

植物学报 ›› 2023, Vol. 58 ›› Issue (6): 926-934.DOI: 10.11983/CBB23060 cstr: 32102.14.CBB23060
收稿日期:2023-05-12
接受日期:2023-09-19
出版日期:2023-11-01
发布日期:2023-11-27
通讯作者:
* E-mail: haitao-hu@zjnu.cn
基金资助:
Chungang Xie1,2, Zhe Liu1, Shusheng Zhang3, Haitao Hu1,*( )
)
Received:2023-05-12
Accepted:2023-09-19
Online:2023-11-01
Published:2023-11-27
Contact:
* E-mail: haitao-hu@zjnu.cn
摘要: 以手指柠檬(Citrus australasica)茎段为外植体, 通过离体器官发生途径诱导形成不定芽, 探讨不同植物生长调节剂组合、培养基类型以及暗培养时间对其愈伤组织诱导和植株再生的影响, 建立手指柠檬离体再生体系。结果表明, 1/2MS+4.0 mg∙L-1 ZT+30.0 g∙L-1蔗糖为手指柠檬茎段不定芽诱导的最佳配方, 14天暗培养后转移到光下培养效果最好, 愈伤组织及不定芽诱导率均为100%, 每外植体平均再生不定芽数达4.83。诱导不定根的适宜培养基配方为1/2MS+0.5 mg∙L-1 NAA, 生根率达94.43%, 平均再生根数为3.9; 在草炭:珍珠岩:蛭石=2:1:1 (v/v/v)的混合基质中组培苗长势最好, 成活率在90%以上。该研究建立了手指柠檬茎段离体再生体系, 为手指柠檬的遗传改良和优良品种快繁奠定了基础。
谢纯刚, 刘哲, 章书声, 胡海涛. 手指柠檬茎段离体再生体系建立. 植物学报, 2023, 58(6): 926-934.
Chungang Xie, Zhe Liu, Shusheng Zhang, Haitao Hu. Establishment of In Vitro Regeneration System of Citrus australasica. Chinese Bulletin of Botany, 2023, 58(6): 926-934.
| No. | NAA (mg?L-1) | 6-BA (mg?L-1) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | ||||
| 1 | 0 | 0 | 61.11 | 55.57±5.57 cde | 1.57±0.29 bc | ++ |
| 2 | 0 | 0.5 | 100.00 | 72.23±5.53 efg | 2.08±0.08 de | ++++ |
| 3 | 0 | 1.0 | 100.00 | 94.43±5.57 g | 2.53±0.03 e | ++++ |
| 4 | 0 | 2.0 | 91.67 | 55.57±5.57 cde | 1.19±0.10 ab | ++ |
| 5 | 0 | 4.0 | 58.33 | 27.73±5.57 abc | 1.17±0.17 ab | + |
| 6 | 0.1 | 0.5 | 58.33 | 61.13±5.57 def | 2.36±0.07 de | +++ |
| 7 | 0.1 | 1.0 | 66.67 | 44.43±5.57 bcde | 1.89±0.11 bce | ++ |
| 8 | 0.1 | 2.0 | 75.00 | 44.47±11.17 bcde | 2.00±0.29 bce | ++ |
| 9 | 0.1 | 4.0 | 41.67 | 16.70±0.00 a | 1.17±0.29 ab | + |
| 10 | 0.5 | 0.5 | 66.67 | 55.57±5.57 cde | 2.33±0.19 de | +++ |
| 11 | 0.5 | 1.0 | 91.67 | 66.67±9.61 ef | 2.57±0.23 e | +++ |
| 12 | 0.5 | 2.0 | 33.33 | 33.33±9.61 abcd | 1.61±0.20 bc | ++ |
