

植物学报 ›› 2025, Vol. 60 ›› Issue (6): 968-977.DOI: 10.11983/CBB24164 cstr: 32102.14.CBB24164
张汝鑫1, 李晨荣1, 王童欣1, 黎洁1, 李霆格1, 许慧娴1, 李梅儿1, 赵莹1, 彭婷2,3,*( ), 王健1,*(
), 王健1,*( )
)
收稿日期:2024-10-28
接受日期:2025-02-22
出版日期:2025-11-10
发布日期:2025-02-26
通讯作者:
彭婷,王健
基金资助:
Ruxin Zhang1, Chenrong Li1, Tongxin Wang1, Jie Li1, Tingge Li1, Huixian Xu1, Meier Li1, Ying Zhao1, Ting Peng2,3,*( ), Jian Wang1,*(
), Jian Wang1,*( )
)
Received:2024-10-28
Accepted:2025-02-22
Online:2025-11-10
Published:2025-02-26
Contact:
Ting Peng, Jian Wang
摘要: 为建立大花三色堇(Viola × wittrockiana)植株再生体系, 以其8个品种的叶片和叶柄为实验材料, 筛选并确定了再生率高的品种和部位。结果表明, PXP是供试品种中用于大花三色堇再生体系建立的最佳品种, 其叶柄是最佳外植体。以品种PXP的叶柄为外植体, 进行再生体系优化。结果表明, 愈伤组织诱导最适培养基为1/2MS (不含糖)+30 g∙L-1蔗糖+1.5 mg∙L-1 2,4-D+1.5 mg∙L-1 KT, 不定芽诱导最优培养基为1/2MS (不含糖)+30 g∙L-1海藻糖+0.05 mg∙L-1 2,4-D+3 mg∙L-1 6-BA, 再生苗经多次诱导分化, 再生率可达(67.33±3.06)%。不定芽增殖最优培养基为MS (不含糖)+30 g∙L-1海藻糖+0.5 mg∙L-1 2,4-D+1 mg∙L-1 6-BA, 增殖系数为3.29±0.22。再生苗在配方为1/2MS (不含糖)+30 g∙L-1海藻糖+0.1 mg∙L-1 NAA的生根培养基上培养2周后开始生根, 生根率为(84.44±6.93)%。该研究建立了分化率较高的大花三色堇再生体系, 解决了大花三色堇不定芽分化困难的难题, 为其育种改良和优良品种快繁提供了技术支持。
张汝鑫, 李晨荣, 王童欣, 黎洁, 李霆格, 许慧娴, 李梅儿, 赵莹, 彭婷, 王健. 大花三色堇再生体系建立. 植物学报, 2025, 60(6): 968-977.
Ruxin Zhang, Chenrong Li, Tongxin Wang, Jie Li, Tingge Li, Huixian Xu, Meier Li, Ying Zhao, Ting Peng, Jian Wang. Establishment of a Regeneration System for Viola × wittrockiana. Chinese Bulletin of Botany, 2025, 60(6): 968-977.
