

植物学报 ›› 2024, Vol. 59 ›› Issue (5): 800-809.DOI: 10.11983/CBB23152 cstr: 32102.14.CBB23152
刘玉泽1, 王一菲1, 任威蓁1, 栗浩2, 路斌1, 路丙社1, 于晓跃1,*( )
)
收稿日期:2023-11-13
接受日期:2024-07-14
出版日期:2024-09-10
发布日期:2024-08-19
通讯作者:
于晓跃
基金资助:
Yuze Liu1, Yifei Wang1, Weizhen Ren1, Hao Li2, Bin Lu1, Bingshe Lu1, Xiaoyue Yu1,*( )
)
Received:2023-11-13
Accepted:2024-07-14
Online:2024-09-10
Published:2024-08-19
Contact:
Xiaoyue Yu
摘要: 以北美豆梨(Pyrus calleryana cv. ‘Cleveland’)杂种幼胚为材料, 系统开展了杂种幼胚灭菌、愈伤组织诱导、不定芽增殖和生根培养基的筛选研究。结果表明, 4°C低温贮藏21天的杂种幼胚萌发率最高, 为67.23%; 幼胚最佳灭菌处理为75%乙醇灭菌30秒, 10% H2O2和0.1% HgCl2分别消毒10和14分钟; 最适幼胚萌发培养基为1/2MS+4.0 mg·L-1 6-BA+0.5 mg·L-1 NAA, 萌发率达89.67%; 最适愈伤组织诱导培养基为1/2MS+1.0 mg·L-1 IBA+1.0 mg·L-1 6-BA, 愈伤组织诱导率为93.33%; 最佳分化培养基为1/2MS+0.2 mg·L-1 IBA+2.0 mg·L-1 6-BA, 再生频率为87.44%; 最佳继代增殖培养基为1/2MS+1.5 mg·L-1 6-BA+0.1 mg·L-1 IBA, 增殖率为100%; 最佳生根培养基为1/2MS+20 g·L-1蔗糖+1.0 g·L-1 活性炭+1.5 mg·L-1 IBA+0.05 mg·L-1 NAA, 生根率为82.63%。该研究为北美豆梨杂交种质资源高效繁殖提供了科学依据和指导。
刘玉泽, 王一菲, 任威蓁, 栗浩, 路斌, 路丙社, 于晓跃. 北美豆梨杂种幼胚挽救及再生体系的建立. 植物学报, 2024, 59(5): 800-809.
Yuze Liu, Yifei Wang, Weizhen Ren, Hao Li, Bin Lu, Bingshe Lu, Xiaoyue Yu. Establishment of Immature Embryo Rescue and Regeneration System for Pyrus calleryana cv. ‘Cleveland’. Chinese Bulletin of Botany, 2024, 59(5): 800-809.
| No. | 75% ethanol (s) | 0.1% HgCl2 (min) | 10% H2O2 (min) |
|---|---|---|---|
| A1 | 30 | 8 | 0 |
| A2 | 30 | 10 | 0 |
| A3 | 30 | 12 | 0 |
| A4 | 30 | 14 | 0 |
| A5 | 30 | 16 | 0 |
| A6 | 30 | 18 | 0 |
| A7 | 30 | 10 | 10 |
| A8 | 30 | 12 | 10 |
| A9 | 30 | 14 | 10 |
| A10 | 30 | 16 | 10 |
表1 不同灭菌处理方法
Table 1 Different sterilization methods
| No. | 75% ethanol (s) | 0.1% HgCl2 (min) | 10% H2O2 (min) |
|---|---|---|---|
| A1 | 30 | 8 | 0 |
| A2 | 30 | 10 | 0 |
| A3 | 30 | 12 | 0 |
| A4 | 30 | 14 | 0 |
| A5 | 30 | 16 | 0 |
| A6 | 30 | 18 | 0 |
| A7 | 30 | 10 | 10 |
| A8 | 30 | 12 | 10 |
| A9 | 30 | 14 | 10 |
| A10 | 30 | 16 | 10 |
| No. | Auxin species | Auxin concentration (mg·L-1) | 6-BA (mg·L-1) |
|---|---|---|---|
| B1 | NAA | 0.2 | 1.0 |
| B2 | NAA | 0.5 | 2.0 |
| B3 | NAA | 1.0 | 3.0 |
| B4 | IBA | 0.2 | 2.0 |
| B5 | IBA | 0.5 | 3.0 |
| B6 | IBA | 1.0 | 1.0 |
| B7 | 2,4-D | 0.2 | 3.0 |
| B8 | 2,4-D | 0.5 | 1.0 |
| B9 | 2,4-D | 1.0 | 2.0 |
表2 启动培养的实验方案
Table 2 Experimental scheme of primary culture
| No. | Auxin species | Auxin concentration (mg·L-1) | 6-BA (mg·L-1) |
|---|---|---|---|
| B1 | NAA | 0.2 | 1.0 |
| B2 | NAA | 0.5 | 2.0 |
| B3 | NAA | 1.0 | 3.0 |
| B4 | IBA | 0.2 | 2.0 |