| 13 | 0.5 | 4.0 | 33.33 | 22.23±5.53 ab | 1.17±0.17 ab | + |
表1 不同浓度NAA与6-BA配比对手指柠檬茎段愈伤组织诱导及不定芽增殖的影响
Table 1 Effects of NAA and 6-BA ratio at different concentrations on Citrus australasica callus induction and adventitious bud proliferation
| No. | NAA (mg?L-1) | 6-BA (mg?L-1) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | ||||
| 1 | 0 | 0 | 61.11 | 55.57±5.57 cde | 1.57±0.29 bc | ++ |
| 2 | 0 | 0.5 | 100.00 | 72.23±5.53 efg | 2.08±0.08 de | ++++ |
| 3 | 0 | 1.0 | 100.00 | 94.43±5.57 g | 2.53±0.03 e | ++++ |
| 4 | 0 | 2.0 | 91.67 | 55.57±5.57 cde | 1.19±0.10 ab | ++ |
| 5 | 0 | 4.0 | 58.33 | 27.73±5.57 abc | 1.17±0.17 ab | + |
| 6 | 0.1 | 0.5 | 58.33 | 61.13±5.57 def | 2.36±0.07 de | +++ |
| 7 | 0.1 | 1.0 | 66.67 | 44.43±5.57 bcde | 1.89±0.11 bce | ++ |
| 8 | 0.1 | 2.0 | 75.00 | 44.47±11.17 bcde | 2.00±0.29 bce | ++ |
| 9 | 0.1 | 4.0 | 41.67 | 16.70±0.00 a | 1.17±0.29 ab | + |
| 10 | 0.5 | 0.5 | 66.67 | 55.57±5.57 cde | 2.33±0.19 de | +++ |
| 11 | 0.5 | 1.0 | 91.67 | 66.67±9.61 ef | 2.57±0.23 e | +++ |
| 12 | 0.5 | 2.0 | 33.33 | 33.33±9.61 abcd | 1.61±0.20 bc | ++ |
| 13 | 0.5 | 4.0 | 33.33 | 22.23±5.53 ab | 1.17±0.17 ab | + |
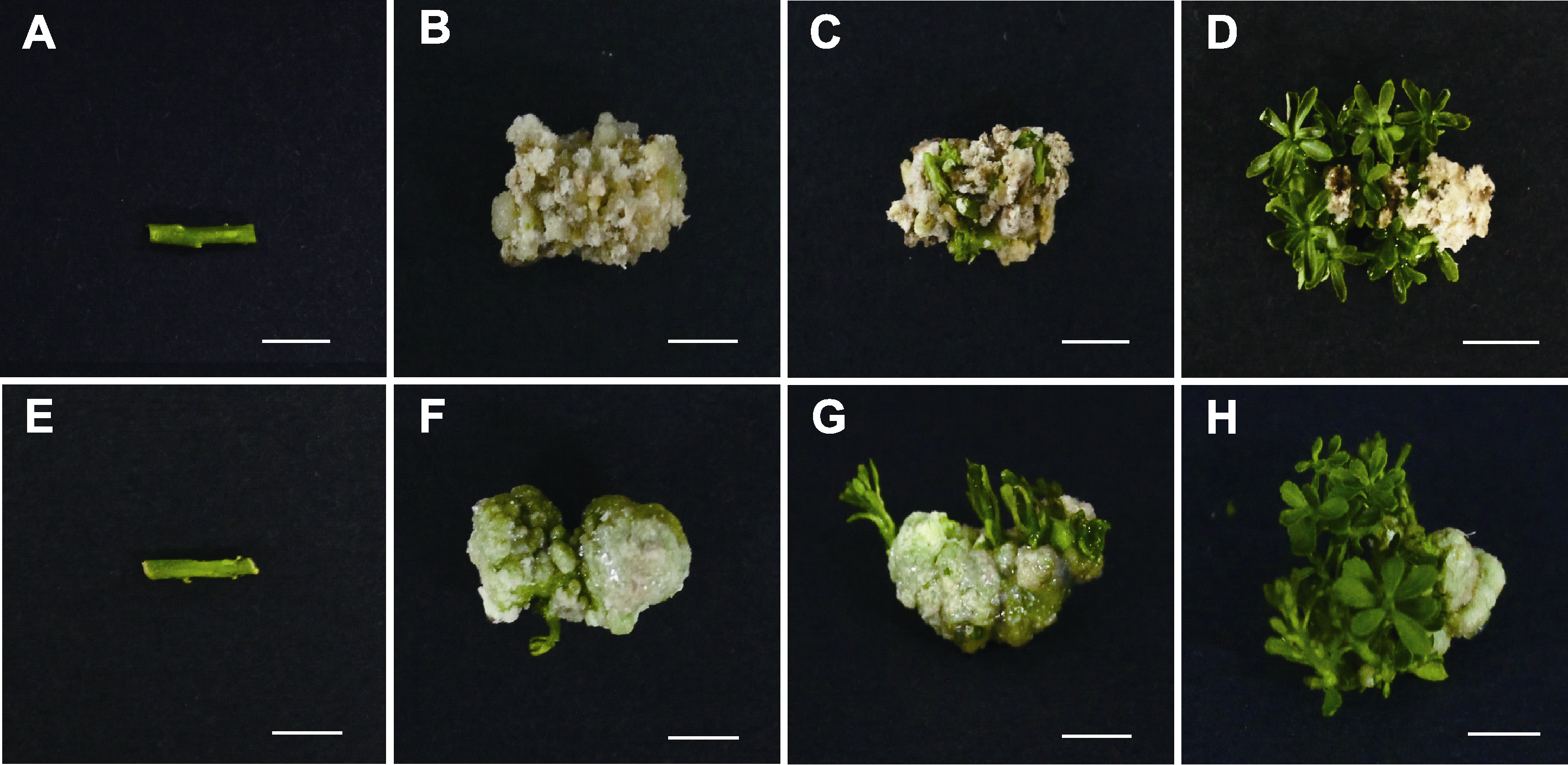
图1 手指柠檬愈伤组织诱导及不定芽分化 (A)-(D) 手指柠檬茎段在添加6-BA的1/2MS培养基上的离体再生过程 (A) 手指柠檬茎段外植体; (B) 培养3周后, 茎段诱导出愈伤组织; (C) 培养4周后, 愈伤组织分化出不定芽; (D) 培养5周后, 诱导出大量不定芽; (E)-(H) 手指柠檬茎段在添加ZT的1/2MS培养基上的离体再生过程 (E) 手指柠檬茎段外植体; (F) 培养3周后, 茎段分化出愈伤组织; (G) 培养4周后, 愈伤组织分化出不定芽; (H) 培养5周后, 诱导出大量不定芽。Bars=0.5 cm
Figure 1 Callus induction and adventitious bud differentiation of Citrus australasica (A)-(D) In vitro regeneration process of C. australasica on 1/2MS medium with 6-BA (A) Stem explant of C. australasica; (B) The stem segments differentiated into callus after 3 weeks; (C) Adventitious buds were induced from the callus after 4 weeks; (D) Adventitious buds were induced after 5 weeks; (E)-(H) In vitro