| Cultivars | Callus formation rate of leaves (%) | Callus formation rate of petioles (%) | Differentiation rate of petiole- derived callus (%) |
|---|---|---|---|
| CJBG | 46.67±4.41 cd | 92.50±1.25 a | 4.17±1.44 cd |
| CJCJBG | 29.44±6.94 e | 91.67±4.16 a | 3.33±1.44 e |
| KJB | 55.56±2.55 b | 90.00±1.25 a | 6.67±1.44 c |
| PXP | 43.33±7.64 cd | 89.38±6.76 a | 15.83±1.44 a |
| LLBL | 73.89±3.47 a | 89.79+3.08 a | 5.83±1.41 cd |
| SX | 41.11±0.96 d | 95.00±2.73 a | 10.00±2.50 b |
| KM | 56.67±1.67 b | 94.58±1.91 a | 6.67±1.44 c |
| ATSL | 50.56±4.19 bc | 92.29±2.95 a | 11.67±1.44 b |
表1 不同三色堇品种的出愈率和分化率
Table 1 Rates of callus induction and differentiation of different cultivars of Viola × wittrockiana
| Cultivars | Callus formation rate of leaves (%) | Callus formation rate of petioles (%) | Differentiation rate of petiole- derived callus (%) |
|---|---|---|---|
| CJBG | 46.67±4.41 cd | 92.50±1.25 a | 4.17±1.44 cd |
| CJCJBG | 29.44±6.94 e | 91.67±4.16 a | 3.33±1.44 e |
| KJB | 55.56±2.55 b | 90.00±1.25 a | 6.67±1.44 c |
| PXP | 43.33±7.64 cd | 89.38±6.76 a | 15.83±1.44 a |
| LLBL | 73.89±3.47 a | 89.79+3.08 a | 5.83±1.41 cd |
| SX | 41.11±0.96 d | 95.00±2.73 a | 10.00±2.50 b |
| KM | 56.67±1.67 b | 94.58±1.91 a | 6.67±1.44 c |
| ATSL | 50.56±4.19 bc | 92.29±2.95 a | 11.67±1.44 b |
| Type | Callus formation rate (%) | Callus growth state | Differentiation rate (%) |
|---|---|---|---|
| Y1 (1/2MS (sugar-free)+30 g∙L-1 sucrose+1.5 mg∙L-1 2,4-D+0.5 mg∙L-1 NAA+3.0 mg∙L-1 GA3) | 93.56±1.16 a | Pale yellow, loose, softer texture, fast growth | 10.37±1.28 b |
| Y2 (1/2MS (sugar-free)+30 g∙L-1 sucrose+1.5 mg∙L-1 2,4-D+1.5 mg∙L-1 KT) | 95.67±3.47 a | Yellow, dense, somewhat hard texture, slow growth | 18.52±1.28 a |
表2 不同培养基对PXP分化率的影响
Table 2 Effect of different media on the differentiation rate of PXP
| Type | Callus formation rate (%) | Callus growth state | Differentiation rate (%) |
|---|---|---|---|
| Y1 (1/2MS (sugar-free)+30 g∙L-1 sucrose+1.5 mg∙L-1 2,4-D+0.5 mg∙L-1 NAA+3.0 mg∙L-1 GA3) | 93.56±1.16 a | Pale yellow, loose, softer texture, fast growth | 10.37±1.28 b |
