

植物学报 ›› 2020, Vol. 55 ›› Issue (1): 83-89.DOI: 10.11983/CBB19213 cstr: 32102.14.CBB19213
收稿日期:2019-10-30
接受日期:2019-12-11
出版日期:2020-01-01
发布日期:2019-12-20
通讯作者:
曲高平
基金资助:Received:2019-10-30
Accepted:2019-12-11
Online:2020-01-01
Published:2019-12-20
Contact:
Gaoping Qu
摘要: SUMO化是一种重要的蛋白质翻译后修饰, 对植物正常生长发育不可或缺。到目前为止已筛选到上千个可能的SUMO底物, 但由于SUMO化修饰水平普遍很低, 其生物学功能研究相对较少。该文详细描述了检测蛋白SUMO化修饰的常用方法, 包括体外和体内SUMO化实验, 以及SUMO化修饰位点的检测方法, 旨在为深入研究植物蛋白SUMO化修饰提供技术支持。
曲高平,金京波. 植物蛋白SUMO化修饰检测方法. 植物学报, 2020, 55(1): 83-89.
Gaoping Qu,Jingbo Jin. Detection of SUMOylation in Plants. Chinese Bulletin of Botany, 2020, 55(1): 83-89.
| Proteins | IPTG concentration (mmol·L-1) | Induction time |
|---|---|---|
| His-SUMO E1 | 1 | 16-20 h (16°C) |
| His-SUMO E2 | 1 | 3-5 h (28°C) |
| His-SUMO1GG | 1 | 16-20 h (16°C) |
| His-SUMO1AA | 1 | 16-20 h (16°C) |
| GST-protein X-Myc | 1 | 3-5 h (28°C) |
表1 SUMO反应相关蛋白的诱导条件
Table 1 Expression of SUMO reaction related proteins
| Proteins | IPTG concentration (mmol·L-1) | Induction time |
|---|---|---|
| His-SUMO E1 | 1 | 16-20 h (16°C) |
| His-SUMO E2 | 1 | 3-5 h (28°C) |
| His-SUMO1GG | 1 | 16-20 h (16°C) |
| His-SUMO1AA | 1 | 16-20 h (16°C) |
| GST-protein X-Myc | 1 | 3-5 h (28°C) |
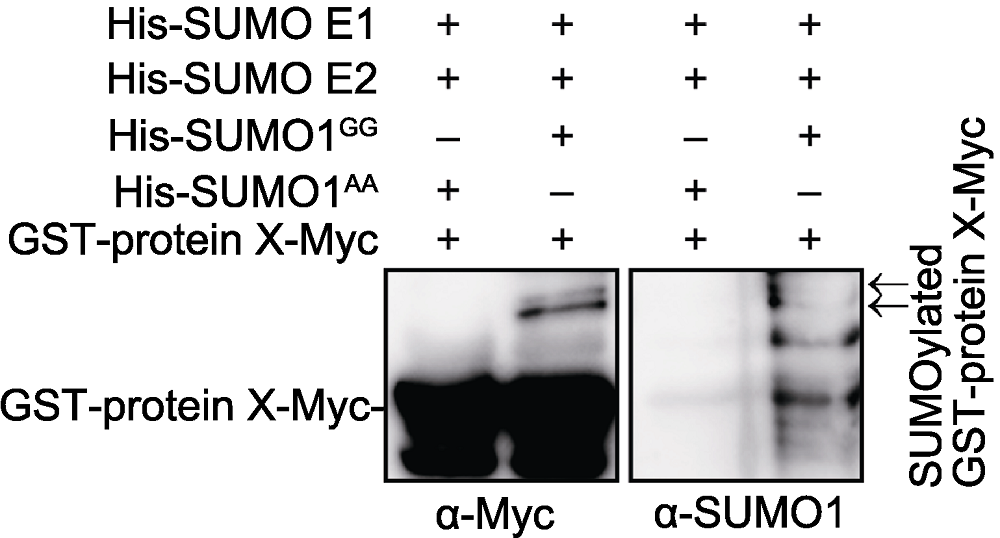
图1 Protein X体外SUMO化修饰 GST-protein X-Myc分别与His-SUMO E1、His-SUMO E2和His- SUMO1GG (His-SUMO1AA作为阴性对照), 进行体外SUMO化反应。反应后用Glutathione beads进一步纯化。Western blot, 分别用anti-Myc和anti-SUMO1抗体进行检测。箭头指示SUMO化修饰的GST-protein X-Myc。
Figure 1 Protein X can be SUMOylated in vitro GST-protein X-Myc was incubated with His-SUMO E1, His- SUMO E2 and His-SUMO1GG (His-SUMO1AA was used as a negative control) at 30°C for 3 h. After reaction, GST-protein X-Myc was purified with Glutathione beads and detected with anti-Myc and anti-SUMO1 antibodies. Arrows represent SUMOylated GST-protein X-Myc.