regeneration process of C. australasica on 1/2MS medium with ZT (E) Stem explant of C. australasica; (F) The stem segments differentiated into callus after 3 weeks; (G) Adventitious buds were induced from the callus after 4 weeks; (H) Adventitious buds were induced after 5 weeks. Bars=0.5 cm
| No. | NAA (mg?L-1) | ZT (mg?L-1) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | ||||
| 1 | 0 | 0 | 61.11 | 55.57±5.57 b | 1.56±0.29 a | ++ |
| 2 | 0 | 0.5 | 77.80 | 77.76±5.53 c | 3.53±0.42 cdef | +++ |
| 3 | 0 | 1.0 | 83.30 | 88.87±5.57 c | 3.63±0.75 cdef | ++++ |
| 4 | 0 | 2.0 | 100.00 | 100.00±0.00 c | 3.94±0.92 def | ++++ |
| 5 | 0 | 3.0 | 100.00 | 100.00±0.00 c | 3.94±0.53 defg | +++ |
| 6 | 0 | 4.0 | 100.00 | 100.00±0.00 c | 4.43±0.50 fg | ++++ |
| 7 | 0 | 5.0 | 100.00 | 100.00±0.00 c | 4.24±0.20 fg | +++ |
| 8 | 0 | 6.0 | 83.33 | 77.77±5.53 c | 3.19±0.19 bcdef | +++ |
| 9 | 0.1 | 0.5 | 88.90 | 77.76±5.53 c | 2.50±0.30 abcde | ++ |
| 10 | 0.1 | 1.0 | 100.00 | 100.00±0.00 c | 3.55±0.48 cdef | ++++ |
| 11 | 0.1 | 2.0 | 100.00 | 100.00±0.00 c | 3.28±0.79 cdef | +++ |
| 12 | 0.1 | 3.0 | 100.00 | 100.00±0.00 c | 3.28±0.46 bcdef | +++ |
| 13 | 0.1 | 4.0 | 94.44 | 94.43±5.57 c | 4.21±0.45 efg | ++++ |
| 14 | 0.1 | 5.0 | 94.44 | 88.87±5.57 c | 3.42±0.14 cdef | +++ |
| 15 | 0.1 | 6.0 | 66.67 | 61.10±5.55 b | 2.69±0.26 abd | +++ |
| 16 | 0.5 | 0.5 | 100.00 | 72.20±11.10 bc | 1.49±0.16 ab | ++ |
| 17 | 0.5 | 1.0 | 85.70 | 33.33±8.33 a | 1.50±0.50 ab | ++ |
| 18 | 0.5 | 2.0 | 88.90 | 50.00±9.64 ab | 1.83±0.29 abc | ++ |
| 19 | 0.5 | 3.0 | 88.89 | 50.00±9.64 ab | 1.83±0.17 ab | ++ |
| 20 | 0.5 | 4.0 | 83.33 | 88.87±5.57 c | 2.86±0.17 abcde | +++ |
| 21 | 0.5 | 5.0 | 50.00 | 44.43±5.57 ab | 2.27±0.37 abc | ++ |
| 22 | 0.5 | 6.0 | 33.33 | 27.77±5.53 a | 2.11±0.11 abc | + |
表2 不同浓度NAA与ZT配比对手指柠檬茎段愈伤组织诱导及不定芽增殖的影响