| Y2 (1/2MS (sugar-free)+30 g∙L-1 sucrose+1.5 mg∙L-1 2,4-D+1.5 mg∙L-1 KT) | 95.67±3.47 a | Yellow, dense, somewhat hard texture, slow growth | 18.52±1.28 a |
| No. | Factor | Differentiation rate (%) | ||
|---|---|---|---|---|
| A (Carbohydrate sources) | B (2,4-D concentration) | C (6-BA concentration) | ||
| 1 | 1 | 1 | 1 | 8.33 |
| 2 | 1 | 2 | 3 | 13.33 |
| 3 | 1 | 3 | 2 | 8.33 |
| 4 | 2 | 1 | 3 | 26.67 |
| 5 | 2 | 2 | 2 | 23.33 |
| 6 | 2 | 3 | 1 | 20.00 |
| 7 | 3 | 1 | 2 | 10.00 |
| 8 | 3 | 2 | 1 | 13.33 |
| 9 | 3 | 3 | 3 | 11.67 |
| K1 | 0.10 | 0.15 | 0.14 | |
| K2 | 0.23 | 0.17 | 0.14 | |
| K3 | 0.12 | 0.13 | 0.17 | |
| Range (R) | 0.13 | 0.02 | 0.03 | |
| Order | A>C>B | |||
| Optimal level | A2 | B2 | C3 | |
| Optimal combination | A2B2C3 | |||
表3 大花三色堇不定芽分化正交试验
Table 3 The orthogonal experiment of adventitious bud differentiation of Viola × wittrockiana
| No. | Factor | Differentiation rate (%) | ||
|---|---|---|---|---|
| A (Carbohydrate sources) | B (2,4-D concentration) | C (6-BA concentration) | ||
| 1 | 1 | 1 | 1 | 8.33 |
| 2 | 1 | 2 | 3 | 13.33 |
| 3 | 1 | 3 | 2 | 8.33 |
| 4 | 2 | 1 | 3 | 26.67 |
| 5 | 2 | 2 | 2 | 23.33 |
| 6 | 2 | 3 | 1 | 20.00 |
| 7 | 3 | 1 | 2 | 10.00 |
| 8 | 3 | 2 | 1 | 13.33 |
| 9 | 3 | 3 | 3 | 11.67 |
| K1 | 0.10 | 0.15 | 0.14 | |
| K2 | 0.23 | 0.17 | 0.14 | |
| K3 | 0.12 | 0.13 | 0.17 | |
| Range (R) | 0.13 | 0.02 | 0.03 | |
| Order | A>C>B | |||
| Optimal level | A2 | B2 | C3 | |
| Optimal combination | A2B2C3 | |||
| Type | 6-BA (mg∙L-1) | 2,4-D (mg∙L-1) | Number of explants | Proliferation coefficient | Growth situation |
|---|---|---|---|---|---|
| Z1 | 0.5 | 0.5 | 45 | 2.20±0.20 c | ++ |
| Z2 | 1.0 | 0.5 | 45 | 3.29±0.22 a | |
| Z3 | 2.0 | 0.5 | 45 | 2.73±0.23 b | ++ |
| Z4 | 3.0 | 0.5 | 45 | 2.18±0.17 c | + |
表4 植物生长调节剂对大花三色堇不定芽增殖的影响
Table 4 Effects of plant growth regulators on the adventitious bud proliferation of Viola × wittrockiana