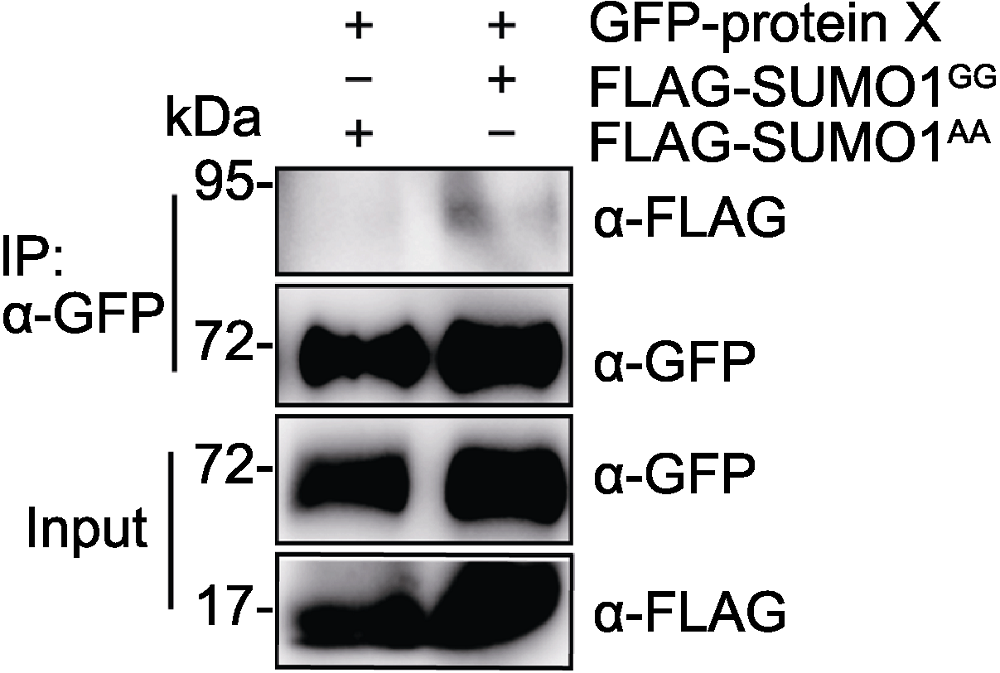
图2 烟草中检测GFP-protein X的SUMO化修饰 GFP-protein X分别与FLAG-SUMO1GG或FLAG-SUMO1AA在烟草叶片中共表达, IP (anti-GFP)产物分别用anti-GFP和anti- FLAG抗体检测。
Figure 2 SUMOylation of GFP-protein X in Nicotiana benthamiana GFP-protein X was transiently co-expressed with FLAG-SUMO1GG or FLAG-SUMO1AA in Nicotiana benthamiana leaves. GFP-protein X was immunoprecipitated with anti-GFP antibody, and IP products were detected with anti-FLAG antibody.