Table 2 Effects of NAA and ZT ratio at different concentrations on Citrus australasica stem callus induction and adventitious bud proliferation
| No. | NAA (mg?L-1) | ZT (mg?L-1) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | ||||
| 1 | 0 | 0 | 61.11 | 55.57±5.57 b | 1.56±0.29 a | ++ |
| 2 | 0 | 0.5 | 77.80 | 77.76±5.53 c | 3.53±0.42 cdef | +++ |
| 3 | 0 | 1.0 | 83.30 | 88.87±5.57 c | 3.63±0.75 cdef | ++++ |
| 4 | 0 | 2.0 | 100.00 | 100.00±0.00 c | 3.94±0.92 def | ++++ |
| 5 | 0 | 3.0 | 100.00 | 100.00±0.00 c | 3.94±0.53 defg | +++ |
| 6 | 0 | 4.0 | 100.00 | 100.00±0.00 c | 4.43±0.50 fg | ++++ |
| 7 | 0 | 5.0 | 100.00 | 100.00±0.00 c | 4.24±0.20 fg | +++ |
| 8 | 0 | 6.0 | 83.33 | 77.77±5.53 c | 3.19±0.19 bcdef | +++ |
| 9 | 0.1 | 0.5 | 88.90 | 77.76±5.53 c | 2.50±0.30 abcde | ++ |
| 10 | 0.1 | 1.0 | 100.00 | 100.00±0.00 c | 3.55±0.48 cdef | ++++ |
| 11 | 0.1 | 2.0 | 100.00 | 100.00±0.00 c | 3.28±0.79 cdef | +++ |
| 12 | 0.1 | 3.0 | 100.00 | 100.00±0.00 c | 3.28±0.46 bcdef | +++ |
| 13 | 0.1 | 4.0 | 94.44 | 94.43±5.57 c | 4.21±0.45 efg | ++++ |
| 14 | 0.1 | 5.0 | 94.44 | 88.87±5.57 c | 3.42±0.14 cdef | +++ |
| 15 | 0.1 | 6.0 | 66.67 | 61.10±5.55 b | 2.69±0.26 abd | +++ |
| 16 | 0.5 | 0.5 | 100.00 | 72.20±11.10 bc | 1.49±0.16 ab | ++ |
| 17 | 0.5 | 1.0 | 85.70 | 33.33±8.33 a | 1.50±0.50 ab | ++ |
| 18 | 0.5 | 2.0 | 88.90 | 50.00±9.64 ab | 1.83±0.29 abc | ++ |
| 19 | 0.5 | 3.0 | 88.89 | 50.00±9.64 ab | 1.83±0.17 ab | ++ |
| 20 | 0.5 | 4.0 | 83.33 | 88.87±5.57 c | 2.86±0.17 abcde | +++ |
| 21 | 0.5 | 5.0 | 50.00 | 44.43±5.57 ab | 2.27±0.37 abc | ++ |
| 22 | 0.5 | 6.0 | 33.33 | 27.77±5.53 a | 2.11±0.11 abc | + |
| No. | Different media | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | |||
| 1 | 1/2MS | 100.00 | 100.00±0.00 a | 4.57±0.33 a | ++++ |
| 2 | MT | 83.30 | 61.13±5.57 b | 3.57±0.24 b | +++ |
| 3 | MS | 100.00 | 88.87±5.57 ab | 3.64±0.38 b | +++ |