| Type | 6-BA (mg∙L-1) | 2,4-D (mg∙L-1) | Number of explants | Proliferation coefficient | Growth situation |
|---|---|---|---|---|---|
| Z1 | 0.5 | 0.5 | 45 | 2.20±0.20 c | ++ |
| Z2 | 1.0 | 0.5 | 45 | 3.29±0.22 a | |
| Z3 | 2.0 | 0.5 | 45 | 2.73±0.23 b | ++ |
| Z4 | 3.0 | 0.5 | 45 | 2.18±0.17 c | + |
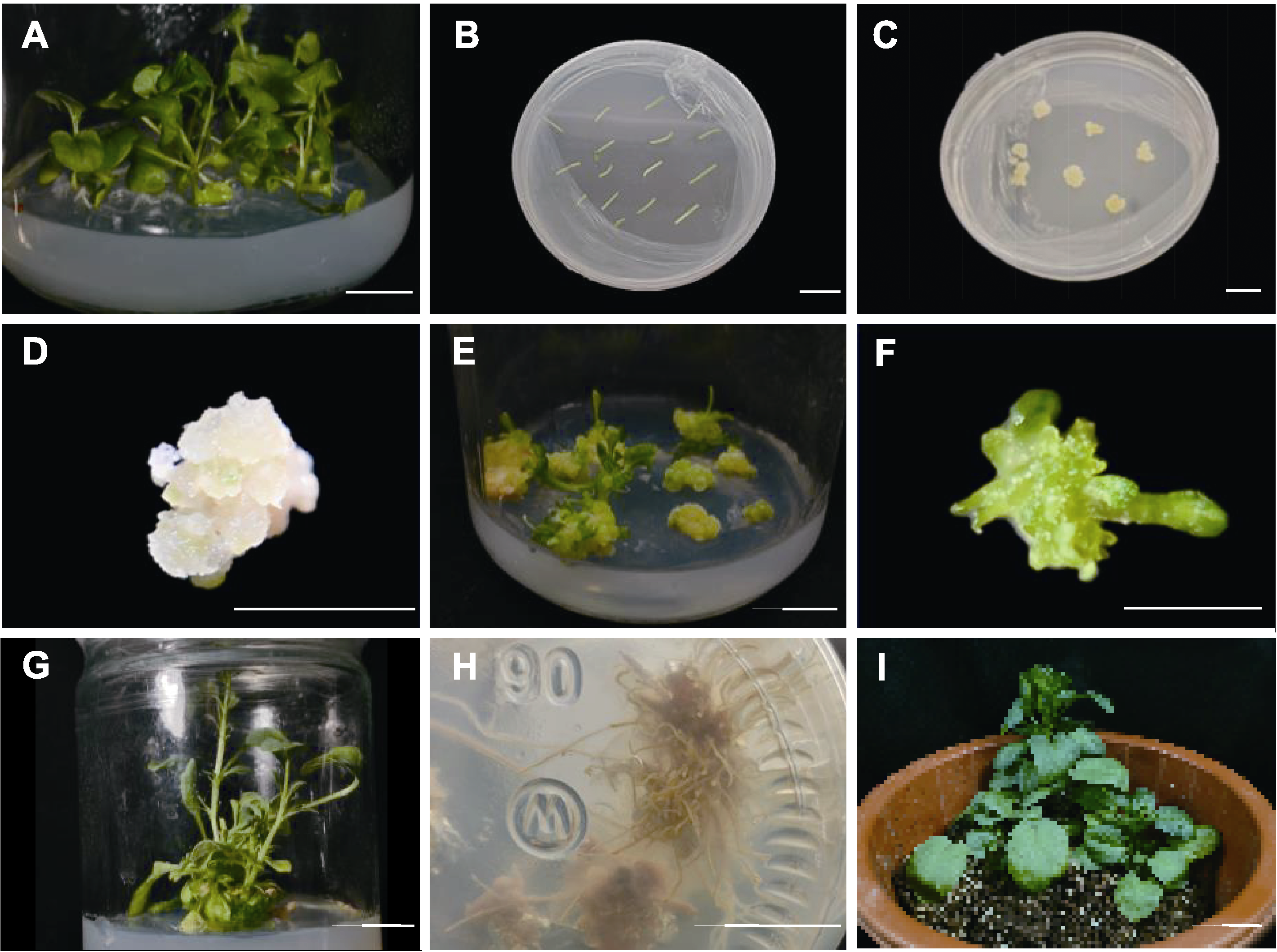
图1 大花三色堇叶柄愈伤组织再生 (A) 无菌苗; (B) 叶柄外植体; (C), (D) 愈伤组织诱导; (E), (F) 不定芽分化; (G) 不定芽增殖; (H) 根诱导; (I) 移栽。Bars=1 cm
Figure 1 Regeneration via petiole-derived callus of Viola × wittrockiana (A) Sterile seedlings; (B) Petioles; (C), (D) Induction of callus; (E), (F) Differentiation of adventitious buds; (G) Proliferation of adventitious buds; (H) Induction of root; (I) Transplanting. Bars=1 cm
| [1] |
Ameri A, Davarynejad GH, Moshtaghi N, Tehranifar A (2020). The role of carbohydrates on the induction of somatic embryogenesis and the biochemical state of the embryogenic callus in Pyrus communis L. cv. ‘Dar Gazi’. Erwerbs-Obstbau 62, 411-419.
DOI |
| [2] | Babber S, Kulbhushan S (1991). Study of anatomy of vitrified structure in Viola tricolour L. Ann Biol 7, 93-95. |
| [3] | Bhardwaj R, Kumar M, Kaushal N, Kamboj AD, Krishnamoorthi A, Singh A, Motla R, Anushi (2024). From lab to bouquet: the biotechnological frontier in modern floriculture for sustainable and resilient flower farming. J Adv Biol Biotechnol 27, 119-137. |
| [4] | Chen YR (2013). The Transformation of Cold Response Gene CBF1 Into Petunia (Petunia hybrida Vilm.) and Pansy (Viola tricolor L.). Master’s thesis. Lanzhou: Lanzhou University. pp. 1-37. (in Chinese) |
| 陈玥如 (2013). 冷响应基因CBF1对矮牵牛和三色堇的遗传转化研究. 硕士论文. 兰州: 兰州大学. pp. 1-37. | |
| [5] | Ding SP, Yan JQ, Ji DF (1998). Effect of sugar sources on plant tissue culture. Chin Bull Bot 15, 42-46. (in Chinese) |
| 丁世萍, 严菊强, 季道藩 (1998). 糖类在植物组织培养中的效应. 植物学通报 15, 42-46. | |
| [6] |
Encina CL, Parisi A, O’Brien C, Mitter N (2014). Enhan-cing somatic embryogenesis in avocado (Persea americana Mill.) using a two-step culture system and including glutamine in the culture medium. Sci Hortic 165, 44-50.