| B5 | IBA | 0.5 | 3.0 |
| B6 | IBA | 1.0 | 1.0 |
| B7 | 2,4-D | 0.2 | 3.0 |
| B8 | 2,4-D | 0.5 | 1.0 |
| B9 | 2,4-D | 1.0 | 2.0 |
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) |
|---|---|---|
| C1 | 1.0 | 0.05 |
| C2 | 1.0 | 0.10 |
| C3 | 1.0 | 0.20 |
| C4 | 1.5 | 0.05 |
| C5 | 1.5 | 0.10 |
| C6 | 1.5 | 0.20 |
| C7 | 2.0 | 0.05 |
| C8 | 2.0 | 0.10 |
| C9 | 2.0 | 0.20 |
| C10 | 3.0 | 0.05 |
| C11 | 3.0 | 0.10 |
| C12 | 3.0 | 0.20 |
| C13 | 4.0 | 0.05 |
| C14 | 4.0 | 0.10 |
| C15 | 4.0 | 0.20 |
表3 继代培养中不同植物生长调节剂组合处理
Table 3 Treatments with different plant growth regulation combinations in subculture
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) |
|---|---|---|
| C1 | 1.0 | 0.05 |
| C2 | 1.0 | 0.10 |
| C3 | 1.0 | 0.20 |
| C4 | 1.5 | 0.05 |
| C5 | 1.5 | 0.10 |
| C6 | 1.5 | 0.20 |
| C7 | 2.0 | 0.05 |
| C8 | 2.0 | 0.10 |
| C9 | 2.0 | 0.20 |
| C10 | 3.0 | 0.05 |
| C11 | 3.0 | 0.10 |
| C12 | 3.0 | 0.20 |
| C13 | 4.0 | 0.05 |
| C14 | 4.0 | 0.10 |
| C15 | 4.0 | 0.20 |
| No. | Basic media | NAA (mg·L-1) | Sugar (mg·L-1) | AC (g·L-1) | IBA (mg·L-1) |
|---|---|---|---|---|---|
| D1 | 1/2MS | 0.05 | 20 | 0 | 1.0 |
| D2 | 1/2MS | 0.05 | 20 | 1.0 | 1.5 |
| D3 | 1/2MS | 0.05 | 20 | 1.5 | 2.0 |
| D4 | 1/2MS | 0.05 | 25 | 0 | 1.5 |
| D5 | 1/2MS | 0.05 | 25 | 1.0 | 1.0 |
| D6 | 1/2MS | 0.05 | 25 | 1.5 | 2.0 |
| D7 | 1/2MS | 0.05 | 30 | 0 | 2.0 |
| D8 | 1/2MS | 0.05 | 30 | 1.0 | 1.0 |
| D9 | 1/2MS | 0.05 | 30 | 1.5 | 1.5 |
表4 生根培养实验方案
Table 4 Experimental scheme for rooting culture
| No. | Basic media | NAA (mg·L-1) | Sugar (mg·L-1) | AC (g·L-1) | IBA (mg·L-1) |
|---|---|---|---|---|---|
| D1 | 1/2MS | 0.05 | 20 | 0 | 1.0 |
| D2 | 1/2MS | 0.05 | 20 | 1.0 | 1.5 |
| D3 | 1/2MS | 0.05 | 20 | 1.5 | 2.0 |
| D4 | 1/2MS | 0.05 | 25 | 0 | 1.5 |
| D5 | 1/2MS | 0.05 | 25 | 1.0 | 1.0 |
| D6 | 1/2MS | 0.05 | 25 | 1.5 | 2.0 |
| D7 | 1/2MS | 0.05 | 30 | 0 | 2.0 |
| D8 | 1/2MS | 0.05 | 30 | 1.0 | 1.0 |
| D9 | 1/2MS | 0.05 | 30 | 1.5 | 1.5 |

图1 不同处理对北美豆梨杂种幼胚灭菌及萌发率的影响 (A) 不同灭菌处理对幼胚污染率与成活率的影响; (B) 不同低温贮藏时间对幼胚萌发率的影响。不同小写字母表示各处理间差异显著(P<0.05)。A1-A10同表1。
Figure 1 Effect of different treatments on sterilization and germination rate of seed embryo (A) Effect of different treatments on sterilization; (B) Effect of different low temperature storage time on germination rate. Different lowercase letters indicate significant differences among different treatmets at P<0.05 level. A1-A10 are the same as shown in Table 1.