| 4 | WPM | 83.30 | 72.23±5.53 bc | 2.83±0.17 bc | +++ |
| 5 | White | 66.70 | 33.33±0.00 e | 1.56±0.29 c | + |
表3 不同基本培养基对手指柠檬茎段愈伤组织诱导及不定芽增殖的影响
Table 3 Effects of different basic media on callus induction and adventitious bud proliferation of Citrus australasica
| No. | Different media | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | |||
| 1 | 1/2MS | 100.00 | 100.00±0.00 a | 4.57±0.33 a | ++++ |
| 2 | MT | 83.30 | 61.13±5.57 b | 3.57±0.24 b | +++ |
| 3 | MS | 100.00 | 88.87±5.57 ab | 3.64±0.38 b | +++ |
| 4 | WPM | 83.30 | 72.23±5.53 bc | 2.83±0.17 bc | +++ |
| 5 | White | 66.70 | 33.33±0.00 e | 1.56±0.29 c | + |
| No. | Dark treatment (day) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | |||
| 1 | 0 | 55.57±5.57 a | 44.43±5.57 a | 2.28±0.15 a | ++ |
| 2 | 7 | 88.87±5.57 b | 83.33±9.61 bc | 3.50±0.21 b | +++ |
| 3 | 14 | 100.00±0.00 b | 100.00±0.00 c | 4.83±0.38 c | ++++ |
| 4 | 21 | 94.43±5.57 b | 66.70±0.00 b | 2.75±0.38 a | ++ |
| 5 | 28 | 100.00±0.00 b | 38.87±5.57 a | 2.33±0.33 a | ++ |
表4 暗培养处理对手指柠檬茎段愈伤组织诱导及不定芽增殖的影响
Table 4 Effects of dark treatment on callus induction and adventitious bud proliferation of Citrus australasica
| No. | Dark treatment (day) | 3 weeks | 5 weeks | Growth condition | |
|---|---|---|---|---|---|
| Callus induction rate (%) | Rate of adventitious bud differentiation (%) | Frequency of bud differentiation | |||
| 1 | 0 | 55.57±5.57 a | 44.43±5.57 a | 2.28±0.15 a | ++ |
| 2 | 7 | 88.87±5.57 b | 83.33±9.61 bc | 3.50±0.21 b | +++ |
| 3 | 14 | 100.00±0.00 b | 100.00±0.00 c | 4.83±0.38 c | ++++ |
| 4 | 21 | 94.43±5.57 b | 66.70±0.00 b | 2.75±0.38 a | ++ |
| 5 | 28 | 100.00±0.00 b | 38.87±5.57 a | 2.33±0.33 a | ++ |
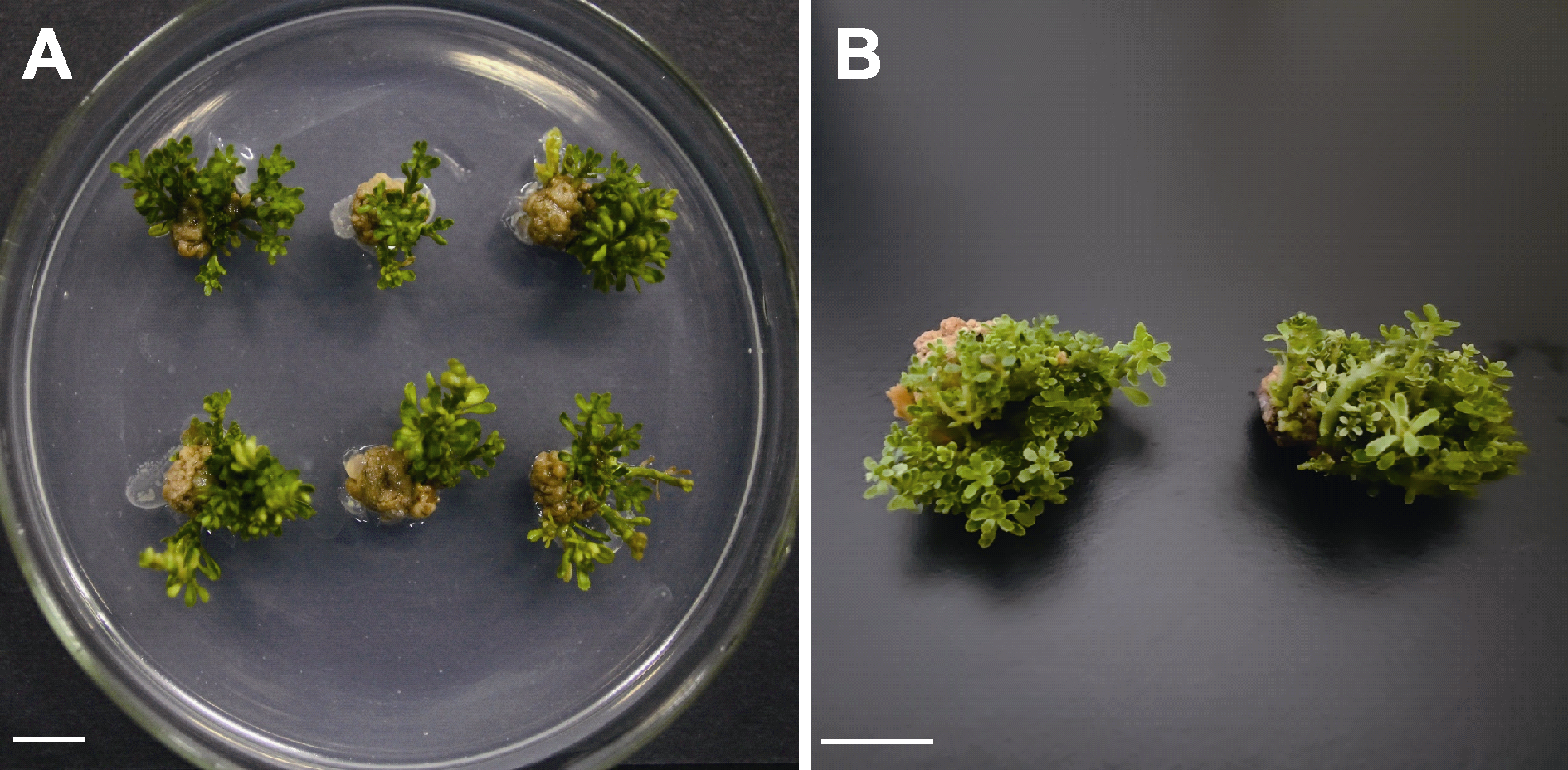
图2 手指柠檬茎段在优化后的培养基上不定芽再生状况 手指柠檬茎段在配方为1/2MS+5 mg?L-1 ZT+30 g?L-1蔗糖的优化培养基上暗处理14天, 光下培养5周(A)和8周(B)离体不定芽的再生情况。ZT: 玉米素。Bars=1 cm
