toxicity in barley seedlings. Plant Soil 362, 301-318. |
| [5] | Christou A, Manganaris GA, Papadopoulos I, Fotopoulos V ( 2013). Hydrogen sulfide induces systemic tolerance to salinity and non-ionic osmotic stress in strawberry plants through modification of reactive species biosynthesis and transcriptional regulation of multiple defence pathways. J Exp Bot 64, 1953-1966. |
| [6] | Dooley FD, Nair SP, Ward PD ( 2013). Increased growth and germination success in plants following hydrogen sulfide administration. PLoS One 8, e62048. |
| [7] | Fang HH, Liu ZQ, Long YP, Liang YL, Jin ZP, Zhang LP, Liu DM, Li H, Zhai JX, Pei YX ( 2017). The Ca 2+/CaM2- binding transcription factor TGA3 elevates LCD expression and H2S production to bolster Cr 6+ tolerance in Arabidopsis. Plant J 91, 1038-1050. |
| [8] | García-Mata C, Lamattina L ( 2010). Hydrogen sulphide, a novel gasotransmitter involved in guard cell signaling. New Phytol 188, 977-984. |
| [9] | Guo HM, Xiao TY, Zhou H, Xie YJ, Shen WB ( 2016). Hydrogen sulfide: a versatile regulator of environmental stress in plants. Acta Physiol Plant 38, 16. |
| [10] | Jia HL, Hu YF, Fan TT, Li JS ( 2015). Hydrogen sulfide modulates actin-dependent auxin transport via regulating ABPs results in changing of root development in Arabidopsis. Sci Rep 5, 8251. |
| [11] | Jin ZP, Shen JJ, Qiao ZJ, Yang GD, Wang R, Pei YX ( 2011). Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochem Biophys Res Commun 414, 481-486. |
| [12] | Jin ZP, Xue SW, Luo YA, Tian BH, Fang HH, Li H, Pei YX ( 2013). Hydrogen sulfide interacting with abscisic acid in stomatal regulation responses to drought stress in Arabidopsis. Plant Physiol Biochem 62, 41-46. |
| [13] | Letunic I, Doerks T, Bork P ( 2015). SMART: recent updates, new developments and status in 2015. Nucleic Acids Res 43, D257-D260. |
| [14] | Li CJ, Liu ZJ, Zhang QR, Wang RZ, Xiao LT, Ma H, Chong K, Xu YY ( 2012). SKP1 is involved in abscisic acid signaling to regulate seed germination, stomatal opening and root growth in Arabidopsis thaliana. Plant Cell Environ 35, 952-965. |
| [15] | Li H, Gao MQ, Xue RL, Wang D, Zhao HJ ( 2015). Effect of hydrogen sulfide on D1 protein in wheat under drought stress. Acta Physiol Plant 37, 225. |
| [16] | Li H, Li M, Wei XL, Zhang X, Xue RL, Zhao YD, Zhao HJ ( 2017). Transcriptome analysis of drought-responsive genes regulated by hydrogen sulfide in wheat ( Triticum aestivum L.) leaves. Mol Genet Genomics 292, 1091-1110. |
| [17] | Ma DY, Ding HN, Wang CY, Qin HX, Han QX, Hou JF, Lu HF, Xie YX, Guo TC ( 2016). Alleviation of drought stress by hydrogen sulfide is partially related to the abscisic acid signaling pathway in wheat. PLoS One 11, e0163082. |
| [18] | Papenbrock J, Riemenschneider A, Kamp A, Schulz- Vogt HN, Schmidt A ( 2007). Characterization of cysteine-degrading and H2S-releasing enzymes of higher plants—from the field to the test tube and back. Plant Biol 9, 582-588. |
| [19] | Shen JJ, Qiao ZJ, Xing TJ, Zhang LP, Liang YL, Jin ZP, Yang GD, Pei YX ( 2012). Cadmium toxicity is alleviated by AtLCD and AtDCD in Escherichia coli. J Appl Microbiol 113, 1130-1138. |