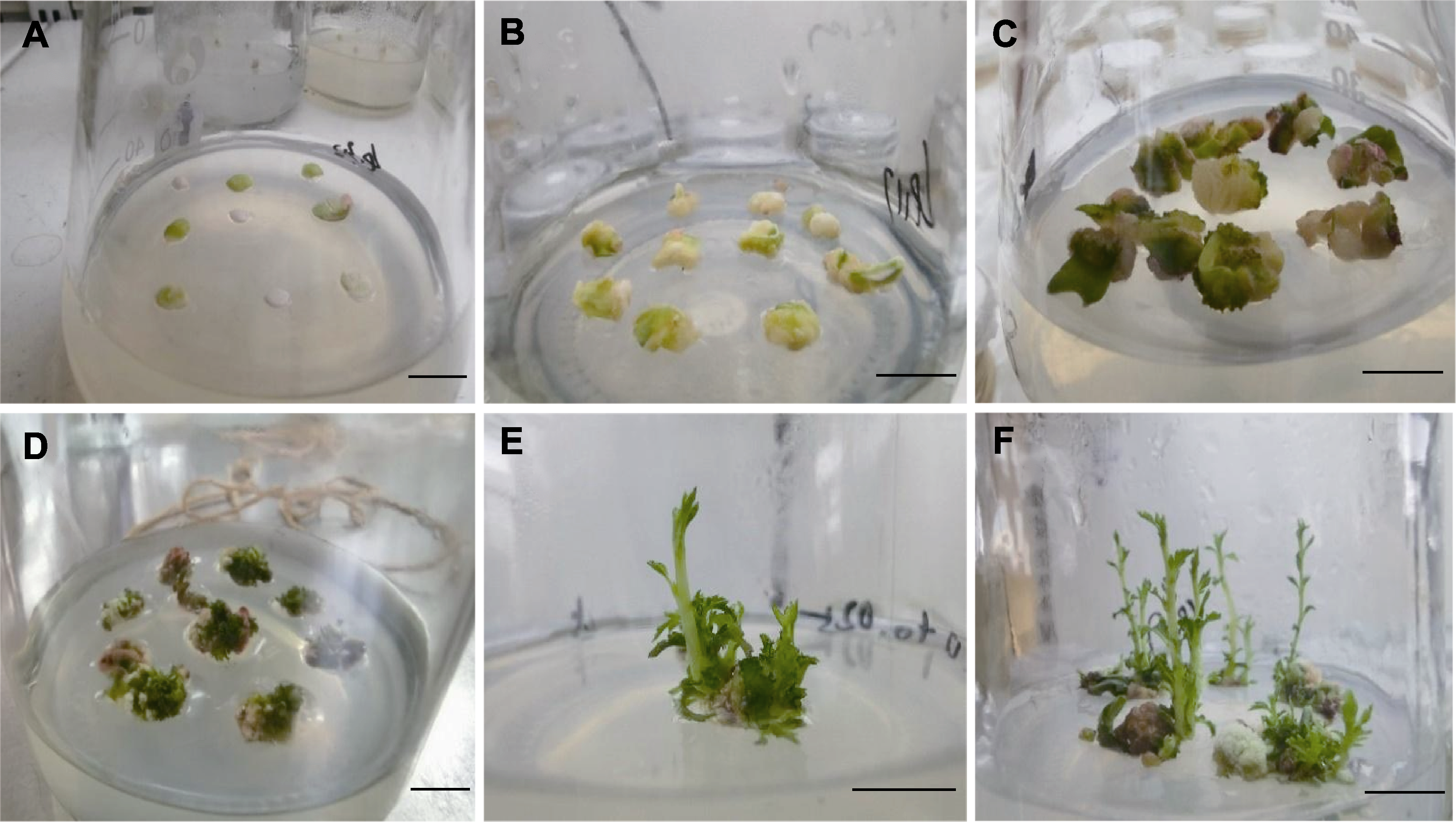
图2 杂种幼胚的诱导分化过程 (A) 暗培养2-4天; (B) 暗培养6-7天; (C) 暗培养14天; (D) 暗培养21天后转为光培养; (E) 接种30天左右; (F) 接种45天左右。Bars=1 cm
Figure 2 Induced differentiation process of embryo (A) Dark culture for 2-4 days; (B) Dark culture for 6-7 days; (C) Dark culture for 14 days; (D) After dark culture for 21 days, it was converted to light culture; (E) Inoculation for about 30 days; (F) About 45 days after inoculation. Bars=1 cm
| No. | Callus rate (%) | Differentiation rate (%) | Number of adventitious buds regenerated | Callus form |
|---|---|---|---|---|
| B1 | 60.56±2.55 e | 77.22±2.55 c | 3.27±0.04 b | Yellowish green, white, sticky lump |
| B2 | 66.11±2.55 d | 72.78±2.55 d | 2.79±0.04 c | Yellowish green, white, loose |
| B3 | 74.44±2.55 c | 70.56±1.92 d | 2.86±0.03 c | Light green, white, ropy |
| B4 | 72.22±2.55 c | 89.44±1.92 a | 3.79±0.06 a | Light green, loose |
| B5 | 85.56±1.92 b | 82.78±1.92 b | 3.86±0.03 a | Chartreuse, white, ropy |
| B6 | 93.33±1.67 a | 63.89±1.92 e | 2.67±0.03 d | Chartreuse, white, ropy |
| B7 | 82.78±2.55 b | 81.67±2.89 b | 3.59±0.04 b | Yellow, white, clumpy and compact |
| B8 | 86.67±3.33 b | 60.56±1.92 e | 2.69±0.05 d | Light yellow, granular sphericity, compact and fragile |
| B9 | 91.67±2.89 a | 61.67±1.67 e | 2.38±0.04 e | Light yellow, white, waterlogged callus |
表5 不同配比植物生长调节物质对杂种幼胚组织初代培养的影响
Table 5 Effect of different plant growth regulator combinations on the primary culture of seed embryos
| No. | Callus rate (%) | Differentiation rate (%) | Number of adventitious buds regenerated | Callus form |
|---|---|---|---|---|
| B1 | 60.56±2.55 e | 77.22±2.55 c | 3.27±0.04 b | Yellowish green, white, sticky lump |
| B2 | 66.11±2.55 d | 72.78±2.55 d | 2.79±0.04 c | Yellowish green, white, loose |
| B3 | 74.44±2.55 c | 70.56±1.92 d | 2.86±0.03 c | Light green, white, ropy |
| B4 | 72.22±2.55 c | 89.44±1.92 a | 3.79±0.06 a | Light green, loose |
| B5 | 85.56±1.92 b | 82.78±1.92 b | 3.86±0.03 a | Chartreuse, white, ropy |
| B6 | 93.33±1.67 a | 63.89±1.92 e | 2.67±0.03 d | Chartreuse, white, ropy |