Figure 2 Adventitious buds from stem explants of Citrus australasica on optimized medium After 14 days dark treatment, adventitious buds from stem explants of C. australasica on 1/2MS+5 mg?L-1 ZT+30 g?L-1 sucrose, 5 weeks (A) and 8 weeks (B) in the light. ZT: Zeatin. Bars=1 cm
| No. | NAA concentration (mg?L-1) | Rooting rate (%) | Average rooting number | Growth condition |
|---|---|---|---|---|
| 1 | 0 | 16.70±0.00 d | 0.97±0.21 d | + |
| 2 | 0.05 | 38.87±9.64 c | 2.02±0.24 c | ++ |
| 3 | 0.1 | 44.43±9.64 c | 2.07±0.15 c | ++ |
| 4 | 0.3 | 77.77±9.58 b | 3.03±0.13 b | ++ |
| 5 | 0.5 | 94.43±9.64 a | 3.90±0.20 a | ++++ |
| 6 | 1 | 61.13±9.64 c | 2.35±0.40 c | +++ |
表5 不同浓度NAA对手指柠檬不定芽生根的影响
Table 5 Effects of NAA concentration on rooting induction of Citrus australasica adventitious buds
| No. | NAA concentration (mg?L-1) | Rooting rate (%) | Average rooting number | Growth condition |
|---|---|---|---|---|
| 1 | 0 | 16.70±0.00 d | 0.97±0.21 d | + |
| 2 | 0.05 | 38.87±9.64 c | 2.02±0.24 c | ++ |
| 3 | 0.1 | 44.43±9.64 c | 2.07±0.15 c | ++ |
| 4 | 0.3 | 77.77±9.58 b | 3.03±0.13 b | ++ |
| 5 | 0.5 | 94.43±9.64 a | 3.90±0.20 a | ++++ |
| 6 | 1 | 61.13±9.64 c | 2.35±0.40 c | +++ |
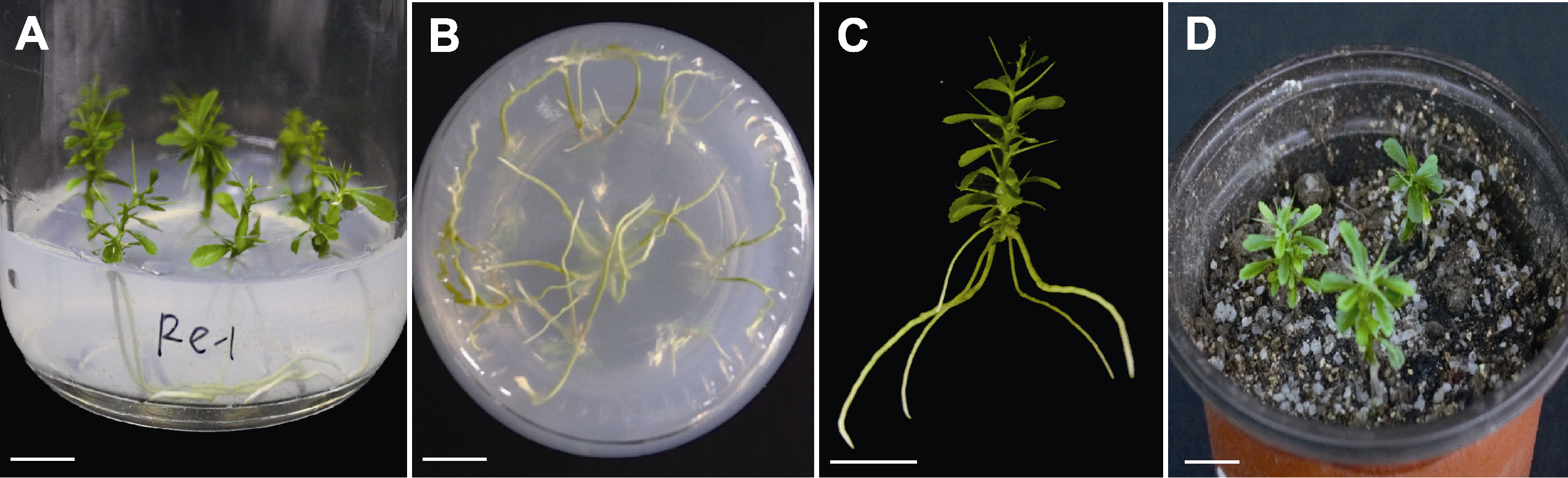
图3 手指柠檬再生植株的生根与移栽 (A), (B) 不定根诱导; (C) 再生植株; (D) 移栽成活的植株。Bars=1 cm
Figure 3 Rooting and transplanting of Citrus australasica regenerated plants (A), (B) Adventitious root induction; (C) Regenerated plants; (D) Transplant survived plants. Bars=1 cm
| [1] | 常贝贝, 屈宜宝, 王智宇, 程晓帆, 杜晓云, 于晓丽, 赵玲玲, 张硕 (2023). 2个苹果新品种高效再生体系建立. 分子植物育种. https://kns.cnki.net/kcms/detail/46.1068.S.20220722.1647.008.html. |
| [2] | 范永梅, 甘霖, 邓秀新 (2003). 冰糖橙胚性愈伤组织的诱导与植株再生. 华中农业大学学报 22, 399-402. |
| [3] |
林颖, 龙自立, 张璐, 叶庆富, 刘永立 (2012). 猕猴桃胚乳再生植株体系的优化. 核农学报 26, 257-261, 310.
DOI |
| [4] |
逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧 (2022). 大花银莲花愈伤组织诱导及再生体系的建立. 植物学报 57, 217-226.