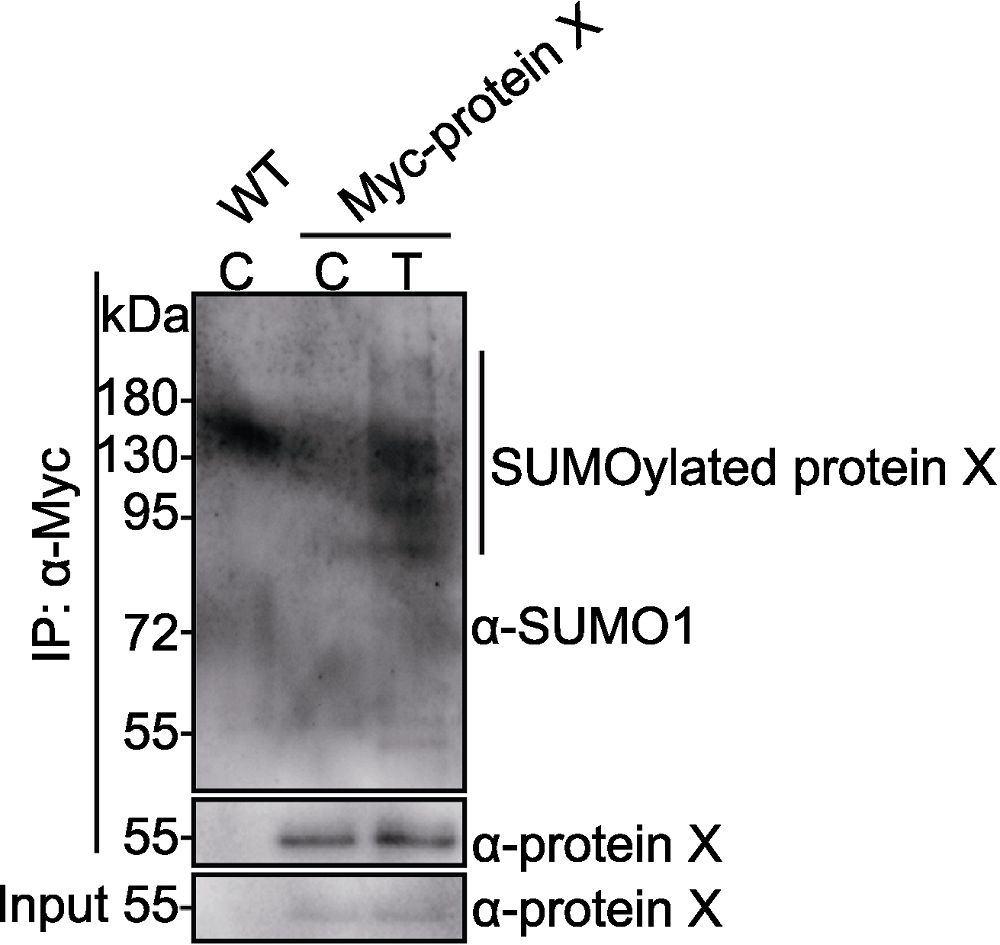
图3 转基因材料中检测Myc-protein X的SUMO化修饰 Myc-protein X转基因材料和野生型(WT)在对照条件(control, C)或处理条件(treatment, T)下生长3天。Myc-protein X和SUMO化修饰的Myc-protein X分别用anti-protein X和anti- SUMO1抗体检测。竖线指示SUMO化修饰的Myc-protein X。
Figure 3 SUMOylation of Myc-protein X in transgenic plants Myc-protein X transgenic plants and wild-type (WT) were grown in control (C) or treatment (T) conditions for 3 days. Myc-protein X and SUMOylated Myc-protein X were detected with anti-protein X and anti-SUMO1 antibodies, respectively. Vertical line indicates SUMOylated Myc-protein X bands.