| B7 | 82.78±2.55 b | 81.67±2.89 b | 3.59±0.04 b | Yellow, white, clumpy and compact |
| B8 | 86.67±3.33 b | 60.56±1.92 e | 2.69±0.05 d | Light yellow, granular sphericity, compact and fragile |
| B9 | 91.67±2.89 a | 61.67±1.67 e | 2.38±0.04 e | Light yellow, white, waterlogged callus |
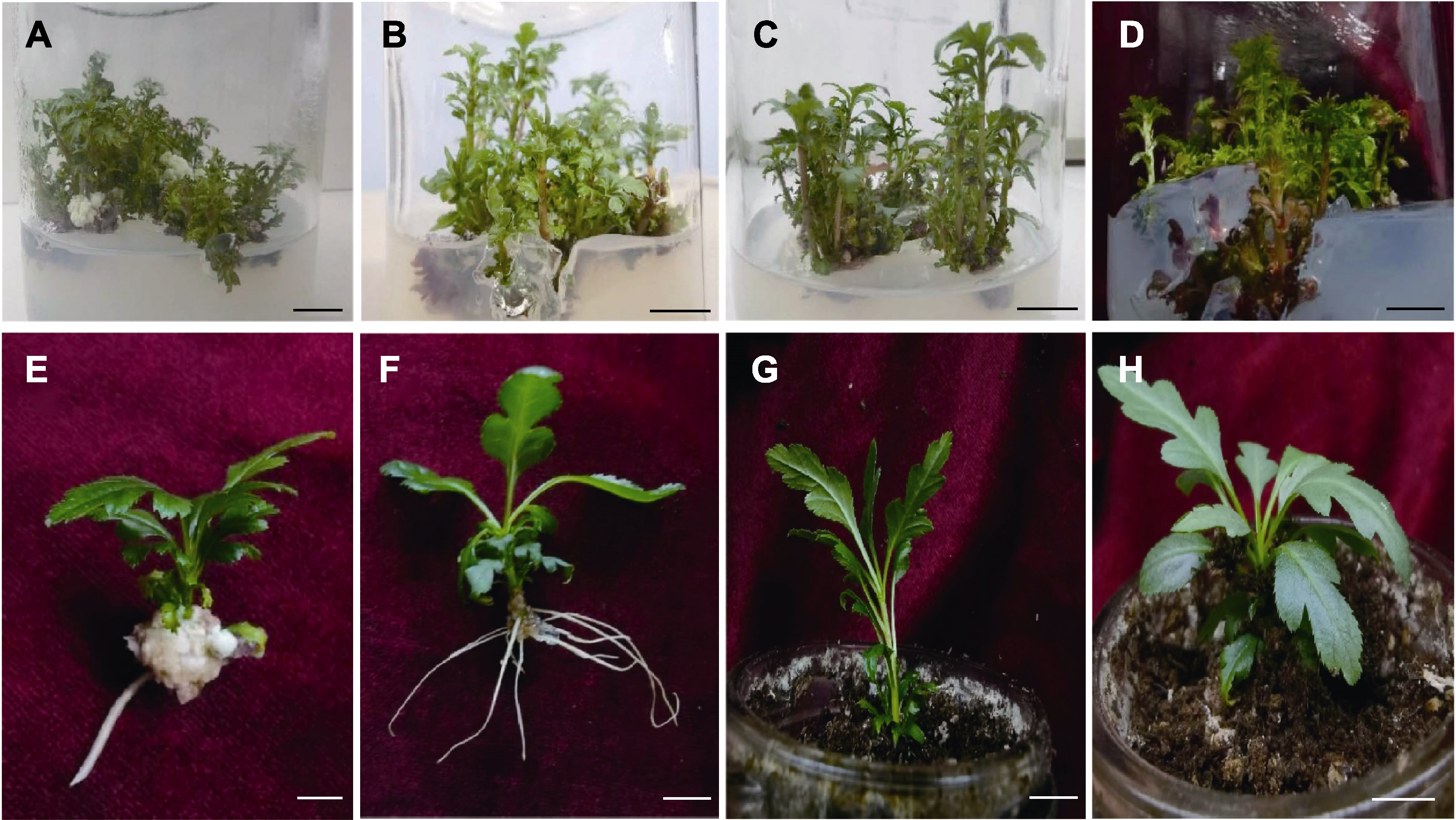
图3 不定芽增殖及组培苗移栽 (A)-(D) 6-BA浓度分别为1.0、1.5、2.0和4.0 mg·L-1时不定芽的增殖情况; (E) 未添加活性炭的处理组生根情况; (F) 添加活性炭的处理组生根情况; (G), (H) 移栽。Bars=1 cm
Figure 3 Adventitious bud proliferation and tissue culture seedling transplanting (A)-(D) Adventitious bud proliferation when 6-BA concentration was 1.0,1.5, 2.0 and 4.0 mg·L-1; (E) Rooting condition of the treatment group without adding active carbon; (F) Rooting condition of the treatment group added active carbon; (G), (H) Transplant. Bars=1 cm
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) | Multiplication rates (%) | Multiplication coefficient | Growth condition |
|---|---|---|---|---|---|
| C1 | 1.0 | 0.05 | 91.11±0.96 d | 3.17±0.17 efg | Average, short |
| C2 | 1.0 | 0.1 | 87.22±0.96 e | 3.00±0.44 fg | Average, short |
| C3 | 1.0 | 0.2 | 95.00±1.67 c | 2.89±0.10 g | Average |
| C4 | 1.5 | 0.05 | 100.00±0.00 a | 4.17±0.44 bc | Stronger |
| C5 | 1.5 | 0.1 | 100.00±0.00 a | 5.22±0.35 a | Stronger, large leaf blade, dark green |
| C6 | 1.5 | 0.2 | 100.00±0.00 a | 4.11±0.10 bcd | Stronger, dark green |
| C7 | 2.0 | 0.05 | 100.00±0.00 a | 3.67±0.17 cde | Stronger, dark green |
| C8 | 2.0 | 0.1 | 100.00±0.00 a | 4.50±0.33 b | Stronger |
| C9 | 2.0 | 0.2 | 100.00±0.00 a | 3.61±0.19 cdef | Stronger |
| C10 | 3.0 | 0.05 | 95.56±0.96 c | 3.61±0.69 cdef | Average growth, small leaf blade |
| C11 | 3.0 | 0.1 | 97.22±0.96 b | 3.50±0.44 defg | Average growth, small leaf blade |
| C12 | 3.0 | 0.2 | 100.00±0.00 a | 3.28±0.19 efg | Average growth, dwarf tufted bud, deformed seedling |
| C13 | 4.0 | 0.05 | 96.11±0.96 bc | 3.94±0.35 bcd | Weak growth, fewer leaves, red stems and leaves, glass seedlings |
| C14 | 4.0 | 0.1 | 92.22±0.96 d | 3.67±0.29 cde | Small leaf blade, dwarf tufted bud, glass seedlings, deformed seedling |