DOI |
| [5] |
任露露, 张有泽, 黄克林, 宛晓春, 张照亮, 朱木兰, 韦朝领 (2023). 茶树茎段不定芽高效发生体系的建立. 植物学报 58, 308-315.
DOI |
| [6] | 魏佳, 路天宇, 王朝胜, 张红叶, 张晶, 王顺利, 李润芝 (2022). 不同粒级园艺基质原料物理性质差异分析. 北京农学院学报 37(4), 13-18. |
| [7] | 杨圣涛, 吕岩, 贺元源, 刘婷婷, 马晓祯 (2021). 基于CT扫描的草炭土孔隙结构分析及渗流模拟. 工程地质学报 29, 1354-1365. |
| [8] | 张郎郎, 张洁, 吕虹霖, 谭彬, 王伟, 程钧, 冯建灿 (2022). 欧洲李叶片再生体系的建立. 果树学报 39, 1945-1953. |
| [9] | 张秀英, 鲁兴凯, 程安富, 胡志芳, 马勉娣, 张丹, 黄国嫣, 陈晨, 全勇, 汪琼 (2022). 基质对苹果砧木M26脱毒组培苗移栽成活率和生长的影响. 中国南方果树 51(5), 150-153. |
| [10] |
张旭红, 王頔, 梁振旭, 孙美玉, 张金政, 石雷 (2018). 欧洲百合愈伤组织诱导及植株再生体系的建立. 植物学报 53, 840-847.
DOI |
| [11] |
Bernula D, Benkő P, Kaszler N, Domonkos I, Valkai I, Szőllősi R, Ferenc G, Ayaydin F, Fehér A, Gémes K (2020). Timely removal of exogenous cytokinin and the prevention of auxin transport from the shoot to the root affect the regeneration potential of Arabidopsis roots. Plant Cell Tissue Organ Cult 140, 327-339.
DOI |
| [12] |
Cioni E, Migone C, Ascrizzi R, Muscatello B, De Leo M, Piras AM, Zambito Y, Flamini G, Pistelli L (2022). Comparing metabolomic and essential oil fingerprints of Citrus australasica F. Muell (Finger Lime) varieties and their in vitro antioxidant activity. Antioxidants (Basel) 11, 2047.
DOI URL |
| [13] |
Conti G, Xoconostle-Cázares B, Marcelino-Pérez G, Hopp HE, Reyes CA (2021). Citrus genetic transformation: an overview of the current strategies and insights on the new emerging technologies. Front Plant Sci 12, 768197.
DOI URL |
| [14] |
Dai WH, Castillo C (2007). Factors affecting plant regeneration from leaf tissues of buddleia species. HortScience 42, 1670-1673.
DOI URL |
| [15] |
Huang T, Peng SL, Dong GF, Zhang LY, Li GG (2002). Plant regeneration from leaf-derived callus in Citrus grandis (pummelo): effects of auxins in callus induction medium. Plant Cell Tissue Organ Cult 69, 141-146.