DOI URL |
| [7] |
Fernandes L, Ramalhosa E, Baptista P, Pereira JA, Saraiva JA, Casal SIP (2019). Nutritional and nutraceutical composition of pansies (Viola × wittrockiana) during flowering. J Food Sci 84, 490-498.
DOI PMID |
| [8] | Fu JM, Guo HJ, Lou YH (2014). Method for improving pe-rennial ryegrass callus regeneration rate. Chinese Patent, CN103314860B. 2014-08-13. (in Chinese) |
| 傅金民, 郭慧娟, 娄燕宏 (2014). 一种提高多年生黑麦草愈伤组织再生率的方法. 中国专利, CN103314860B. 2014-08-13. | |
| [9] | Gandolfo E, Hakim G, Geraci J, Feuring V, Giardina E, Di Benedetto A (2016). Responses of pansy (Viola wittrockiana Gams.) to the quality of the growing media. J Exp Agric Int 12, 1-10. |
| [10] |
Gonçalves J, Borges JCF, Carlos LDA, Silva APCM, Souza FAD (2019). Bioactive compounds in edible flowers of garden pansy in response to irrigation and mycorrhizal inoculation. Rev Ceres 66, 407-415.
DOI URL |
| [11] |
González-Barrio R, Periago MJ, Luna-Recio C, Garcia- Alonso FJ, Navarro-González I (2018). Chemical composition of the edible flowers, pansy (Viola wittrockiana) and snapdragon (Antirrhinum majus) as new sources of bioactive compounds. Food Chem 252, 373-380.
DOI PMID |
| [12] |
Jha SR, Naz R, Asif A, Okla MK, Soufan W, Al-Ghamdi AA, Ahmad A (2020). Development of an in vitro propagation protocol and a sequence characterized amplified region (SCAR) marker of Viola serpens Wall. ex Ging. Plants 9, 246.
DOI URL |
| [13] |
Jheng FY, Do YY, Liauh YW, Chung JP, Huang PL (2006). Enhancement of growth and regeneration efficiency from embryogenic callus cultures of Oncidium ‘Gower Ramsey’ by adjusting carbohydrate sources. Plant Sci 170, 1133-1140.
DOI URL |
| [14] |
Khajuria AK, Hano C, Bisht NS (2021). Somatic embryogenesis and plant regeneration in Viola canescens Wall. ex. Roxb.: an endangered himalayan herb. Plants 10, 761.
DOI URL |
| [15] | Li XQ, Sheng YH, Fu YG, Zhou Y, Zhao Y, Ling P, Song XQ, Wang J (2020). Establishment of efficient regeneration system of Oncidium. J South Agric 51, 1169-1175. (in Chinese) |
| 李雪青, 盛玉辉, 付瑛格, 周扬, 赵莹, 凌鹏, 宋希强, 王健 (2020). 文心兰高效再生体系的建立. 南方农业学报 51, 1169-1175. | |
| [16] | Long Y, Yang Y, Pan GT, Shen YO (2022). New insights into tissue culture plant-regeneration mechanisms. Front Plant Sci 13, 9267s52. |
| [17] |
Ma J, Li Q, Zhang L, Cai S, Liu YY, Lin JC, Huang RF, Yu YQ, Wen MZ, Xu TD (2022). High auxin stimulates callus through SDG8-mediated histone H3K36 methylation in Arabidopsis. J Integr Plant Biol 64, 2425-2437.