| C15 | 4.0 | 0.2 | 100.00±0.00 a | 3.56±0.19 cdef | Malformed leaf, glass seedlings |
表6 不同植物生长调节物质组合对不定芽增殖的影响
Table 6 Effect of different plant growth regulator combinations on adventitious bud proliferation
| No. | 6-BA (mg·L-1) | IBA (mg·L-1) | Multiplication rates (%) | Multiplication coefficient | Growth condition |
|---|---|---|---|---|---|
| C1 | 1.0 | 0.05 | 91.11±0.96 d | 3.17±0.17 efg | Average, short |
| C2 | 1.0 | 0.1 | 87.22±0.96 e | 3.00±0.44 fg | Average, short |
| C3 | 1.0 | 0.2 | 95.00±1.67 c | 2.89±0.10 g | Average |
| C4 | 1.5 | 0.05 | 100.00±0.00 a | 4.17±0.44 bc | Stronger |
| C5 | 1.5 | 0.1 | 100.00±0.00 a | 5.22±0.35 a | Stronger, large leaf blade, dark green |
| C6 | 1.5 | 0.2 | 100.00±0.00 a | 4.11±0.10 bcd | Stronger, dark green |
| C7 | 2.0 | 0.05 | 100.00±0.00 a | 3.67±0.17 cde | Stronger, dark green |
| C8 | 2.0 | 0.1 | 100.00±0.00 a | 4.50±0.33 b | Stronger |
| C9 | 2.0 | 0.2 | 100.00±0.00 a | 3.61±0.19 cdef | Stronger |
| C10 | 3.0 | 0.05 | 95.56±0.96 c | 3.61±0.69 cdef | Average growth, small leaf blade |
| C11 | 3.0 | 0.1 | 97.22±0.96 b | 3.50±0.44 defg | Average growth, small leaf blade |
| C12 | 3.0 | 0.2 | 100.00±0.00 a | 3.28±0.19 efg | Average growth, dwarf tufted bud, deformed seedling |
| C13 | 4.0 | 0.05 | 96.11±0.96 bc | 3.94±0.35 bcd | Weak growth, fewer leaves, red stems and leaves, glass seedlings |
| C14 | 4.0 | 0.1 | 92.22±0.96 d | 3.67±0.29 cde | Small leaf blade, dwarf tufted bud, glass seedlings, deformed seedling |
| C15 | 4.0 | 0.2 | 100.00±0.00 a | 3.56±0.19 cdef | Malformed leaf, glass seedlings |
| No. | Rooting rate (%) | Average number of roots | Maximum root length (cm) | Rooting situation | The base case |
|---|---|---|---|---|---|
| D1 | 15.74±3.21 f | 0.43±0.04 g | 3.10 | Rooting is rare, taproot is short and thick | More callus, white |
| D2 | 82.63±4.24 ab | 3.63±0.08 a | 10.50 | Taproot is long, more fibrous roots | Callus is rare or absent |
| D3 | 73.15±4.24 b | 2.19±0.06 c | 14.90 | Taproot is long, more fibrous roots | Minor callus |
| D4 | 28.70±3.21 e | 1.19±0.06 e | 2.60 | Rarely rooting | More callus, white |