DOI URL |
| [16] |
Jardak R, Boubakri H, Zemni H, Gandoura S, Mejri S, Mliki A, Ghorbel A (2020). Establishment of an in vitro regeneration system and genetic transformation of the Tunisian ‘Maltese half-blood’ (Citrus sinensis): an agro- economically important variety. 3 Biotech 10, 99.
DOI PMID |
| [17] |
Long Y, Yang Y, Pan GT, Shen YO (2022). New insights into tissue culture plant-regeneration mechanisms. Front Plant Sci 13, 926752.
DOI URL |
| [18] | Loyola-Vargas VM, Ochoa-Alejo N (2018). An introduction to plant tissue culture:advances and perspectives. In: Loyola-Vargas VM, Ochoa-Alejo N, eds. Plant Cell Culture Protocols. New York: Humana Press. pp. 3-13. |
| [19] |
Peña L, Pérez RM, Cervera M, Juárez JA, Navarro L (2004). Early events in Agrobacterium-mediated genetic transformation of citrus explants. Ann Bot 94, 67-74.
DOI URL |
| [20] |
Poles L, Licciardello C, Distefano G, Nicolosi E, Gentile A, La Malfa S (2020). Recent advances of in vitro culture for the application of new breeding techniques in citrus. Plants (Basel) 9, 938.
DOI URL |
| [21] |
Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A, Ćosić T (2021). Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. Int J Mol Sci 22, 8554.
DOI URL |
| [22] | Skoog F, Miller CO (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp Soc Exp Biol 11, 118-130. |
| [23] |
Zenser N, Ellsmore A, Leasure C, Callis J (2001). Auxin modulates the degradation rate of Aux/IAA proteins. Proc Natl Acad Sci USA 98, 11795-11800.
DOI PMID |
| [1] | 李彤, 李楚然, 张芷瑜, 付晓熳, 刘云, 张颖君, 杨力颖, 赵平. 西印度醋栗组培快繁技术初探[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 冯雯, 王玉国. 栽培薯蓣茎段离体再生体系的建立[J]. 植物学报, 2024, 59(5): 792-799. |
| [3] | 刘玉泽, 王一菲, 任威蓁, 栗浩, 路斌, 路丙社, 于晓跃. 北美豆梨杂种幼胚挽救及再生体系的建立[J]. 植物学报, 2024, 59(5): 800-809. |
| [4] | 曾浩, 李佩芳, 郭至辉, 刘春林, 阮颖. 银扇草再生体系的建立[J]. 植物学报, 2024, 59(3): 433-440. |
| [5] | 任露露, 张有泽, 黄克林, 宛晓春, 张照亮, 朱木兰, 韦朝领. 茶树茎段不定芽高效发生体系的建立[J]. 植物学报, 2023, 58(2): 308-315. |
| [6] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧. 大花银莲花愈伤组织诱导及再生体系的建立[J]. 植物学报, 2022, 57(2): 217-226. |
| [7] | 李孟悦, 刘柳, 刘艳, 张晓曼. 毛报春(Primula × pubescens)腋芽再生组织培养体系的建立[J]. 植物学报, 2021, 56(6): 732-739. |
| [8] | 罗钱, 张燕莎, 欧静. 郁金樱愈伤组织诱导及植株再生[J]. 植物学报, 2021, 56(4): 451-461. |
| [9] | 罗虹, 温小蕙, 周圆圆, 戴思兰. 芳香堆心菊离体再生体系的建立[J]. 植物学报, 2020, 55(3): 318-328. |
| [10] | 邓莎, 吴艳妮, 吴坤林, 房林, 李琳, 曾宋君. 14种中国典型极小种群野生植物繁育特性和人工繁殖研究进展[J]. 生物多样性, 2020, 28(3): 385-400. |
| [11] | 张文婷,何燕红,舒宁,邢景景,刘宝骏,包满珠,刘国锋. 金黄花滇百合植株再生与离体快繁技术体系的建立[J]. 植物学报, 2019, 54(6): 773-778. |
| [12] | 唐凤鸾,赵健,赵志国,夏科,仇硕. 走马胎的组织培养与快速繁殖[J]. 植物学报, 2019, 54(3): 378-384. |
| [13] | 咸洋,董昕,解孝满,吴丹,韩彪,王艳. 光照和温度对红花槭限制生长保存的影响[J]. 植物学报, 2019, 54(1): 64-71. |
| [14] | 安佰义, 郭才南, 包文慧, 李凤飞, 赵赫, 陈丽, 安丰云. 白檀离体快繁技术[J]. 植物学报, 2018, 53(5): 693-699. |
| [15] | 王燕, 牟豪杰, 吕永平, 李海营, 汪一婷, 陈剑平. 寿锦的离体植株再生及组培产业化增殖[J]. 植物学报, 2017, 52(3): 331-336. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||