| [20] | Shi HT, Ye TT, Chan ZL ( 2013). Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon(L). Pers.). Plant Physiol Biochem 71, 226-234. |
| [21] | Shi HT, Ye TT, Han N, Bian HW, Liu XD, Chan ZL ( 2015). Hydrogen sulfide regulates abiotic stress tolerance and biotic stress resistance in Arabidopsis. J Integr Plant Biol 57, 628-640. |
| [22] | Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S ( 2011). MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28, 2731-2739. |
| [23] | Wang YQ, Li L, Cui WT, Xu S, Shen WB, Wang R ( 2012). Hydrogen sulfide enhances alfalfa ( Medicago sativa) tolerance against salinity during seed germination by nitric oxide pathway. Plant Soil 351, 107-119. |
| [24] | Xie YJ, Lai DW, Mao Y, Zhang W, Shen WB, Guan RZ ( 2013). Molecular cloning, characterization, and expression analysis of a novel gene encoding L-cysteine desulfhydrase from Brassica napus. Mol Biotechnol 54, 737-746. |
| [25] | Zhang H, Hu LY, Hu KD, He YD, Wang SH, Luo JP ( 2008). Hydrogen sulfide promotes wheat seed germination and alleviates oxidative damage against copper stress. J Integr Plant Biol 50, 1518-1529. |
| [26] | Zhang H, Hu LY, Li P, Hu KD, Jiang CX, Luo JP ( 2010a). Hydrogen sulfide alleviated chromium toxicity in wheat. Biol Plantarum 54, 743-747. |
| [27] | Zhang H, Hu SL, Zhang ZJ, Hu LY, Jiang CX, Wei ZJ, Liu J, Wang HL, Jiang ST ( 2011). Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biol Technol 60, 251-257. |
| [28] | Zhang H, Jiao H, Jiang CX, Wang SH, Wei ZJ, Luo JP, Jones RL ( 2010b). Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol Plant 32, 849-857. |
| [1] | 陈唯,曾晓贤,谢楚萍,田长恩,周玉萍. 植物内源ABA水平的动态调控机制[J]. 植物学报, 2019, 54(6): 677-687. |
| [2] | 郜怀峰,张亚飞,王国栋,孙希武,贺月,彭福田,肖元松. 钼在桃树干旱胁迫响应中的作用解析[J]. 植物学报, 2019, 54(2): 227-236. |
| [3] | 曹云, 沈文静, 陈炼, 胡飞龙, 周蕾, 徐海根. Metabarcoding技术在真菌多样性研究中的应用[J]. 生物多样性, 2016, 24(8): 932-939. |
| [4] | 杨瑞雪, 刘海洋, 刘胜利, 于婷乔, 陈玉珍, 卢存福. 基于等温滴定微量热技术的玉米脱落酸受体检测体系[J]. 植物学报, 2016, 51(6): 790-800. |
| [5] | 刘晓东, 王若仲, 焦彬彬, 代培红, 李月. 拟南芥IAA酰胺合成酶GH3-6负调控干旱和盐胁迫的反应[J]. 植物学报, 2016, 51(5): 586-593. |
| [6] | 苗红霞, 王园, 徐碧玉, 刘菊华, 贾彩红, 张建斌, 王卓, 孙佩光, 金志强. 香蕉MaASR1基因的抗干旱作用[J]. 植物学报, 2014, 49(5): 548-559. |
| [7] | 易文凯, 王佳, 杨辉, 田云, 卢向阳. 植物ABA受体及其介导的信号转导通路[J]. 植物学报, 2012, 47(5): 515-524. |
| [8] | 柏新富, 卜庆梅, 谭永芹, 朱建军, 刘林德. NaCl对渗透胁迫下三角叶滨藜光合作用和水分状况的调节[J]. 植物学报, 2012, 47(5): 500-507. |
| [9] | 张盛春, 鞠常亮, 王小菁. 拟南芥漆酶基因AtLAC4参与生长及非生物胁迫响应[J]. 植物学报, 2012, 47(4): 357-365. |
| [10] | 张兰军, 姬飞腾, 王丽丽, 亓岽东, 朱燕, 邓馨. 复苏植物旋蒴苣苔C2结构域小蛋白BhC2DP1参与植物对ABA的反应[J]. 植物学报, 2012, 47(1): 11-27. |
| [11] | 张大鹏. 始于质体/叶绿体的ABA信号通路[J]. 植物学报, 2011, 46(4): 361-369. |
| [12] | 李大红, 张冬平, 曹丹丹, 梁建生. 植物RACK1蛋白研究进展[J]. 植物学报, 2011, 46(2): 224-232. |
| [13] | 路国辉, 武文华, 王瑞珍, 李新亮, 王英强. 野牡丹异型雄蕊的功能分化[J]. 生物多样性, 2009, 17(2): 174-181. |
| [14] | 郭华军;焦远年;邸超;姚冬霞;张盖华;郑雪;刘岚;张群莲;郭蔼光*;苏震*. 拟南芥转录因子GRAS 家族基因群响应渗透和干旱胁迫的初步探索[J]. 植物学报, 2009, 44(03): 290-299. |
| [15] | 赵春章;刘庆*;姚晓芹;汪明;龚良春. 长期喷施ABA对云杉幼苗生长和生理特性的影响[J]. 植物学报, 2008, 25(03): 284-291. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||