| D5 | 85.19±4.24 a | 2.37±0.07 b | 12.90 | Taproot is long, fibrous roots are well developed | Callus is rare or absent |
| D6 | 60.19±4.24 c | 1.26±0.04 de | 2.50 | Taproot is thin and long, minuscule fibrous roots | Minor callus |
| D7 | 25.93±4.24 e | 0.84±0.04 f | 11.90 | Rarely rooting, a few taproots are thick and short | Large callus, white |
| D8 | 51.85±4.24 d | 1.31±0.06 d | 12.50 | Taproot is slender with a few fibrous roots | Moderate callus |
| D9 | 65.74±4.24 c | 3.59±0.08 a | 9.40 | Taproot is slender with a few fibrous roots | Moderate callus |
表7 不同浓度蔗糖、活性炭(AC)和IBA对不定芽生根的影响
Table 7 Effects of different concentrations of sucrose, active carbon (AC) and IBA on the rooting of adventitious buds
| No. | Rooting rate (%) | Average number of roots | Maximum root length (cm) | Rooting situation | The base case |
|---|---|---|---|---|---|
| D1 | 15.74±3.21 f | 0.43±0.04 g | 3.10 | Rooting is rare, taproot is short and thick | More callus, white |
| D2 | 82.63±4.24 ab | 3.63±0.08 a | 10.50 | Taproot is long, more fibrous roots | Callus is rare or absent |
| D3 | 73.15±4.24 b | 2.19±0.06 c | 14.90 | Taproot is long, more fibrous roots | Minor callus |
| D4 | 28.70±3.21 e | 1.19±0.06 e | 2.60 | Rarely rooting | More callus, white |
| D5 | 85.19±4.24 a | 2.37±0.07 b | 12.90 | Taproot is long, fibrous roots are well developed | Callus is rare or absent |
| D6 | 60.19±4.24 c | 1.26±0.04 de | 2.50 | Taproot is thin and long, minuscule fibrous roots | Minor callus |
| D7 | 25.93±4.24 e | 0.84±0.04 f | 11.90 | Rarely rooting, a few taproots are thick and short | Large callus, white |
| D8 | 51.85±4.24 d | 1.31±0.06 d | 12.50 | Taproot is slender with a few fibrous roots | Moderate callus |
| D9 | 65.74±4.24 c | 3.59±0.08 a | 9.40 | Taproot is slender with a few fibrous roots | Moderate callus |
| [1] | 邓彬 (2019). 玛瑙红樱桃幼胚培养及其遗传转化. 硕士论文. 贵阳: 贵州大学. pp. 1-71. |
| [2] | 邓小敏 (2020). 君子兰未成熟胚离体培养的研究. 农业技术与装备 (08), 55, 57. |
| [3] | 李克壮, 梅梅, 洪晓松, 葛根塔娜, 陆秀君 (2014). 美国红枫组织培养初步研究. 见:第十六届中国科协年会——分11森林培育技术创新与特色资源产业发展学术研讨会论文集. 昆明: 中国科学技术协会, 云南省人民政府. pp. 7. |