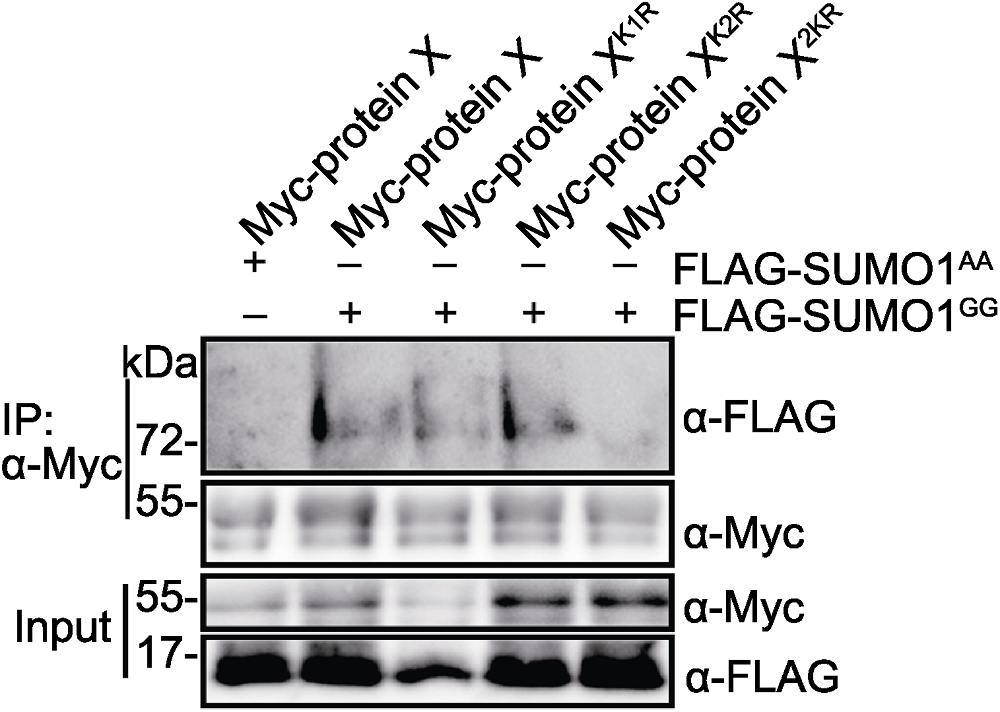
图4 K1和K2是protein X主要的SUMO化修饰结合位点 Myc-protein X、Myc-protein XK1R、Myc-protein XK2R或Myc- protein X2KR分别与FLAG-SUMO1GG或FLAG-SUMO1AA在烟草中瞬时共表达。
Figure 4 K1 and K2 are the primary SUMOylation sites of protein X Myc-protein X, Myc-protein XK1R, Myc-protein XK2R or Myc- protein X2KR was transiently co-expressed with FLAG-SUMO1GG or FLAG-SUMO1AA in Nicotiana benthamiana leaves, respectively.
| [1] | 徐庞连, 曾棉炜, 黄丽霞, 阳成伟 (2008). 植物SUMO化修饰及其生物学功能. 植物学通报 25, 608-615. |
| [2] | Bernier-Villamor V, Sampson DA, Matunis MJ, Lima CD (2002). Structural basis for E2-mediated SUMO conjugation revealed by a complex between ubiquitin-conjugating enzyme Ubc9 and RanGAP1. Cell 108, 345-356. |
| [3] | Catala R, Ouyang J, Abreu IA, Hu YX, Seo H, Zhang XR, Chua NH (2007). The Arabidopsis E3 SUMO ligase SIZ1 regulates plant growth and drought responses. Plant Cell 19, 2952-2966. |
| [4] | Colby T, Matthäi A, Boeckelmann A, Stuible HP (2006). SUMO-conjugating and SUMO-deconjugating enzymes from Arabidopsis. Plant Physiol 142, 318-332. |
| [5] | Conti L, Price G, O'Donnell E, Schwessinger B, Dominy P, Sadanandom A (2008). Small ubiquitin-like modifier proteases OVERLY TOLERANT TO SALT1 and -2 regulate salt stress responses in Arabidopsis. Plant Cell 20, 2894-2908. |
| [6] | Elrouby N, Coupland G (2010). Proteome-wide screens for small ubiquitin-like modifier (SUMO) substrates identify Arabidopsis proteins implicated in diverse biological processes. Proc Natl Acad Sci USA 107, 17415-17420. |
| [7] | Hermkes R, Fu YF, Nürrenberg K, Budhiraja R, Schmelzer E, Elrouby N, Dohmen RJ, Bachmair A, Coupland G (2011). Distinct roles for Arabidopsis SUMO protease ESD4 and its closest homolog ELS1. Planta 233, 63-73. |
| [8] | Ishida T, Yoshimura M, Miura K, Sugimoto K (2012). MMS21/HPY2 and SIZ1, two Arabidopsis SUMO E3 ligases, have distinct functions in development. PLoS One 7, e46897. |
| [9] | Kong XX, Luo X, Qu GP, Liu P, Jin JB (2017). Arabidopsis SUMO protease ASP1 positively regulates flowering time partially through regulating FLC stability. J Integr Plant Biol 59, 15-29. |