DOI URL |
| [18] |
Mercuri A, Sacchetti A, De Benedetti L, Schiva T, Alberti S (2002). Green fluorescent flowers. Plant Sci 162, 647-654.
DOI URL |
| [19] |
Nanjaraj Urs AN, Hu YL, Li PW, Yuchi Z, Chen YH, Zhang Y (2019). Cloning and expression of a nonribosomal peptide synthetase to generate blue rose. ACS Synth Biol 8, 1698-1704.
DOI PMID |
| [20] |
Nauenburg JD, Buttler KP (2007). Validierung des namens Viola wittrockiana. Kochia 2, 37-41.
DOI URL |
| [21] | Niu YY, Wang M, Li WJ, Li B, Fan MJ, Cheng YH, Wang CF (2023). Cultivation technical regulations of Viola tricolor. Hortic Seed 43(7), 45-46. (in Chinese) |
| 牛燕燕, 王梅, 李文静, 李冰, 范明杰, 成永慧, 王超凡 (2023). 三色堇栽培技术规程. 园艺与种苗 43(7), 45-46. | |
| [22] |
Núñez S, López V, Moliner C, Valero MS, Gómez-Rincón C (2023). Lipid lowering and anti-ageing effects of edible flowers of Viola × wittrockiana Gams in a Caenorhabditis elegans obese model. Food Funct 14, 8854-8864.
DOI URL |
| [23] |
Satyavathi VV, Jauhar PP, Elias EM, Rao MB (2004). Effects of growth regulators on in vitro plant regeneration in durum wheat. Crop Sci 44, 1839-1846.
DOI URL |
| [24] |
Tang J, Wang CK, Pan XL, Yan H, Zeng GZ, Xu WY, He WJ, Daly NL, Craik DJ, Tan NH (2010). Isolation and characterization of cytotoxic cyclotides from Viola tricolor. Peptides 31, 1434-1440.
DOI PMID |
| [25] |
Vukics V, Kery A, Guttman A (2008). Analysis of polar antioxidants in heartsease (Viola tricolor L.) and garden pansy (Viola × wittrockiana Gams.). J Chromatogr Sci 46, 823-827.
DOI PMID |
| [26] |
Wang J, Bao MZ (2007). Plant regeneration of pansy (Viola wittrockiana) ‘Caidie’ via petiole-derived callus. Sci Hortic 111, 266-270.
DOI URL |
| [27] | Wijowska M, Kuta E, Przywara L (1999). In vitro culture of unfertilized ovules of Viola odorata L. Acta Biol Cracov Ser Bot 41, 95-101. |
| [28] | Wu Q, Qin JJ, Chen W, Su X (2016). Research on the tissue culture and rapid propagation of medicinal plant Viola philippica. J Tradit Chin Vet Med 35(3), 5-8. (in Chinese) |
| 吴琼, 秦晶晶, 陈纹, 苏雪 (2016). 药用植物紫花地丁组织培养与快速繁殖技术研究. 中兽医医药杂志 35(3), 5-8. | |
| [29] |
Xu JP, Naing AH, Bunch H, Jeong J, Kim H, Kim CK (2021). Enhancement of the flower longevity of petunia by CRISPR/Cas9-mediated targeted editing of ethylene biosynthesis genes. Postharvest Biol Technol 174, 111460.
DOI URL |
| [30] | Yang JL, Rao YF, Zhang RH, Zhou GL, Lin CF, He YH, Ning GG (2024). Establishment of an efficient leaf regeneration system for Pinguicula. Chin Bull Bot 59, 626-634. (in Chinese) |
|
杨佳丽, 饶羽菲, 张润花, 周国林, 林处发, 何燕红, 宁国贵 (2024). 捕虫堇叶片高效再生体系建立. 植物学报 59, 626-634.