| [4] | 李林山, 张黎 (2020). 植物激素对如意皇后粗肋草离体培养的影响. 农业科学研究 41(3), 89-92. |
| [5] | 李孟悦, 刘柳, 刘艳, 张晓曼 (2021). 毛报春(Primula×pubescens)腋芽再生组织培养体系的建立. 植物学报 56, 732- 739. |
| [6] | 李清亚, 路斌, 赵佳伟, 栗浩, 李艳, 苗胜越, 路丙社 (2020). 不同豆梨品种对低温胁迫的生理响应及抗寒性评价. 西北农林科技大学学报(自然科学版) 48, 86-94, 110. |
| [7] | 李学馨 (2020). 梨继代体系优化及再生体系探索的研究. 硕士论文. 南京: 南京农业大学. pp. 1-60. |
| [8] | 廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰 (2023). 平潭野菊混合瓣型株系再生体系的建立. 植物学报 58, 449-460. |
| [9] | 龙达, 刘卫东, 张艳萍, 张雕 (2019). 观赏桃未成熟胚子叶再生植株的研究. 经济林研究 37(3), 173-179. |
| [10] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧 (2022). 大花银莲花愈伤组织诱导及再生体系的建立. 植物学报 57, 217-226. |
| [11] | 栾晓龙, 史昊, 许波, 张倩男, 刘莉 (2023). 活性炭对秋子梨组培幼苗生根的影响. 分子植物育种 (2), 589-593. |
| [12] | 吕德任, 云勇, 潘梅, 王景飞, 戚华沙 (2015). 花叶艳山姜组培苗不定根诱导研究. 中国园艺文摘 (12), 11-12, 52. |
| [13] | 潘晓, 陈瑞丹 (2010). ‘淡丰后’梅胚培养影响因素的研究. 北京林业大学学报 32(S2), 88-92. |
| [14] | 石丽敏, 宋费玲, 许巧贤, 卢华兵, 陶正明, 姜武, 吴志刚 (2021). 蔗糖浓度对白芨种子培养的影响. 浙江农业科学 62, 297-298, 338. |
| [15] | 孙大宽, 马莉, 杨宏艳, 马焕成, 郑晟靖, 唐军荣 (2022). 3种因素对木棉组培苗生根的影响. 云南农业大学学报(自然科学) 37, 137-144. |
| [16] | 孙清荣, 关秋竹, 陶吉寒, 孙洪雁 (2020). 碳源和细胞分裂素对‘库尔勒香梨’离体叶片再生不定芽的影响. 山东农业科学 52(8), 17-20. |
| [17] | 王德芬, 张梅, 李鼎立, 王然, 马春晖, 宋健坤 (2016). 秋子梨叶片高效再生体系的构建. 北方园艺 (4), 97-101. |
| [18] | 王亚琴, 韦陆丹, 王文静, 刘宝骏, 张春玲, 张俊卫, 何燕红 (2020). 万寿菊再生体系的建立及优化. 植物学报 55, 749-759. |
| [19] | 吴翠云, 阿依买木, 刘丽, 王新建 (2009). 影响野生杜梨种胚离体培养的因素. 西北农业学报 18(2), 209-212, 216. |
| [20] | 徐丽娟, 董梅, 王永香, 王倩 (2016). 激素浓度和配比对蝴蝶兰离体培养的影响. 青岛农业大学学报(自然科学版) 33, 20-23. |
| [21] | 袁振, 廖荣君, 邢刚, 刘卫东, 刘汝成, 冯殿齐 (2020). 欧李无糖组培生根研究. 山东林业科技 50(4), 39-42. |
| [22] | 张仕超 (2022). 南红梨和杜梨多倍体诱导及鉴定. 硕士论文. 杨凌: 西北农林科技大学. pp. 1-63. |
| [23] | 赵亚楠, 张懿, 谭旭, 王胜男, 姜峰, 李天忠, 朱元娣 (2021). 杜梨组培苗两步生根技术体系优化. 中国农业大学学报 26(11), 105-112. |
| [24] | 钟颖, 冯建荣, 樊新民, 任欢喜, 张秀抗, 许竹叶 (2018). 库尔勒香梨离体叶片再生体系的建立. 新疆农业科学 55, 829-836. |
| [25] | 宗宇, 孙萍, 牛庆丰, 滕元文 (2013). 中国北方野生杜梨分布现状及其形态多样性评价. 果树学报 30, 918-923. |
| [26] | Antonelli M, Druart P (1990). The use of a brief 2,4-D treatment to induce leaf regeneration on Prunus canescens Bois. Acta Hortic (280), 45-50. |