| [10] | Lin XL, Niu D, Hu ZL, Kim DH, Jin YH, Cai B, Liu P, Miura K, Yun DJ, Kim WY, Lin RC, Jin JB (2016). An Arabidopsis SUMO E3 ligase, SIZ1, negatively regulates photomorphogenesis by promoting COP1 activity. PLoS Genet 12, e1006016. |
| [11] | Liu LP, Jiang Y, Zhang XM, Wang X, Wang YB, Han YZ, Coupland G, Jin JB, Searle I, Fu YF, Chen FL (2017). Two SUMO proteases SUMO PROTEASE RELATED TO FERTILITY1 and 2 are required for fertility in Arabidopsis. Plant Physiol 175, 1703-1719. |
| [12] | Miller MJ, Barrett-Wilt GA, Hua ZH, Vierstra RD (2010). Proteomic analyses identify a diverse array of nuclear processes affected by small ubiquitin-like modifier conjugation in Arabidopsis. Proc Natl Acad Sci USA 107, 16512-16517. |
| [13] | Murtas G, Reeves PH, Fu YF, Bancroft I, Dean C, Coupland G (2003). A nuclear protease required for flowering-time regulation in Arabidopsis reduces the abundance of SMALL UBIQUITIN-RELATED MODIFIER conjugates. Plant Cell 15, 2308-2319. |
| [14] | Rodriguez MS (2016). SUMO: Methods and Protocols . New York: Humana Press. |
| [15] | Rytz TC, Miller MJ, McLoughlin F, Augustine RC, Marshall RS, Juan YT, Charng YY, Scalf M, Smith LM, Vierstra RD (2018). SUMOylome profiling reveals a diverse array of nuclear targets modified by the SUMO ligase SIZ1 during heat stress. Plant Cell 30, 1077-1099. |
| [16] | Sadanandom A, Ádám É, Orosa B, Viczián A, Klose C, Zhang CJ, Josse EM, Kozma-Bognár L, Nagy F (2015). SUMOylation of phytochrome-B negatively regulates light- induced signaling in Arabidopsis thaliana. Proc Natl Acad Sci USA 112, 11108-11113. |
| [17] | Saleh A, Withers J, Mohan R, Marqués J, Gu YN, Yan SP, Zavaliev R, Nomoto M, Tada Y, Dong XN (2015). Posttranslational modifications of the master transcriptional regulator NPR1 enable dynamic but tight control of plant immune responses. Cell Host Microbe 18, 169-182. |
| [18] | Saracco SA, Miller MJ, Kurepa J, Vierstra RD (2007). Genetic analysis of SUMOylation in Arabidopsis: conjugation of SUMO1 and SUMO2 to nuclear proteins is essential. Plant Physiol 145, 119-134. |