DOI |
|
| [31] | Zhang ML (2021). Establishment of Suspension Cells in Chinese Jujube (Ziziphus jujuba Mill.) and Its Application in cAMP Synthesis and Regulation. Master’s thesis. Baoding: Hebei Agricultural University. pp. 1-35. (in Chinese) |
| 张梦玲 (2021). 枣悬浮细胞系的建立及其在cAMP合成调控中的应用. 硕士论文. 保定: 河北农业大学. pp. 1-35. | |
| [32] | Zhang QS (2009). Studies on Inheritance of Flower Color and Flower Color Patter and Tissue Culture of Pansy (Viola × wittrockiana) and Viola (V. cornuta). Master’s thesis. Wuhan: Huazhong Agricultural University. pp. 1-63. (in Chinese) |
| 张其生 (2009). 三色堇与角堇花色、花斑遗传规律及组织培养的研究. 硕士论文. 武汉: 华中农业大学. pp. 1-63. |
| [1] | 冯帅帅, 乔峰, 李爱花, 何璇, 姜婷婷, 韩葳, 央宗, 李全希, 黄爱玲, 刘进, 谭德云. 黑莓‘APF-190T’茎段离体再生体系的建立[J]. 植物学报, 2026, 61(1): 1-0. |
| [2] | 周立茹, 敖妍, 仲静. 文冠果种仁不定芽诱导及褐化抑制[J]. 植物学报, 2025, 60(6): 957-967. |
| [3] | 李晶晶, 李艳飞, 王安琪, 王佳颖, 邓成燕, 卢敏, 马剑英, 戴思兰. 菊花品种万代风光再生及遗传转化体系的建立[J]. 植物学报, 2025, 60(4): 597-610. |
| [4] | 李彤, 李楚然, 张芷瑜, 付晓熳, 刘云, 张颖君, 杨力颖, 赵平. 西印度醋栗组培快繁技术初探[J]. 植物学报, 2025, 60(4): 611-620. |
| [5] | 刘玉泽, 王一菲, 任威蓁, 栗浩, 路斌, 路丙社, 于晓跃. 北美豆梨杂种幼胚挽救及再生体系的建立[J]. 植物学报, 2024, 59(5): 800-809. |
| [6] | 冯雯, 王玉国. 栽培薯蓣茎段离体再生体系的建立[J]. 植物学报, 2024, 59(5): 792-799. |
| [7] | 曾浩, 李佩芳, 郭至辉, 刘春林, 阮颖. 银扇草再生体系的建立[J]. 植物学报, 2024, 59(3): 433-440. |
| [8] | 武晓云, 廖敏凌, 李雪茹, 舒梓淳, 辛佳潼, 张伯晗, 戴思兰. 毛华菊3种瓣型株系再生体系的建立[J]. 植物学报, 2024, 59(2): 245-256. |
| [9] | 谢纯刚, 刘哲, 章书声, 胡海涛. 手指柠檬茎段离体再生体系建立[J]. 植物学报, 2023, 58(6): 926-934. |
| [10] | 廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰. 平潭野菊混合瓣型株系再生体系的建立[J]. 植物学报, 2023, 58(3): 449-460. |
| [11] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧. 大花银莲花愈伤组织诱导及再生体系的建立[J]. 植物学报, 2022, 57(2): 217-226. |
| [12] | 李孟悦, 刘柳, 刘艳, 张晓曼. 毛报春(Primula × pubescens)腋芽再生组织培养体系的建立[J]. 植物学报, 2021, 56(6): 732-739. |
| [13] | 罗钱, 张燕莎, 欧静. 郁金樱愈伤组织诱导及植株再生[J]. 植物学报, 2021, 56(4): 451-461. |
| [14] | 张冬瑞, 卜志刚, 陈玲玲, 常缨. 香鳞毛蕨的组织培养和快速繁殖体系构建[J]. 植物学报, 2020, 55(6): 760-767. |
| [15] | 罗虹, 温小蕙, 周圆圆, 戴思兰. 芳香堆心菊离体再生体系的建立[J]. 植物学报, 2020, 55(3): 318-328. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||