| [27] | Caboni E, Tonelli MG, Lauri P, D’Angeli S, Damiano C (1999). In vitro shoot regeneration from leaves of wild pear. Plant Cell Tissue Organ Cult 59, 1-7. |
| [28] | Long Y, Yang Y, Pan GT, Shen YQ (2022). New insights into tissue culture plant regeneration mechanisms. Front Plant Sci 13, 926752. |
| [29] | Phillips GC, Garda M (2019). Plant tissue culture media and practices: an overview. In Vitro Cell Dev Biol Plant 55, 242-257. |
| [30] | Raspor M, Motyka V, Kaleri AR, Ninković S, Tubić L, Cingel A, Ćosić T (2021). Integrating the roles for cytokinin and auxin in de novo shoot organogenesis: from hormone uptake to signaling outputs. Int J Mol Sci 22, 8554. |
| [31] | Thomas TD (2008). The role of activated charcoal in plant tissue culture. Biotechnol Adv 26, 618-631. |
| [1] | 李晶晶, 李艳飞, 王安琪, 王佳颖, 邓成燕, 卢敏, 马剑英, 戴思兰. 菊花品种‘万代风光’再生及遗传转化体系的建立[J]. 植物学报, 2025, 60(4): 1-0. |
| [2] | 李彤, 李楚然, 张芷瑜, 付晓熳, 刘云, 张颖君, 杨力颖, 赵平. 西印度醋栗组培快繁技术初探[J]. 植物学报, 2025, 60(4): 1-0. |
| [3] | 冯雯, 王玉国. 栽培薯蓣茎段离体再生体系的建立[J]. 植物学报, 2024, 59(5): 792-799. |
| [4] | 曾浩, 李佩芳, 郭至辉, 刘春林, 阮颖. 银扇草再生体系的建立[J]. 植物学报, 2024, 59(3): 433-440. |
| [5] | 武晓云, 廖敏凌, 李雪茹, 舒梓淳, 辛佳潼, 张伯晗, 戴思兰. 毛华菊3种瓣型株系再生体系的建立[J]. 植物学报, 2024, 59(2): 245-256. |
| [6] | 谢纯刚, 刘哲, 章书声, 胡海涛. 手指柠檬茎段离体再生体系建立[J]. 植物学报, 2023, 58(6): 926-934. |
| [7] | 廖敏凌, 蒲娅, 武晓云, 马朝峰, 王文奎, 戴思兰. 平潭野菊混合瓣型株系再生体系的建立[J]. 植物学报, 2023, 58(3): 449-460. |
| [8] | 逯锦春, 曹丽娜, 佟冠杰, 王鑫颖, 张利英, 喻锌, 李荟芳, 李彦慧. 大花银莲花愈伤组织诱导及再生体系的建立[J]. 植物学报, 2022, 57(2): 217-226. |
| [9] | 李孟悦, 刘柳, 刘艳, 张晓曼. 毛报春(Primula × pubescens)腋芽再生组织培养体系的建立[J]. 植物学报, 2021, 56(6): 732-739. |
| [10] | 罗钱, 张燕莎, 欧静. 郁金樱愈伤组织诱导及植株再生[J]. 植物学报, 2021, 56(4): 451-461. |
| [11] | 张冬瑞, 卜志刚, 陈玲玲, 常缨. 香鳞毛蕨的组织培养和快速繁殖体系构建[J]. 植物学报, 2020, 55(6): 760-767. |
| [12] | 罗虹, 温小蕙, 周圆圆, 戴思兰. 芳香堆心菊离体再生体系的建立[J]. 植物学报, 2020, 55(3): 318-328. |
| [13] | 邓莎, 吴艳妮, 吴坤林, 房林, 李琳, 曾宋君. 14种中国典型极小种群野生植物繁育特性和人工繁殖研究进展[J]. 生物多样性, 2020, 28(3): 385-400. |
| [14] | 张文婷,何燕红,舒宁,邢景景,刘宝骏,包满珠,刘国锋. 金黄花滇百合植株再生与离体快繁技术体系的建立[J]. 植物学报, 2019, 54(6): 773-778. |
| [15] | 徐悦,曹英萍,王玉,付春祥,戴绍军. 发根农杆菌介导的菠菜毛状根遗传转化体系的建立[J]. 植物学报, 2019, 54(4): 515-521. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||