| [19] | Tomanov K, Zeschmann A, Hermkes R, Eifler K, Ziba I, Grieco M, Novatchkova M, Hofmann K, Hesse H, Bachmair A (2014). Arabidopsis PIAL1 and 2 promote SUMO chain formation as E4-type SUMO ligases and are involved in stress responses and sulfur metabolism. Plant Cell 26, 4547-4560. |
| [20] | Yates G, Srivastava AK, Sadanandom A (2016). SUMO proteases: uncovering the roles of deSUMOylation in plants. J Exp Bot 67, 2541-2548. |
| [21] | Yoo SD, Cho YH, Sheen J (2007). Arabidopsis mesophyll protoplasts: a versatile cell system for transient gene expression analysis. Nat Protoc 2, 1565-1572. |
| [22] | Zhao Q, Xie YB, Zheng YY, Jiang S, Liu WZ, Mu WP, Liu ZX, Zhao Y, Xue Y, Ren J (2014). GPS-SUMO: a tool for the prediction of SUMOylation sites and SUMO-interaction motifs. Nucleic Acids Res 42, W325-W330. |
| [1] | 刘雨函, 曹启江, 张诗晗, 李益慧, 王菁, 谭晓萌, 刘筱儒, 王显玲. 拟南芥AtFTCD-L参与根系响应土壤紧实度的机制研究[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 景艳军, 林荣呈. 蓝光受体CRY2化身“暗黑舞者”[J]. 植物学报, 2024, 59(6): 878-882. |
| [3] | 罗燕, 刘奇源, 吕元兵, 吴越, 田耀宇, 安田, 李振华. 拟南芥光敏色素突变体种子萌发的光温敏感性[J]. 植物学报, 2024, 59(5): 752-762. |
| [4] | 陈艳晓, 李亚萍, 周晋军, 解丽霞, 彭永彬, 孙伟, 和亚男, 蒋聪慧, 王增兰, 郑崇珂, 谢先芝. 拟南芥光敏色素B氨基酸位点突变对其结构与功能的影响[J]. 植物学报, 2024, 59(3): 481-494. |
| [5] | 杨继轩, 王雪霏, 顾红雅. 西藏野生拟南芥开花时间变异的遗传基础[J]. 植物学报, 2024, 59(3): 373-382. |
| [6] | 王钢, 王二涛. “卫青不败由天幸”——WeiTsing的广谱抗根肿病机理被揭示[J]. 植物学报, 2023, 58(3): 356-358. |
| [7] | 杨永青, 郭岩. 植物细胞质外体pH感受机制的解析[J]. 植物学报, 2022, 57(4): 409-411. |
| [8] | 黄俊文, 冯琦伊, 郑凯勇, 黄俊杰, 王林博, 赖瑞强, 赖建彬, 阳成伟. 植物蛋白质SUMO化修饰体外高效检测系统[J]. 植物学报, 2022, 57(4): 490-499. |
| [9] | 支添添, 周舟, 韩成云, 任春梅. PAD4突变加速拟南芥酪氨酸降解缺陷突变体sscd1的程序性细胞死亡[J]. 植物学报, 2022, 57(3): 288-298. |
| [10] | 李艳艳, 齐艳华. 植物Aux/IAA基因家族生物学功能研究进展[J]. 植物学报, 2022, 57(1): 30-41. |
| [11] | 车永梅, 孙艳君, 卢松冲, 侯丽霞, 范欣欣, 刘新. AtMYB77促进NO合成参与调控干旱胁迫下拟南芥侧根发育[J]. 植物学报, 2021, 56(4): 404-413. |
| [12] | 李秋信, 迟伟, 季代丽. CURT1调控类囊体膜弯曲的研究进展[J]. 植物学报, 2021, 56(4): 462-469. |
| [13] | 王婷, 羊欢欢, 赵弘巍, JosefVoglmeir, 刘丽. 蛋白质N-糖基化在拟南芥生长周期中的变化规律及去糖基化对根发育的影响[J]. 植物学报, 2021, 56(3): 262-274. |
| [14] | 林雨晴, 齐艳华. 生长素输出载体PIN家族研究进展[J]. 植物学报, 2021, 56(2): 151-165. |
| [15] | 马龙, 李桂林, 李师鹏, 蒋苏. 根尖整体透明技术改良[J]. 植物学报, 2020, 55(5): 596-604. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
